Copyright
©The Author(s) 2018.
World J Hepatol. Jan 27, 2018; 10(1): 142-154
Published online Jan 27, 2018. doi: 10.4254/wjh.v10.i1.142
Published online Jan 27, 2018. doi: 10.4254/wjh.v10.i1.142
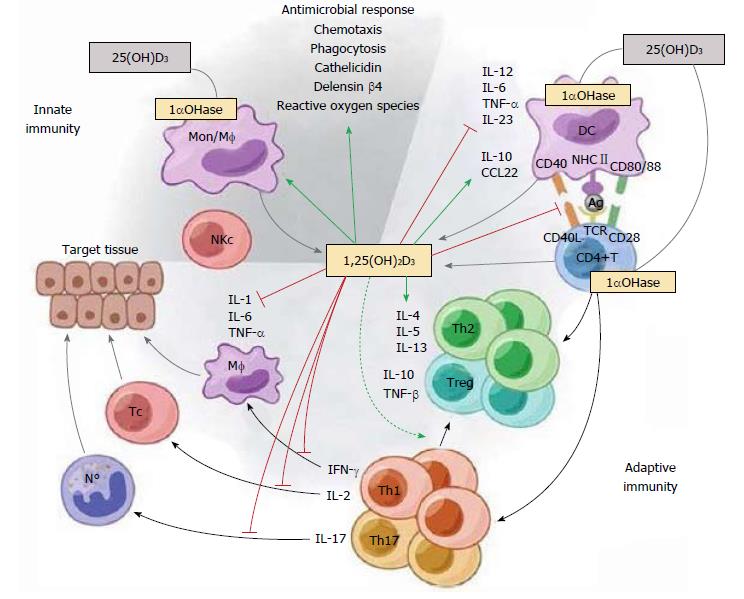
Figure 1 The immunomodulatory effects of 1,25(OH)2D3.
1,25(OH)2D3 targets different players of the innate and adaptive immune compartment. 1,25(OH)2D3 stimulates innate immune responses by enhancing the chemotactic and phagocytotic responses of macrophages, as well as the production of antimicrobial proteins such as cathelicidin. On the other hand, 1,25(OH)2D3 also modulates adaptive immunity. At the level of the APC (like the DC), 1,25(OH)2D3 inhibits the surface expression of the MHC-II-complexed antigen and co-stimulatory molecules, in addition to the production of the cytokines IL-12 and IL-23, thereby indirectly shifting the polarization of T cells from a Th1 and Th17 phenotype towards a Th2 phenotype. In addition, 1,25(OH)2D3 directly affects T cell responses, by inhibiting the production of Th1 cytokines (IL-2 and IFN-γ) and Th17 cytokines (IL-17 and IL-21), and by stimulating Th2 cytokine production (IL-4). Moreover, 1,25(OH)2D3 favors Treg cell development via modulation of DCs and by directly targeting T cells. Finally, 1,25(OH)2D3 blocks plasma cell differentiation, IgG and IgM production, and B cell proliferation. Reproduced with the permission of the Nature Publishing Group[52].
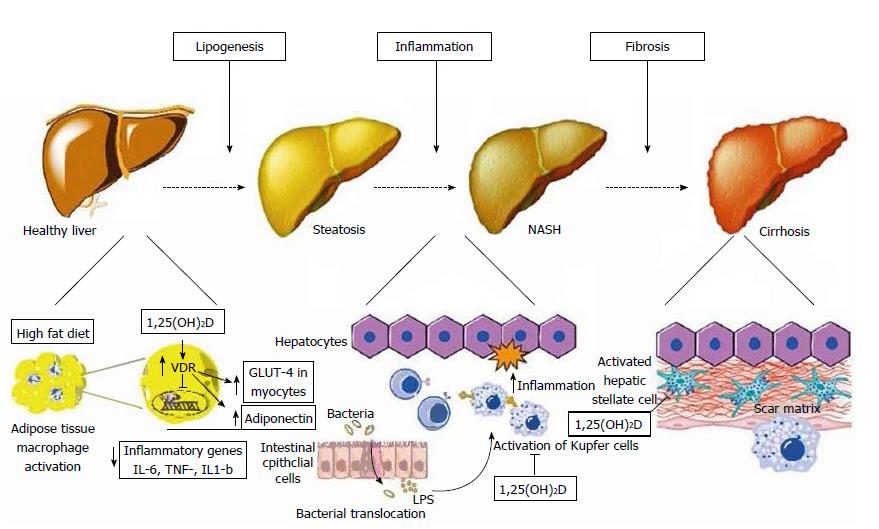
Figure 2 Schematic representation of metabolic, anti-inflammatory, and anti-fibrotic effects of vitamin D on hepatocytes and non-parenchymal hepatic cells (hepatic stellate cells, Kupffer cells) in non-alcoholic fatty liver disease.
Left: At the initial stage of lipogenesis, 1,25(OH)D acts on adipocytes and inhibits NF-κB transcription, known as the pro-inflammatory “master switch”, and thus inhibits the expression of the inflammatory cytokines IL-6, TNF-α, and IL-1β. It also increases adiponectin secretion from adipocytes and enhances GLUT-4 receptor expression in myocytes, both of which improve insulin resistance; Middle: Increased gut permeability allows the translocation of bacterial pathogens which can activate Toll-like receptors (TLR) on Kupffer cells. 1,25(OH)D downregulates the expression of TLR-2, TLR-4, and TLR-9 in these cells, thus ameliorating inflammation; Right: 1,25(OH)D acts on hepatic stellate cells by binding to VDR, which reduces the proliferation of these cells that play a major role in inducing fibrosis. VDR: Vitamin D receptor; TLR: Toll-like receptor; LPS: Lipopolysaccharide. Reproduced in compliance with Creative Commons in PubMed Central Open Access to Reproduced with the permission of the Baishideng Publishing Group Inc[9].
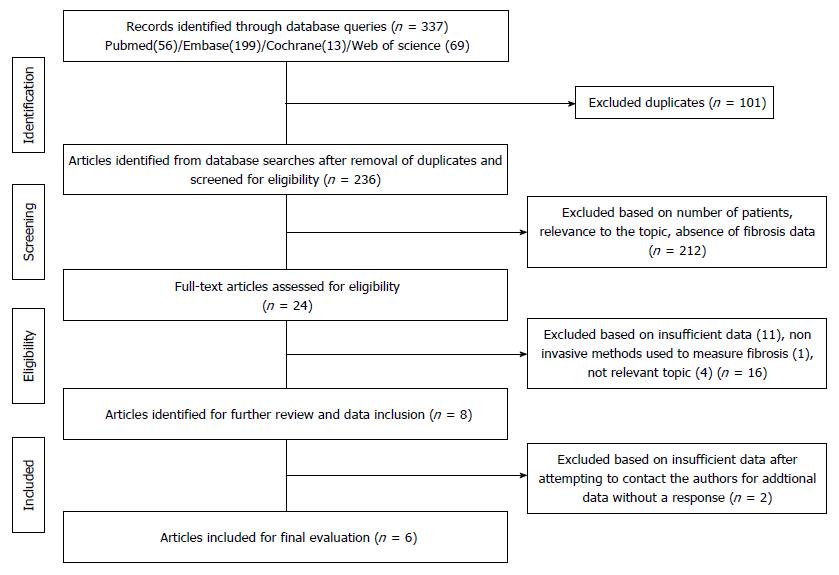
Figure 3 Flowchart illustrating the process for the selection of the included articles.
Three hundred and thirty-seven articles were identified using PubMed (n = 56)/EMBASE (n = 199)/Cochrane (n = 13)/Web of Science (n = 69) search engines. A detailed evaluation of the articles by at least two independent reviewers (total of three) assessed the sufficiency of data, the method of fibrosis qualification, and relevance to the topic in order to narrow the studies to six.
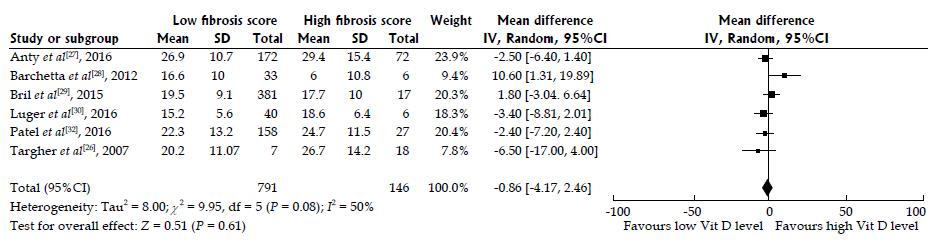
Figure 4 Random effects pooled the mean difference of 25-hydroxyvitamin D levels in nonalcoholic fatty liver disease patients with high and low fibrosis scores.
A meta-analysis of the pooled data of the six included studies according to fibrosis scores of low F0-2 vs high F3-4. Figure 4 illustrates the forest plot of the results of the six included studies, with 95%CI, and the overall effect (under the random-effects model) with 95%CI are illustrated in this forest plot. The six included studies[26-30,32] assessed the association of 25-hydroxyvitamin D among patients with nonalcoholic fatty liver disease (NAFLD). We used a random-effects model to assess the pooled data in a meta-analysis as previously described[36]. The statistical heterogeneity was not significant with I2 of 37.8% (Pheterogeneity = 0.0766); however, we observed a trend towards high heterogeneity. We found no difference in 25-hydroxyvitamin D among NAFLD patients with high (F3-4) vs low (F0-2) fibrosis, with the summary effect size of 0.95 representing mean differences between F0-2 and F3-4 NAFLD patients. Overall, our analysis confirmed that there was no association between serum 25-hydroxyvitamin D and low vs high fibrosis score in NAFLD patients from the six included studies.
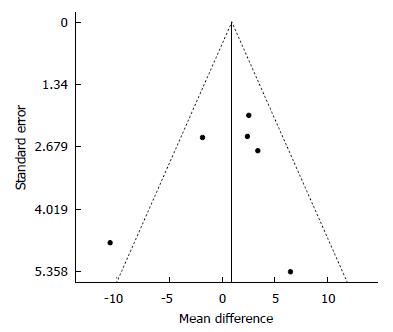
Figure 5 Funnel plot of standard error by differences in Means for 25(OH)D.
We analyzed the data for a possible publication bias. The circles represent observed published studies. The funnel plot was asymmetric, thereby suggesting a possible publication bias.
- Citation: Saberi B, Dadabhai AS, Nanavati J, Wang L, Shinohara RT, Mullin GE. Vitamin D levels do not predict the stage of hepatic fibrosis in patients with non-alcoholic fatty liver disease: A PRISMA compliant systematic review and meta-analysis of pooled data. World J Hepatol 2018; 10(1): 142-154
- URL: https://www.wjgnet.com/1948-5182/full/v10/i1/142.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i1.142