Published online Oct 26, 2019. doi: 10.4252/wjsc.v11.i10.764
Peer-review started: March 20, 2019
First decision: April 16, 2019
Revised: May 17, 2019
Accepted: August 27, 2019
Article in press: August 27, 2019
Published online: October 26, 2019
Processing time: 219 Days and 11.4 Hours
Mesenchymal stem cells (MSCs) are stromal multipotent stem cells that can differentiate into multiple cell types, including fibroblasts, osteoblasts, chondrocytes, adipocytes, and myoblasts, thus allowing them to contribute to the regeneration of various tissues, especially bone tissue. MSCs are now considered one of the most promising cell types in the field of tissue engineering. Traditional petri dish-based culture of MSCs generate heterogeneity, which leads to inconsistent efficacy of MSC applications. Biodegradable and biocompatible polymers, poly(3-hydroxyalkanoates) (PHAs), are actively used for the manufacture of scaffolds that serve as carriers for MSC growth. The growth and differentiation of MSCs grown on PHA scaffolds depend on the physicochemical properties of the polymers, the 3D and surface microstructure of the scaffolds, and the biological activity of PHAs, which was discovered in a series of investigations. The mechanisms of the biological activity of PHAs in relation to MSCs remain insufficiently studied. We suggest that this effect on MSCs could be associated with the natural properties of bacteria-derived PHAs, especially the most widespread representative poly(3-hydroxybutyrate) (PHB). This biopolymer is present in the bacteria of mammalian microbiota, whereas endogenous poly(3-hydroxybutyrate) is found in mammalian tissues. The possible association of PHA effects on MSCs with various biological functions of poly(3-hydroxybutyrate) in bacteria and eukaryotes, including in humans, is discussed in this paper.
Core tip: Biodegradable and biocompatible polymers, poly(3-hydroxyalkanoates) (PHAs), are actively used for the manufacture of scaffolds that serve as carriers for mesenchymal stem cell growth in tissue engineering. It was shown that PHAs have their own biological activity affecting mesenchymal stem cell growth and differentiation. However, the mechanisms of the biological activity of PHAs remain unclear. In this review, we discuss the possible association of the effects of bacteria-originating PHAs on mesenchymal stem cells with various biological functions of PHAs in bacteria and eukaryotes, including in humans.
- Citation: Voinova V, Bonartseva G, Bonartsev A. Effect of poly(3-hydroxyalkanoates) as natural polymers on mesenchymal stem cells. World J Stem Cells 2019; 11(10): 764-786
- URL: https://www.wjgnet.com/1948-0210/full/v11/i10/764.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i10.764
Mesenchymal stem cells (MSCs) are multipotent progenitor stem cells that can differentiate into multiple mesenchyme cell types, including fibroblasts, osteoblasts, chondrocytes, adipocytes, and myoblasts, and this capacity contributes to the regeneration of mesenchymal tissues such as bone, cartilage, muscle, ligament, tendon, adipose, and stroma. Due to these properties, MSCs are now considered one of the most promising cell types in the field of tissue engineering[1,2].
Tissue engineering is a multidisciplinary scientific, technological direction that combines the latest developments in engineering, materials science, cellular biology, biochemistry, and medicine, thus offering new approaches for the restoration of tissue functions. Tissue engineering is a fundamentally different paradigm in relation to surgery and transplantation. It is based not on approaches related to the substitution or functional compensation of tissue but on tissue regeneration: the organism itself can repair the damaged tissue if the appropriate conditions are provided. Tissue engineering involves an appropriate combination of cells (e.g., MSCs), scaffolds, and bioactive molecules. Together, these interactions allow intercellular communication and cell-biomaterial interactions, which work toward achieving the desired therapeutic response[3,4].
Different types of biomaterials can be used for the development of scaffolds for tissue engineering: metals, ceramics, and synthetic or natural polymers have been used. Nevertheless, biodegradable and biocompatible polymers of natural or synthetic origin, polyhydroxyalkanoates, are believed to be one of the most promising biomaterials for developing scaffolds for bone regeneration. Since the beginning of the 21st century, medical devices and dosage forms based on polyhydroxyalkanoates have been actively introduced into medical practices. The following types of chemically synthetic polyhydroxyalkanoates are actively used in clinical practice and scientific research: poly(2-hydroxypropanoic) (polylactic, PLA, polylactides), poly(2-hydroxyacetic) (polyglycolic, PGA, polyglycolides), poly(6-hydroxycaprolactone), and their copolymers, for example, polylactide-co-glycolides (PLGAs) and polymers similar in structure, such as poly-p-dioxanone[5]. Natural polyhydroxyalkanoates – poly(3-hydroxyalkanoates) (PHAs) represent polyesters of 3-hydroxyalkanoic acids, e.g., poly(3-hydroxybutyrate) (PHB) is a linear polyester of 3-hydroxybutyric acid. The biopolymer includes only the R-form of 3-hydroxybutyric acid due to which it is a partially crystalline polyester in an isolated and purified form (the PHB crystallinity is 55%-80%). For industry and research purposes, natural PHAs can be produced biotechnologically by bacterial producer strains[6]. The following natural PHAs can be distinguished depending on the length of the alkane side radical: PHB, poly(3-hydroxyvalerate), poly(3-hydroxyhexanoate), poly(3-hydroxyoctanoate), etc. During bacterial biosynthesis, as a rule, it is not homopolymers of other PHAs but their block-copolymers with 3-hydroxybutyrate that are the most frequently obtained: poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx), poly(3-hydroxybutyrate-co-3-hydroxyoctanoate), etc. All of them are quite different in their physicochemical properties, such as crystallinity, melting temperature, glass transition temperature, hydrophobicity, plasticity, elastic modulus, and others[7,8].
The chemical structure and the main properties of synthetic poly(2-hydroxyalkanoates) (PLA, PGA, and their copolymers PLGA) are similar to the chemical structure and the physicochemical and biological properties of natural PHAs: the ability to biodegrade in an organism without the formation of toxic products; biocompatibility with human organs and tissues; optimal mechanical properties (relatively high strength, plasticity); other physicochemical properties (thermoplasticity, specific diffusion properties); and the opportunity to use efficient technological processes for their production[5-9]. Therefore, the study of natural PHAs (including PHB) as biomaterials for the regeneration of different tissues and organs is of paramount importance. Chemically synthetic PHAs can be considered biomimetic analogs of PHB[5,9]. However, it should be taken into account that the properties of natural biopolymers are determined by their functions in nature[10]. In particular, the properties of bacteria-origin biopolymers are closely associated with their role in the bacterial world. In most cases, this important information is not taken into account in research on the biomedical applications of various biomaterials. However, why should we not give it proper importance? In this review, we attempt to fill these gaps in the current scientific field by considering the natural properties of polymers in connection with their effect on cells.
A high in vitro biocompatibility of PHB has also been shown in studies using cell cultures. Therefore, PHB is a promising material for tissue engineering. Cell cultures of various origins, including murine and human fibroblasts, rat, rabbit, and human MSCs, human osteogenic sarcoma cells, human epithelial cells, human endothelial cells, rabbit articular cartilage chondrocytes, rabbit smooth muscle cells, and human neurons in direct contact with PHB when cultured on polymer films and scaffolds exhibited satisfactory levels of cell adhesion, viability, and proliferation. However, the study of the effect of PHAs on the growth and differentiation of MSCs is of particular importance for the use of PHAs as biomaterials for tissue engineering[8,11].
Osteogenic differentiation of MSCs in vitro
It was shown that human[12,13], rat[14-17], and rabbit[18] MSCs isolated from both adipose tissue[14,15] and bone marrow[14-17], murine calvarial preosteoblast cells MC3T3-E1 S14[19], and human induced pluripotent stem cells[20] grown on scaffolds from PHB and its copolymers PHBV and PHBHHx undergo spontaneous differentiation in the osteogenic direction in regular medium. Osteogenic differentiation in osteogenic medium is more pronounced (Table 1). The osteogenic differentiation of MSCs and osteoblastic cells grown on PHA scaffolds was confirmed by a change in cell morphology[13,15,17,18,21], inhibition of their proliferation[14,15,17], an increase in alkaline phosphatase (ALP) activity, deposition of calcium salts[12,15,22], and the expression of markers of osteogenic differentiation and bone formation (ALP, type 1 collagen, Runx2, osteocalcin, osteopontin) determined by enzyme immunoassay and PCR[12,15,16,20].
| The type of PHA | Surface topography/modification, 3D-scaffolds microstructure | The type of stem cells | The time of cell cultivation | The effect of PHAs on the proliferation of stem cells | The effect of PHAs on the morphology of stem cells | The effect of PHAs on the differentiation of stem cells | Ref. |
| Osteogenic differentiation | |||||||
| PHB (Mw = 150000) doped with PEG 1000 | Porous flat and cubic scaffolds produced by salt leaching from polymer solution using sucrose crystals and ammonium carbonate as porogens | Rat bone marrow MSCs | 1, 3, 6, 14 d (proliferation tests); 6, 14 d (differentiation tests) | Suppressed cell proliferation in comparison with TCPS (XTT assay) | Good quality of cell adhesion and spread with developed filopodia (SEM) | Spontaneous osteogenic differentiation in regular medium (increased ALP activity on the 7th and 14th days by up to 10 times and CD45 expression in comparison with TCPS) (ALP activity assay, immunocytochemistry using flow cytometer) | [14,28] |
| PHBV (with 5 mol % 3-HV) | Porous flat scaffolds produced by salt leaching from polymer solution using sucrose crystals as porogens; oxygen plasma treated and untreated | Rat bone marrow stromal osteoblastic cells (rat bone marrow MSCs) | 7, 14, 21, 28 d | Low cell proliferation (60% at day 7 for untreated scaffolds). Treatment with oxygen plasma slightly increased (up to 50%) cell growth (MTS) | - | Spontaneous osteogenic differentiation in regular medium (increased ALP activity on the 28th day by up to 10 times). Treatment with oxygen plasma did not change this effect (ALP activity assay) | [22] |
| PHBV (with 8 mol % 3-HV) | Porous flat scaffolds produced by salt leaching from polymer solution using sucrose crystals as porogens; oxygen plasma treated and untreated | Rat bone marrow stromal osteoblastic cells (rat bone marrow MSCs) | 7, 14, 21, 29, 60 d | Low cell proliferation (2.2 times at day 60 for untreated scaffolds). Treatment with oxygen plasma slightly increased (up to 23%) cell growth (MTS) | Spindle shaped cells on the 29th day with cytoplasmic extensions; large ovoid cells with osteoblast-like morphology on the 60th day. Mineralization from day 21 to day 60. A close connection between the cell boundary and the scaffold (SEM, histology, CM) | Spontaneous osteogenic differentiation in regular medium (increased ALP activity and osteocalcin expression at day 60 by up to 12 times and 4 times, respectively). Treatment with oxygen plasma did not change the induction of ALP activity or increase the induction of osteocalcin expression (ALP activity assay, test for osteocalcin) | [17] |
| PHBV | Porous scaffolds produced by freeze-drying | Human adipose-derived MSCs | 2, 7, 14, 21, 28 d | Increased cell proliferation from days 7 to 28 (MTT) | Good quality of cell adhesion and spread with developed cytoplasmic extensions (SEM) | Signs of spontaneous osteogenic differentiation in regular medium (slightly increased ALP activity at day 28 by up to 10 times). Inhibition of osteogenic differentiation (ALP activity assay) or not (proteins expression assays) in osteogenic medium (osteopontin, collagen I type, osteocalcin indirect immunofluorescence) | [12] |
| PHB | Porous cubic scaffolds produced by salt leaching from polymer solution using sucrose crystals as porogens | Rat bone marrow and adipose-derived MSCs | 3, 7, 14, 21 d (proliferation tests); 7, 14, 21 d (differentiation tests) | Low cell proliferation (up to 2 times at day 21) (MTT assay) | Spherically shaped cells grouped within cell clusters at day 21; calcium deposition (SEM) | Signs of spontaneous osteogenic differentiation in regular medium (a very slight increase in ALP activity and osteocalcin expression at day 21 by up to 4 times); osteocalcin expression; and calcium deposition at day 21 (ALP activity assay, von Kossa staining, PCR for osteocalcin). | [15] |
| PHBV (with 12 mol % 3-HV) doped with poly(ethylene oxide) (Mw ≈ 1000000) with a mass ratio of 9:1 | Scaffolds produced by electrospinning with randomly oriented nanofibers | Rat bone marrow MSCs | 1, 4, 7 d (proliferation tests); 7, 14 d (differentiation tests) | Relatively high cell proliferation rate (up to 3-fold at day 7 (CCK-8 test) | Good quality of cell spread in random directions, filopodia extend along the fibers (SEM) | Slight signs of spontaneous osteogenic differentiation in regular medium (a very slight increase in ALP expression at day 14) (ALP staining, PCR), or no effect (staining and expression of osteocalcin, calcium deposition) | [16,21] |
| PHB, PHBHHx (Mw = 470000) | Porous flat scaffolds produced by salt leaching from polymer solution using salt crystals as porogens | Rabbit bone marrow MSCs | 10 d | Higher proliferation in comparison with PLA (MTT assay) | Typical osteoblast phenotype, calcium deposition at day 10 (SEM) | Spontaneous osteogenic differentiation in regular medium at day 10 (ALP activity assay) | [18] |
| PHBV (with 5 mol % 3-HV) | Films casted from polymer solution | Murine calvarial preosteoblast cells MC3T3-E1 S14 line | 21 d | - | A dense monolayer of cuboidal-shape cells with obvious areas of mineralization (CM) | Higher stimulation of osteogenic differentiation in comparison with cells grown on TCPS in osteogenic medium (Calcium C test, von Kossa staining) | [19] |
| PHBV (with 12 mol % 3-HV; Mw = 530000) | Scaffolds produced by electrospinning with randomly oriented fibers | Human bone marrow MSCs | 1, 7, 11 d (proliferation tests); 7 d (differentiation tests) | No difference between cell proliferation on scaffolds from PHBV and poly-ε-caprolactone (trypan blue assay) | Good quality of cell adhesion and spread with developed filopodia. Cells have a nearly spherical shape (CM) | Higher modulation of osteogenic differentiation in comparison with MSCs grown on poly-ε-caprolactone scaffolds in osteogenic medium (calcein and Alizarin red staining assay) | [13] |
| PHBV (with 5 mol % 3-HV, Mw = 680000) | Scaffolds produced by electrospinning with randomly oriented nanofibers | Human induced pluripotent stem cells | 1, 3, 5, 7, 10 d (proliferation tests); 7, 14 d (differentiation tests) | The higher proliferation rate in comparison with TCPS (MTT assay) | Original-like MSCs formed colonies at day 5 (LM). | Higher stimulation of osteogenic differentiation in comparison with cells grown on TCPS in osteogenic medium (ALP activity assay, RT-PCR for measure of ranx-2, col-1, ALP, osteonectin, osteocalcin expression levels; Western blot assay for osteocalcin and osteopontin expression levels) | [20] |
| PHBHHx (with 12 mol % 3-HHx), and PHBVHHx | Films casted from polymer solution | Human bone marrow MSCs | 4, 72 h (proliferation tests); 14, 21 d (differentiation tests) | Higher cell proliferation rate (for PHBHHx and PHBVHHx) in comparison with PLA and with TCPS (for PHBVHHx) | - | No effect on cell differentiation in regular medium (FM: ALP and van Kossa staining) | [23] |
| PHBHHx (with 8.3 mol % 3-HHx, Mw = 1210000) | Smooth compression-molded films, porous scaffolds casted from solution films, electrospun scaffolds | Human bone marrow MSCs | 5, 6, 7, 14 d (proliferation tests); 14 d, 5 wk (differentiation tests) | The same (for compression-molded films) and higher (up to 2-fold for solution-casted and electrospun films) cell proliferation in comparison with TCPS | Spindle-like, similar to original MSCs, good cell adhesion and spreading in regular medium (FM, SEM). | No differentiation in regular medium. Inhibition of differentiation in osteogenic medium (osteocalcin assay, Alizarin red S staining, RT-PCR for measure of collagen I and osteonectin expression level) | [24] |
| PHBHHx (Mw = 300000) | Scaffolds produced by electrospinning with randomly oriented fibers | Rat bone marrow MSCs | 3 d | - | The well-developed stress fibers spanned the entire cell body and supermature focal adhesions (CM, immunofluorescence) | No osteogenic differentiation: no significant expression of osteocalcin, osteopontin, osteonectin, runx2, in regular and osteogenic media at day 3 (RT-PCR) | [25] |
| Chondrogenic differentiation | |||||||
| PHB and PHBHHx in a ratio of 1:2 (by weight) | Porous flat scaffolds with pores of 200–300 μm in diameter produced by salt leaching from polymer solution using salt crystals as porogens and lyophilization | Human adipose-derived stromal cells | 7 d (proliferation tests); 14 d, 5 wk (differentiation tests) | 100% cells viability at day 7 (FM) | After 1 d, the differentiated cells attached to scaffolds. At 7 d and 14 d, the differentiated cells produced extracellular matrices to fill the voids of the scaffolds (SEM) | Chondrogenic differentiation in chondrogenic medium: increased glycosaminoglycan and collagen content. No chondrogenic differentiation in regular medium: a very slight increase in collagen content (biochemical glycosaminoglycan and collagen content assays) | [27] |
| PHBHHx (Mw = 600000) | Films casted from polymer solution | Murine bone marrow MSCs; chondrocytes isolated from cartilage from knees of mice | 1 d (24 h) | No change in cell proliferation in comparison with TCPS (RT-PCR analysis of proliferating cell nuclear antigen) | - | Spontaneous chondrogenic differentiation in regular medium at day 1 (as in chondrocytes with the exception of col1): upregulation of aggrecan, col2, sox9, col10, pthrp, and col1 genes, downregulation of osteocalcin, Cbfa1/Runx2, MMP13 genes, microRNAs miR-29a and miR-29b (alcian blue staining for glycosaminoglycans; immunostaining for type II collagen; RT-PCR analysis of chondrogenic markers) | [26] |
| PHBV | Porous cylindrical (5 mm diameter, 2 mm thick) scaffolds with a pore size of 30-300 μm produced by salt leaching | Swine bone marrow MSCs, cartilage progenitor cells, and chondrocytes | After 1 wk of in vitro culture subcutaneous implantation in vivo into nude mice for 6 wk. | Higher wet weight and volume of the cell-scaffold constructs seeded with cartilage progenitor cells and chondrocytes in comparison with MSCs 6 wk after implantation. | Good compatibility between the cells and the scaffold and production of considerable amounts of extracellular matrix after 1 wk of in vitro culture (SEM) | Higher chondrogenic differentiation in vivo of cartilage progenitor cells and chondrocytes: increased expression of sox-9, collagen II, aggrecan, safranin-O, glycosaminoglycans in comparison with MSCs (alcian blue staining, immunostaining, RT-PCR) | [29] |
| PHBHHx | Films casted from polymer solution | Human umbilical cord MSCs | 3, 7, 14 d (proliferation tests); 14, 28 d (differentiation tests) | Higher MSCs proliferation in chondrogenic medium in comparison with TCPS (MTT assay) | Good spreading of cells and their proliferation (SEM) | Chondrogenic differentiation in chondrogenic medium: increased glycosaminoglycan, proteoglycan, and collagen content, upregulation of collagen II and aggrecan, genes (amino-sugars and hydroxyproline assays, toluidine blue staining, RT-PCR) | [28] |
| Epidermal differentiation | |||||||
| PHBV, (Mw = 450 kDa) | Scaffolds produced by electrospinning with randomly oriented nanofibers | Bone marrow MSCs | 1, 3, 7, 14 d (proliferation tests); 3, 7, 14 d (differentiation tests) | 100% viability of cells. Higher cell proliferation in epidermal-induction medium in comparison with regular medium. Lower cell proliferation on PHBV scaffolds in regular and epidermal-induced medium in comparison with TCPS (CM, MTS assay) | Good adhesion and spreading of cells tended to start forming a monolayer at day 7. The spindle-like, fully extended morphology of MSCs at day 3. The keratinocytic morphology of MSCs characterized by polygonal cells at days 7 and 14 (SEM, CM) | The epidermogenic differentiation in epidermal-induction medium: increased expression of keratin, filaggrin, and involucrin. No epidermogenic differentiation in regular medium (CM, RT-PCR) | [38] |
| Adipogenic differentiation | |||||||
| PHBHHx (Mw = 300000) | Scaffolds produced by electrospinning with randomly oriented fibers | Rat bone marrow MSCs | 3 d | - | Well-developed stress fibers spanned the entire cell body and supermature focal adhesions (CM, immunofluorescence) | No adipogenic differentiation: no significant expression of PPARg, Lpl, ADFP, CD36 in regular and adipogenic media at day 3 (RT-PCR) | [25] |
| Endotheliogenic differentiation | |||||||
| PHB/PHBV composite (30:70) | Scaffolds produced by electrospinning with randomly oriented nanofibers | Human adipose tissue-derived MSCs | 7, 14, 21 d (proliferation tests); 7, 14, 21 d (differentiation tests) | Lower cell proliferation on scaffolds in endothelial-induction medium in comparison with TCPS. Higher cell proliferation on scaffolds in regular medium in comparison with TCPS (CM, MTT assay) | Good adhesion and spread, typical spindle-shape morphology, and cell-to-cell interactions. Good distribution of cells in regular medium. MSCs in endothelial-induction medium formed circle-like structures characteristic of endothelial cell organization, mimicking the tubular organization of blood vessels at day 21 (SEM, CM, calcein-AM staining) | Endotheliogenic differentiation in endothelial-induction medium: increased expression of VE-Cadherin, vWF factor, and VEGFR2 (immunostaining, flow cytometry, RT-PCR) | [39] |
| Neurogenic differentiation | |||||||
| PHBHHx and PHBVHHx | Films casted from polymer solution, porous scaffolds with a pore size of 110-170 μm produced by in solution phase separation | Human bone marrow MSCs | 2, 3 d (proliferation tests); 7, 14, 21 d (differentiation tests) | Slightly higher cell proliferation on PHBHHx and PHBVHHx films (33% and 31%, respectively) in comparison with PLA films in regular medium at day 3 (CCK-8 assay) | Good adhesion, spread, and proliferation in PHBHHx and PHBVHHx films and PHBVHHx scaffolds in regular medium (SEM, CM) | Neurogenic differentiation of neural stem cells in neurogenic medium: increased expression of nestin, β-tubulin III and anti-glial fibrillary acidic protein (slightly higher expression levels of these markers in cells grown on PHBHHx and PHBVHHx films in comparison with PLA) | [34] |
| PHBHHx and PHBVHHx | Films casted from polymer solution | Rat neural embryonic stem cells | 1, 3, 5 d (proliferation tests); 3, 7 d (differentiation tests) | Same cell proliferation and viability on PHBHHx and PHBVHHx films in comparison with PLA films in neurogenic medium (CCK-8 assay) | Bipolar or even monopolar morphology of the cells with relatively short neuritis at day 3 in neurogenic medium (CM) | Neurogenic differentiation of neural stem cells in neurogenic medium: increased expression of β-tubulin III and anti-glial fibrillary acidic protein (the same level of this marker expression in cells grown on PLA, PHBHHx and PHBVHHx films) | [36] |
| PHB, PHBV, P3HB4HB, and PHBHHx | Films casted from polymer solution, porous scaffolds produced by in solution phase separation and freeze-drying | Rat neural embryonic stem cells | 7, 10 d (proliferation tests); 7, 14, 21 d (differentiation tests) | Slightly higher cell proliferation on PHB, PHBHHx and PHB4HB films in comparison with PLA films in neurogenic medium (CCK-8 assay) | Cells with extended processes and plausible neurite connections at days 3 and 7 in neurogenic medium (CM, SEM) | Neurogenic differentiation of neural stem cells in neurogenic medium: increased expression of β-tubulin III (higher expression level of this marker in cells grown on PHB4HB and PHBHHx in comparison with PHB) | [37] |
Chondrogenic differentiation of MSCs in vitro
However, other studies have not confirmed the induction of osteogenic differentiation of MSCs on PHBHHx scaffolds[23-25]. It was shown that the spontaneous differentiation of MSCs grown on PHBHHx scaffolds occurs in the chondrogenic direction, which was revealed by a change in the expression of the chondrogenic marker genes of MSCs: Aggreggan, col2, sox9, col10 and pthrp[26]. PHB, PHBV, and PHBHHx scaffolds support chondrogenic differentiation in the chondrogenic medium[27,28]. Chondrogenic differentiation was also demonstrated in vivo after implantation of PHB scaffolds seeded with MSCs and was associated with the expression of sox-9, collagen II, aggrecan, safranin-O, and glycosaminoglycans[29].
Proliferation of MSCs in vitro
In spite of stimulation of MSC osteogenic differentiation in regular medium, the proliferation of MSCs grown on scaffolds from PHB and PHBV can both have a higher[18,20,21,30] or lower rate[15,17,28,31] in comparison with controls (e.g., tissue culture plastic) in contrast to the suppression of MSC proliferation in osteogenic or chondrogenic media where MSC differentiation was pronounced[17-20]. PHBHHx scaffolds contribute to increasing cell growth[23,24,30,32] but can also cause apoptosis[33] (Table 1).
Other types of MSC differentiation in vitro
Moreover, in neurogenic medium PHB[35,37], PHBV[37], PHBHHx[34,36,37], PHBVHHx[34,36], poly(3-hydroxybutyrate-co-3-hydroxyoctanoate)[35], PHB4HB[37], and their composites support the differentiation of MSCs in the neurogenic direction in vitro, which results in a change in cell morphology and expression of genes of specific proteins: Nestin, glial fibrillary acidic protein, and βIII-tubulin[34-37]. The differentiation of MSCs in the epidermal (to keratinocytes) and endothelial (to vascular endothelial cells) directions was also demonstrated in appropriate special stimulating media using nanofibrous scaffolds produced by electrospinning as a substrate for cell growth[38,39].
The osteoinductive properties of PHB and its copolymers were displayed not only in vitro but also in vivo when scaffolds derived from these polymers were used as bone-substituting biomaterials and were implanted into bone defects. PHB, the main polymer of a homologous series of the family of PHAs, is the best-known microbiological polyester, which is a promising alternative to biodegradable synthetic thermoplastics and is actively used in regenerative medicine and tissue engineering. The biodegradation rate of PHB and its copolymers is much lower than the biodegradation rate of synthetic PLA, PGA, and their copolymers with the very intensive process of bone tissue reconstruction, which makes PHB use for the regeneration of bone tissue more relevant. Polymer biodegradation mainly occurs due to the phagocytic activity of specialized cells (macrophages, foreign body giant cells, and osteoclasts), i.e. specialized cellular biodegradation of these biopolymers takes place. The greater biocompatibility of PHB and its copolymers compared with synthetic PHAs due to the absence of the effect of acidification of surrounding tissues by the polymer biodegradation products is also of great importance. When the tissue reaction to PHB was compared with tissue reactions to synthetic PLA, PGA polyesters, and their copolymers, several studies found mild or moderate tissue reactions to PHB, while severe chronic inflammatory reactions were not infrequently observed for PLA, PGA, or PLGA[8,11].
Porous scaffolds and patches from PHB were shown to promote bone tissue regeneration, which was demonstrated in experimental models of critical (parietal bone of the rat skull) and noncritical (rat femur) bone defects. At all stages of the process of bone defect regeneration, a minimally pronounced tissue reaction to implantation was observed. This reaction was associated with the gradual bioresorption of the polymeric material by osteoclasts, active vascularization of the scaffolds, and sprouting of the newly formed bone tissue into the pores of the PHB scaffolds[8,11]. Regeneration of bone tissue in the PHB scaffolds was also indicated by the elevated expression of osteogenic markers, e.g., collagen type I[15,40]. or their expression was in opposite reduced[41]. We observed the appearance of the newly formed bone tissue evenly throughout the volume of the porous biopolymer scaffolds in the form of islands, not from its edges, while a fibrous capsule was not formed around the material, indicating complete integration of the biopolymer material with the bone tissue[42]. Moreover, it was shown that other bone tissue devices based on PHB (nonwoven patches, porous scaffolds) could contribute to the regeneration of other tissues of various organs, including nerves, intestine, heart, and vessels. A high level of vascularization (angiogenesis) and the increased expression of vascular endothelial growth factor were shown in the tissue defect area during regeneration using PHB devices[8,11,15,41]. It should be noted, however, that PHB and its copolymers can activate immune cells during implantation causing the secretion of proinflammatory cytokines by these cells[43,44], which is a typical tissue reaction to implantation of virtually any material.
The problem of the interaction of PHAs and MSCs is closely related to the use of these polymers for the manufacture of medical devices for regenerative surgery and tissue engineering of the intestine using devices from synthetic polyhydroxyalka-noates. The successful transplantation of tubular prostheses on the basis of PGA nonwoven material was carried out to replace a dog esophagus. For this purpose, the prostheses were seeded with fibroblasts and keratinocytes and placed in the abdominal cavity to develop the tissue engineering construction in situ. Development of the mucosa and muscular membrane was observed after the esophageal substitution, while the prosthesis that was not seeded with cells was rejected[45].
Successful use of the tissue engineering construction based on a surgical mesh fabricated of PGA with a PLA cover as a patch for the stomach wall was demonstrated in a model of stomach wall defects in rats. The surgical mesh was seeded with embryonic epithelial cells, and patch fusion with the stomach wall tissues and the development of mucous and smooth muscle membranes were demonstrated[46]. Japanese researchers developed a tissue engineering construction based on a porous tubular prosthesis from PGA seeded with embryonic epithelial intestine cells. In rats, the anastomosis of a tubular prosthesis with rat jejunum was surgically generated to analyze the regenerative potential of the construction, and the development of mucosa with villous epithelium on a polymer scaffold and its integration with the intestine wall tissues were observed[47]. With improvements in the endoscopic suturing technique, new opportunities for the application of tissue engineering techniques arise for the regeneration of gastrointestinal tract tissues; good results have already been demonstrated, proving the techniques to be safe and reliable. Takeshita et al[48] successfully used a poly(6-hydroxycaprolactone) scaffold produced by 3D printing and seeded with fibroblasts to close intestinal wall defects by an endoscopic suturing technique.
Intestinal progenitor stem cells are intensively used for tissue engineering of the stomach, small intestine, and bowel in combination with polymer scaffolds[49,50]. However, investigation of intestinal stem cells on scaffolds from PHAs or even synthetic polyhydroxyalkanoates or the development of tissue-engineered devices based on PHA scaffolds seeded with intestinal stem cells are very rare. A good example of such rare studies of particular interest is the work of Costello CM[51] and Shaffiey SA[52]. Their porous scaffolds based on PLGA of a complex microarchitecture (modeling the small intestine mucosa villi) were developed for tissue engineering of the intestinal mucosa. The active growth of the intestinal epithelial cells was demonstrated on the obtained scaffolds using both the commercial Caco-2 line and cells isolated from the human intestinal mucosa. The development of villus-like, polymer-cellular hybrid structures, the differentiation of cultivated cells in functional cells of the intestinal mucosa (enterocytes, goblet cells, and Paneth cells), and mucus production was demonstrated[51]. Then, the researchers developed a tissue-engineered construction of such a complicated structure seeded with coculture of intestinal stem cells, myofibroblasts, macrophages, and probiotic bacteria and demonstrated enhanced mucosal regeneration in the dog rectum upon using this construction[52].
The microstructure and topography of the surface on which cells grow can play particularly important roles in the growth and differentiation of MSCs. Moreover, these features can even reverse the effects of bioactive molecules on cell growth or differentiation[24,25,53]. The effect of the biological activity of polymers on the growth and differentiation of MSCs both in vitro and in vivo depends on the microstructure of 3D devices made from them: surface topography[24], 3D-microstructure[25], porosity, pore size, and shape[9,54]. Different cells prefer different surfaces. For example, MSCs and osteoblasts prefer rougher surfaces with the appropriate size of pores[55,56], while fibroblasts prefer smoother surfaces, and epithelial cells attach only to the smoothest surface[57]. This appropriate roughness affects cell attachment because it provides the right space for the growth of MSCs or supplies solid anchors for their filopodia. A scaffold with the appropriate size of pores provides better surface properties for anchoring type II collagen filaments and for their penetration into internal layers of the scaffolds seeded with chondrocytes, which is supported by the interaction of extracellular matrix proteins with the material surface[24,25].
However, the study of the proliferation and differentiation of MSCs grown on electrospun scaffolds with randomly oriented and aligned fibers yields contradictory data: the aligned fibers stimulate the osteogenic differentiation of MSCs[25], or there was no significant difference in cell differentiation compared with randomly oriented fibers[16]. Moreover, scaffolds with porous surfaces can inhibit the osteogenic differentiation of MSCs cultivated in the osteogenic medium[24]. The appropriate surface properties may also promote cell attachment and proliferation by providing more spaces for better gas/nutrient exchange or more serum protein adsorption[9,54].
The scaffolds from PHAs, which are manufactured by a series of techniques (salt leaching, electrospinning, phase separation, blending, etc) have a fibrous porous microstructure, which is a biomimetic structure similar to the extracellular matrix[58]. Surprisingly, PHAs are easily processed by various methods to produce such biomimetic structures (Table 1). It may also be related to the natural properties of PHAs, such as the complex structure of bacterial carbonosomes, where the synthesis of PHB is carried out[59].
It is generally accepted that the effect of PHAs on the growth and differentiation of MSCs is connected with the physicochemical properties of the polymeric material and the 3D and surface microstructure of scaffolds made from this polymer. Indeed, the chemical composition, hydrophilicity, and charge of the polymer surface have significant influences on cell attachment, viability, and proliferation[60]. For example, physical or chemical modifications of the surface of PHA devices can improve the attachment and growth of cells on the surface. Lipase treatment increases the viable cell number on PHB films by 100-200 times compared with untreated PHB film. Treatment of PHB films with NaOH also led to a 25-fold increase in the viable cell number compared with untreated PHB film[61]. It was shown that treatment of PHB film surfaces with low-pressure ammonia or oxygen plasma improved the growth of human fibroblasts, epithelial cells of respiratory mucosa, and rat bone marrow stromal osteoblastic cells (rat bone marrow MSCs) due to increased hydrophilicity (but with no change in microstructure) of the polymer surface[17,22,62]. It was suggested that the improved hydrophilicity of the films after PHB treatment with lipases, alkali, and plasma could allow cells in suspension to more easily attach to the polymer films compared with untreated films. The influence of the hydrophilicity of the biomaterial surface on cell adhesion was demonstrated previously[63]. Some researchers used PHAs only as a framework to cover biomaterials and investigated the effect on the growth and differentiation of MSCs. Thus, collagen type I, containing or not containing chondroitin sulfate, was covered on the textile scaffold from PHB threads, and the osteogenic differentiation of human MSCs isolated from bone marrow was studied[64].
Surprisingly, some PHAs (including PHB and PHBV) have piezoelectric properties similar to those of natural bone. Mechanical stress in bone tissue produces electrical signals that stimulate bone growth and remodeling[13]. Electrical stimulation resulted in significant increases in the osteogenic differentiation of MSCs in vitro and improved the regeneration of critical bone defects in vivo[65]. Thus, the osteogenic activity of PHB and its copolymers could be due to the piezoelectric properties of these biopolymers.
The physicochemical properties of PHAs are related entirely to the function of natural PHAs as storage polymers in a bacterial cell. The relative hydrophobicity of PHB is due to the elimination of the effect of high osmotic pressure on the storage polymer[6,7,10]. The semicrystalline structure and thermoplasticity of PHAs are connected with the necessity to maintain the particular state of the polymer in the cell, which should be simultaneously stable in water medium and available for controlled digestion by PHA depolymerases. Chemical inertness is the main reason for PHA biocompatibility and is associated with the nontoxicity of storage polymers for bacterial cell[6,10,66].
The ability of PHAs to biodegrade may be the stand-alone reason for the biological activity of these polymers, including the relation with MSCs. As biodegradable polymers, natural PHAs cause targeted activation of macrophages and osteoclasts – cells that directly cause biodegradation of the polymers. It was shown that macrophages and osteoclasts adhere strongly to and proliferate on polymer films. On the polymer surface, macrophages are activated by a polymeric material, which contributes to cell phagocytic activity[67,68]. The adhesion of macrophages to the surface of a polymeric material plays a key role: it was shown that biodegradation of polymeric membranes occurs only when macrophages adhere to their surface, and if macrophages cannot adhere to the membrane, the polymer does not degrade[68].
Macrophages can uptake microparticles from low-molecular PHB with a size of 1-10 μm by phagocytosis[67]. At high concentrations of PHB particles (> 10 µg/mL), phagocytosis is accompanied by toxic effects and changes in the functional state of macrophages[67], while nanoparticles (15-250 nm) of PHB and its copolymers do not show significant cytotoxic effects on macrophages even at a high concentration of 1 mg/mL unlike nanoparticles from PLA[69]. Phagocytosis of microparticles from PHB is accompanied by an increase in the production of nitric oxide and tumor necrosis factor alpha in activated macrophages. Macrophages died after the uptake of a large number of microparticles. It was also demonstrated that phagocytosis of particles from PHB decreases with time due to the active process of PHB biodegradation[67].
In addition to macrophages, fibroblasts and osteoblasts are also capable of uptaking PHB microparticles in vitro. Even at high concentrations of PHB particles (> 10 μg/mL), phagocytosis is not accompanied by toxic effects on fibroblasts[67]. The endocytosis of microparticles by osteoblasts is accompanied by the differentiation of cells in the osteogenic direction as indicated by the increase in ALP activity[70]. The PHBHHx nanoparticles were used to study the mechanism of stimulation of cell proliferation. In concentrations from 0.02 to 0.1 g/L, they stimulated an increase in the calcium ion current in the cell cytoplasm, which is one of the main signaling pathways of activation of their division[71-73].
Oligomers of PHAs may also have their own biological activity. Therefore, it was shown that oligomers of PHB and its copolymers with 4-hydroxybutyrate and 3-hydroxyhexanoate (with a chain length of 20-25 monomers) are not toxic to cells (up to a concentration of 20 μg/mL) and have biological activity. They stimulate proliferation, inhibit apoptosis, and suppress the release of calcium into the cytoplasm and the formation of intercellular contacts between the B cells of the pancreas of mice[74].
The process of biodegradation of material by osteoclasts or macrophages can stimulate MSC differentiation in the osteogenic direction. Active osteoclasts grown on biodegradable ceramic scaffolds were shown to influence the development of MSCs into osteoblasts when cocultured in vitro by STAT3 activation[75,76]. Therefore, the ability of PHA to biodegrade itself may be an indirect cause of the induction of MSC differentiation. The ability of hydrolytic and enzymatic degradation (with slow rate) is strongly connected with the need for controlled consumption of this polymer as an energy supply by enzymatic cleavage in special granules of a bacterial cell[6,10,59].
Moreover, the products of PHA biodegradation are common substances in human organisms because of the presence of PHB and its copolymers in bacteria of the human microbiota. A series of symbiotic and infectious bacteria of mammals (including human) of the genuses Agrobacterium, Clostridium, Ralstonia, Bacillus, Burkholderia, Vibrio, Legionella, Pseudomonas, Mycobacterium, Acinetobacter, Sphingomonas, Fusobacterium, Neisseria, Streptomyces, and Bordetella, Rickettsia can synthesize this biopolymer or have the enzymes (or their genes) of PHB biosynthesis, PHA-polymerase.
Some of these bacteria, such as Pseudomonas sp. are capable of synthesizing not only PHB but also various PHB copolymers[66]. The role of this biopolymer in the bacteria of animal microbiota remains a very poorly understood problem. However, a number of papers have suggested the participation of PHB in the relationship between bacteria and the host organism. Kim et al[77] suggested that the PHB synthesis facility allows symbiotic bacteria of the Burkholderia genus to survive in the intestine of the Riptortus pedestris bean bug in the stress conditions induced by the host organism immune system to regulate the number of these bacteria. Apparently, PHB synthesis by bacteria from Rhodobacterales modulated the gastrointestinal tract microbiota in sea cucumbers Apostichopus japonicus contributing to an increase in the animal size multiple times[78].
Moreover, the efficiency of PHB in the fight against infectious diseases in animals was demonstrated: the use of PHB powder as an additive to feed protected Artemia nauplii crustaceans from infectious disease caused by pathogenic Vibrio campbellii bacteria. The efficiency of PHB was 100 times larger than that of the 3HB monomeric precursor[79]. In addition, PHB has the ability to suppress pathogenic bacteria, not only Vibrio sp. but also E. coli and Salmonella sp.[80]. PHB oligomers (with a length of approximately 80 3HB monomers) possess effective concentration-dependent antibacterial and antifungal properties against Staphylococcus aureus, Klebsiella pneumoniae, and Candida albicans through disruption of the bacterial wall/membrane and changes in the transmembrane potential[81].
At the genus level, dietary PHB increased the abundance of beneficial bacteria in the intestine of Pacific white shrimp Litopenaeus vannamei, such as Bacillus, Lactobacillus, Lactococcus, Clostridium, and Bdellovibrio. This beneficial effect was associated with activation of the rapamycin (mTOR) signaling pathway, which plays a crucial role in intestinal inflammation and epithelial morphogenesis[82]. However, the mTOR signaling pathway is involved in MSC proliferation and differentiation. Suppression of the mTOR signaling pathway enhances the osteogenic capacity of stem cells, while mTOR activation causes MSC hyperproliferation[83,84].
Moreover, the modified products of PHB biodegradation methyl-esterified dimers and trimers have pronounced antioxidant activity: 3-fold greater hydroxyl radical–scavenging activity than glutathione and 11-fold higher activity than vitamin C or 3HB monomer[85]. Unfortunately, the role of possible antioxidant properties in biocompatibility or biological activity of PHAs is unexplored, while it can be also be connected with their effect on MSCs. Oxidative stress is one of the regulatory mechanisms for MSC osteogenic and adipogenic differentiation. For example, antioxidant vitamin D3 promotes the osteogenic differentiation of MSCs under normal conditions and partly protects cells from oxidative stress damage by activating the endogenous antioxidant system[86,87]. Maybe this is one of the main reasons for PHA biocompatibility, especially with stem cells. But if PHAs have their antioxidant properties as their natural functions, e.g., in bacterial cells[85], it can explain the mechanism of interaction of PHAs with stem cells.
However, the possible effect of intestinal microbiota bacteria on PHA polymeric devices (and the effect of the polymeric material of these devices on symbiotic bacteria) is not always taken into account when polyhydroxyalkanoates are used to regenerate intestinal tissues. In particular, the roles of natural PHAs and the degradation products in the interaction with intestinal progenitor stem cells and microbiota bacteria remain entirely unexplored. However, the fundamental roles and functions of PHB in relation to human intestinal microbiota bacteria should be taken into account in the development of medical devices based not only on PHB and other natural PHAs but also on their biomimetic analogs, such as synthetic polyhydroxyalkanoates, especially devices based on these polymers for intestine surgery.
The effect of PHAs on the differentiation of MSCs can also be associated with the bioactivity of 3HB that is produced during PHB and its copolymer biodegradation as the primary monomeric precursor of these polymers. Thus, 3HB at a concentration of 0.01 to 0.1 g/L (0.1-1 mmol/L) can cause activation of the proliferation of human keratinocytes of the HaCaT line and mouse L929 fibroblasts by increasing the concentration of calcium ions in the cell cytoplasm and by inhibiting the apoptosis and necrosis of fibroblasts[71-73]. Such bioactivity of 3HB is not surprising because 3HB is a natural metabolite in the mammal body. It is the so-called ketone body with a pronounced versatile biological activity. For example, 3HB performs regulatory functions under various special conditions such as diabetes, starvation, and a ketogenic diet[88,89]. 3HB at a concentration of 0.005-0.1 g/L (0.05-1 mmol/L) induced osteogenic differentiation of mouse MC3T3-E1 preosteoblast cells (unfortunately, not MSCs), which was determined by an increase in ALP activity, calcium deposition (Alizarin red S dye assay), and osteocalcin expression. It should be noted that PHAs enhanced osteogenic differentiation of both MSCs and exactly this cell line of preosteoblast cells.
Moreover, it was demonstrated that 3HB has in vivo osteoinductive activity that was shown in an osteoporosis model in female rats with removed ovaries. However, at lower concentrations, 3HB did not have a similar effect. For slower PHA biodegradation, 3HB formed at concentrations much lower than 0.05 mmol/L[90]. It was also shown that 3HB stimulates the formation of gap junctions between neurons for the transmission of electrical signals, which is associated with improved memory and learning[91]. The ability of PHAs to cause neurogenic differentiation of MSCs could also be related to the previously shown neuroprotective effect of 3HB. 3HB manifests itself at very high doses because the effect of 3HB on the nervous system is due to the nutrient (energy) function of fatty acids for neurons[92].
Furthermore, if PHAs cause differentiation of MSCs only by 3HB biological activity, it is difficult to explain the data of a series of investigations in which the growth and differentiation of MSCs were compared among scaffolds fabricated of different PHAs (PHB and its copolymers usually). The effect of different PHAs on the growth and differentiation of MSCs differs significantly and even dramatically, while the content of 3HB monomers in PHB copolymers is rarely lower than 80 mol %[18,23,33,93]. It was shown that 3HB functions through multiple mechanisms: inhibition of class I histone deacetylases; binding and activation of cell surface G-protein-coupled receptors, hydroxycarboxylic acid receptors, and free fatty acid receptor 3; histone modification by covalent binding with proteins (β-hydroxybutyrylation); and membrane channel and transporter regulation[89]. The product of biodegradation of synthetic PLA and PLGA - L-lactate (2-hydroxypropanoate) was shown to be a natural ligand for Gi-coupled G-protein receptor 81 inhibiting cAMP-mediated intracellular signaling events such as lipolysis[94].
It was also revealed that the biodegradation products of some natural PHAs (e.g., 3-hydroxyoctanoate) have antimicrobial activity against a number of infectious gram-negative and gram-positive bacteria. They inhibit the production of metabolites associated with their pathogenic activity. However, their cytotoxic activity against human fibroblasts is manifested at much higher concentrations[86].
Short-chain fatty acids (including 3HB and L-lactate) are the critical metabolites of mammalian microbiota by which symbiotic bacteria execute the diet-based microbial influence on the host. They affect various physiological processes in the gastrointestinal, nervous, and immune systems and may contribute to health and disease (colitis, diabetes, cancer, asthma)[95]. Thus, the observed effect of 3HB on MSC growth and differentiation could be associated with one of the mechanisms of microbiota influence on mammalian host organisms.
Notwithstanding the above, data from a series of studies provide evidence of the biological activity of PHAs toward cells (including MSCs) through receptor or cellular signaling molecule-associated mechanisms[25,26,33,96]. As we can see in Table 1 PHAs induce osteogenic and chondrogenic differentiation and support MSC differentiation in these and other directions: epitheliogenic, adipogenic, endotheliogenic, and neurogenic. It also can change the MSC proliferation rate, which is associated with change in cell morphology, physiological state, extracellular matrix synthesis, and expression of a series of differentiation markers: ALP, collagen I and II types, osteopontin, osteocalcin, glycosaminoglycan, aggrecan, sox-9, etc. The possible mechanism of the implementation of the effects of PHAs on cell proliferation, differentiation, and apoptosis can be accomplished through integrins – intercellular macromolecules for contact and recognition. It was shown that MSC differentiation and apoptosis of osteoblasts is carried out by a cascade mechanism that includes regulator proteins such as peroxisome proliferator-activated receptor gamma, which is triggered by the interaction of PHAs with integrins on the cell surface[25,33]. It was also shown that the osteogenic and chondrogenic differentiation of MSCs induced by the polymer surface of PHAs could be regulated by microRNAs[26,96]. The microRNAs miR-29a and miR-29b were confirmed to be significantly downregulated in MSCs grown on PHBHHx films through cell-material interactions. They can directly target the 3’ UTR of col2a1 and are suppressed by the chondrogenic protein Sox9 to induce chondrogenesis in MSCs[26].
Some researchers attribute the biological activity of PHB and its copolymers obtained by bacterial biosynthesis to the fact that the polymer biomaterials may be poorly purified from bacterial lipopolysaccharide or DNA. However, a previous study made clear that even a very well-purified polymer is able to activate the cellular response[68].
The problem of the biological activity of PHAs, e.g., their ability to effect stem cell growth and differentiation, seems to be closely related to the problem of endogenous PHB in mammalian tissues. The finding of bacterial PHB in various tissues of mammals was one of the most exciting stages in the history of PHA research. It should be immediately noted that it is not a high-molecular reserve polymer. The synthesis is typical for a number of bacteria, but the so-called short-chain complex-forming PHB (cPHB) and low-molecular oligo-PHB (oPHB) were found[59]. While specific PHB biosynthesis enzymes are available only in prokaryotes, this biopolymer was found in organisms of almost all types. Short- and medium-chain PHBs were found in many different mammalian organs and tissues (including in humans), i.e. in the blood plasma, heart, kidneys, liver, vessels (aorta), nerves, lipoprotein particles, platelets, etc. It was demonstrated that oPHB is located in eukaryotic cell membranes in the form of a PHB–polyphosphate–calcium complex. The oPHB has a molecular weight of 12200, which corresponds to a length of approximately 140 elements of 3HB. The oPHB concentration in human blood plasma can change in a rather wide range, from 0.6 to 18.2 mg/L at an average value of 3.5 mg/L[97].
Thus, what function does PHB have in the mammalian organism? In addition to the role of PHB as a reserve substance and energy depot in bacteria, the researchers who discovered endogenous PHB in mammalian tissues suggested some of its functions. The functions of endogenous PHB for both prokaryotic and eukaryotic organisms are apparently coupled with the regulation of different proteins due to the production by short-chain cPHB and oPHB of both noncovalent and covalent bonds with other biopolymers (proteins, nonorganic polyphosphates, and DNA). In the form of PHB-Ca-polyphosphate complexes, cPHB can function as nonprotein channels capable of conducting inorganic ions through the plasma membrane; these structures also form noncovalent complexes with channels proteins and become part of them)[97]. It was demonstrated that cPHB binds to one of the proteins from the group of melastatin receptors (TRPM8) of mammals (and humans), which leads to a change in its functioning[97,98]. TRPM8 is a membrane calcium channel that functions as a temperature sensor of neurons of the mammalian peripheral nervous system. The binding of the protein TRPM8 receptor channel with cPHB is required for normal functionality of this receptor, which is associated with a change in the conformation state of PHB when passing through this polymer’s glass transition temperature, which is approximately 10 °С[59,97,98].
Another case of the modification of proteins is the OmpA protein of E. coli. The covalent attachment of cPHB to the specific serine residues of OmpA maintains the correct integration and orientation of this protein in the outer membrane of the bacterium. It was proposed that such modification of proteins by cPHB involves the folding and sorting of certain proteins[99]. Similar processes have been described for mammals. Thus, it was shown that PHB oligomers are covalently bound to the calcium ATPase of the plasma membrane of human erythrocytes, simultaneously forming a complex with inorganic phosphates[100].
Other researchers have indirectly confirmed that such conjugation of cPHB with proteins may have a specific physiological function. It was shown that conjugation of the antitumor peptides DP18L with 3-hydroxydecanoate enhances their antitumor activity[101,102]. The formation of covalent bonds of PHB with proteins during biochemical processes can be indirectly confirmed by the ability of many bacterial strains, producers of high-molecular-weight PHB, to utilize other polymers added to the culture medium, which often differ significantly in their physicochemical properties (for example, hydrophilic PEG) by covalent crosslinking with the end of the synthesizing PHB chain and the formation of diblock copolymers (for example, PHB-PEG)[103].
There is a striking example of other biopolymers with such functionality that, in addition, are quite similar to PHB in chemical structure: polyprenols (in bacteria, plants, and fungi) and dolichols (in bacteria and animals). Polyprenylation (covalent binding to polyprenols) plays an essential role in the posttranslational modification of a number of proteins to anchor them in the membrane. All organisms (both eukaryotes and prokaryotes) have biochemical mechanisms for the synthesis of these biopolymers and their conjugation with proteins[88]. Appropriately, there is evidence suggesting that the functionality of cPHB and oPHB in eukaryotes could be similar to the functionality of polyprenols and dolichols. Thus, according to these data, PHB can theoretically be used to regulate the effect of some cytokines or growth factors on stem cells (including nonspecifically) by covalent modification of appropriate receptors by this biopolymer.
Could PHB perform some type of signaling function for microbiota bacteria when communicating with eukaryotic cells (including stem cells) of the host organism? In general, can bacteria of animal microbiota affect stem cells? Very little is known about the influence of microbiota bacteria of human and laboratory animals on the growth and differentiation of stem cells (including MSCs), but some mechanisms of these interactions have been revealed. It was shown that peptidoglycans and polysaccharides of the bacterial cell wall could alter the functional activity of MSCs. They can stimulate the immunomodulatory activity of MSCs (production of cytokines), accelerate or inhibit the growth of MSCs, and regulate the differentiation of MSCs in adipogenic, chondrogenic or osteogenic directions[104-107]. It was revealed that this biochemical response is realized through the activation of specific intracellular MSC receptors, NOD1 and NOD2 and receptors of the TLR family[104,106,108,109]. It should be noted that in cells of the immune system (for example, macrophages), activation of these receptors led to stimulation of the production of proinflammatory cytokines and an antibacterial response. It was shown that the microbiota of the oral mucosa stimulated the differentiation and development of osteoclasts by activating the regulatory genes TLR2, TLR4, NOD1, and NOD2 by peptidoglycans and polysaccharides of the bacterial cell wall. This led, to bone resorption, which plays an essential role in the pathogenesis and treatment of periodontal disease[110-113]. In contrast, in MSCs, activation of these receptors leads to more complex and diverse physiological responses, such as the suppression of chronic inflammation, which can contribute to the treatment of colitis and dermatitis[108].
Some signaling substances of microbiota bacteria can simultaneously act as intermediaries in the regulation of PHB biosynthesis with bacterial communication with the host organism that deserves attention. Thus, the ability of histamine to regulate the synthesis of low-molecular-weight cPHB in E. coli has been demonstrated. Histamine plays an important role as a means of bacterial communication with the host organism and a regulator of the gastrointestinal tract immune system allowing bacteria to be considered “native” to the host organism. Therefore, the effect of histamine on cPHB synthesis can indicate the involvement of this biopolymer in the processes of adaptation and coexistence with the host organism[114,115]. However, it is well known that histamine is one of the most important mediators of an immune response to foreign pathogens that is produced by basophils and mast cells. Histamine is involved in the inflammatory response and has a central role as a mediator of itching ( it acts as a neurotransmitter). Surprisingly, it was shown that the decrease in expression of histamine H2R receptors in human MSCs led to the suppression of cell osteogenic differentiation, which was detected by decreased expression of the osteogenic markers, osteocalcin, bone morphogenetic protein 2, and runt-related transcription factor 2[116].
Moreover, storage PHB and short-chain PHB as bacterial signal molecules can directly affect the differentiation of intestinal stem cells into mucosa enterocytes (absorptive cells). It was shown that intestinal stem cells grown in intestinal organoids in vitro readily uptake nanoparticles from synthetic PHAs (PLGA)[117], whereas PLGA scaffolds support their differentiation in enterocytes, goblet cells, and Paneth cells[51,52]. It should be noted that alkaline phosphatase and bone morphogenic protein are the markers of differentiation both of intestinal stem cells into enterocytes and MSCs into osteoblasts[2,4,118]. Maybe the effect of PHA scaffolds on MSC differentiation is caused by mimic action of PHAs on ALP- and BMP-pathways.
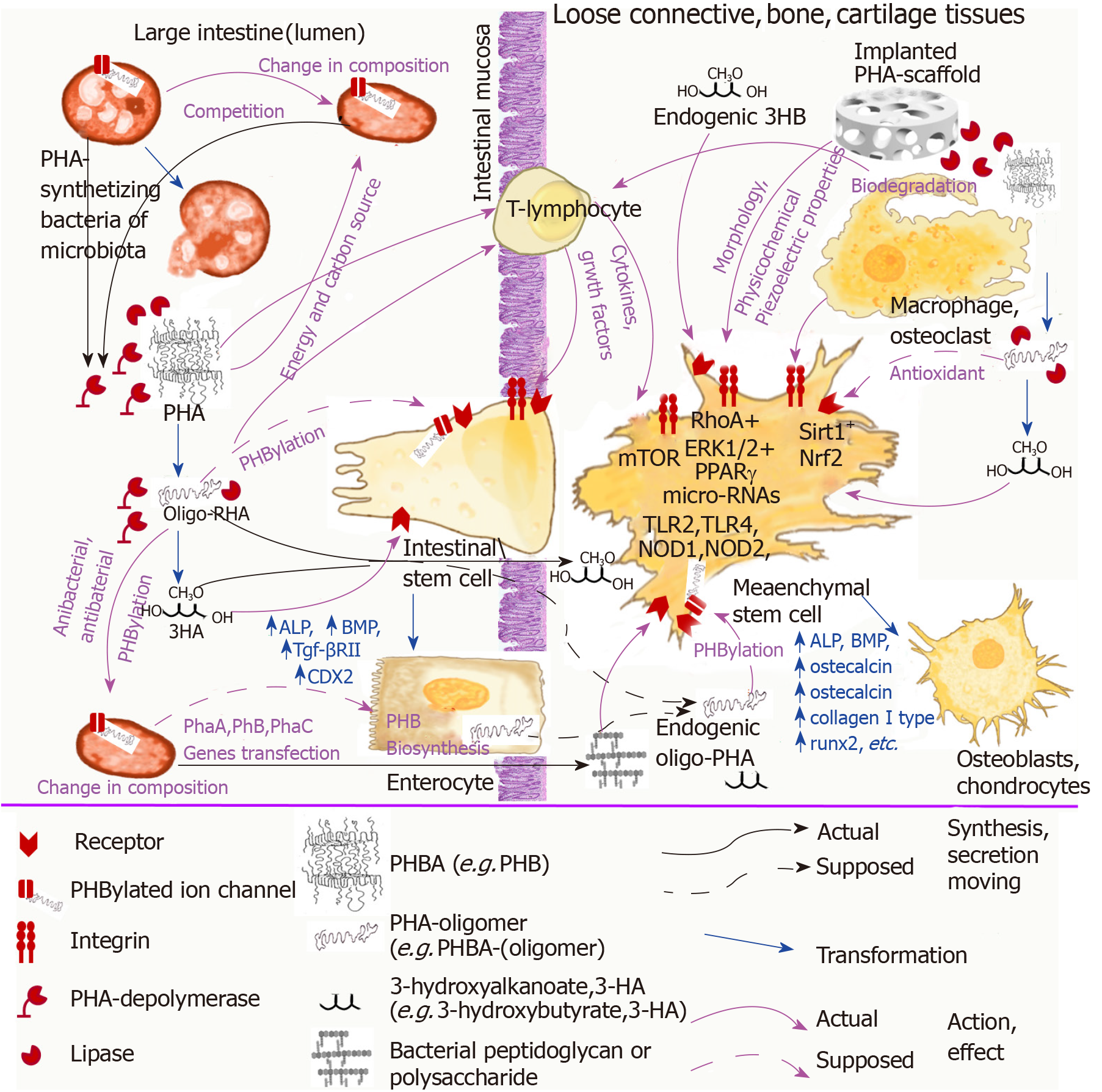
In Figure 1 we summarized the above speculations about the association of PHA natural functions and their effect on stem cells. The possible interaction of PHAs as bacteria-origin signal molecules with microbiota bacteria, intestinal stem cells, and immune cells was demonstrated in association with the effect of implanted devices from PHAs on MSCs.
The piezoelectric properties of PHB and its copolymers could also be associated with the natural functions of these biopolymers for bacteria of mammalian microbiota. There are a number of electroactive bacteria in animal microbiota, and they use electrical stimuli not only for energetic purposes but also for communication[119,120]. Moreover, some of the electroactive bacteria of mammalian microbiota, such as Pseudomonas aeruginosa, can synthesize and accumulate PHB and its various copolymers[121]. Considering that PHB can be covalently linked to receptors of both bacterial and eukaryotic cells[98-100], it cannot be excluded that the piezoelectric properties of the polymer can be used by microbiota both for communication among bacteria of the microbiota and with other cells of the host organism, including intestinal stem cells.
The possibility that PHB can perform some type of signaling function in mammalian tissues can be perceptualized by the fact that the dimers and trimers of 3-hydroxybutyrate are sex pheromones in spiders[122]. Moreover, it is possible that these pheromones could also be products of the bacterial biosynthesis of the arthropod microbiota. For example, in the beetle Costelytra zealandica, the sex pheromone is phenol, which is produced from tyrosine in special glands by symbiotic bacteria Morganella morganii[123]. Although the dimers and trimers of 3-hydroxybutyrate were found in the fungus Hypoxylon truncatum, the mechanism of their synthesis was not disclosed[124].
A better understanding of the mechanisms of interaction of PHAs with MSCs is the key to developing new therapeutic agents based on them. For example, if PHB causes the osteogenic differentiation through activation of some receptor of MSCs and PHBHHx causes the chondrogenic differentiation of MSCs through activation of another receptor of MSCs, then the medical devices based on PHB should be developed specifically for bone regeneration and medical devices based on PHBHHx–specifically for cartilage regeneration. But if the osteogenic differentiation of MSCs is caused by the main product of PHAs biodegradation – 3-hydroxybutyrate, and chondrogenic differentiation of MSCs is caused by some kind of microstructure of PHBHHx medical devices, then it is better to use any type of PHA depending on their physicochemical properties (the strength and elasticity of PHB and PHBHHx differ significantly) for bone regeneration and medical devices based on any type of PHAs with desired microstructure for cartilage regeneration.
At the first case of receptor-mediated induction of MSC differentiation, it can be connected with the similar receptor-mediated action of bacteria-origin PHAs when bacteria interact with each other or with the cells of the host organism that can help to discover mechanisms of PHAs action regarding signal functions and binding with receptors of PHB oligomers. At the second case, the bone regeneration process is mediated by internal product of lipid metabolism (3-hydroxybutyrate and connected with mechanisms of its metabolism). Maybe it is better to regulate 3-hydroxybutyrate metabolism to improve bone regeneration. The same is with the microstructure of PHAs devices because in this case, it is necessary to find the specific structural element regulating chondrogenic MSCs differentiation independent of the type of biomaterial at all. We should keep in mind that elasticity of biomaterials can also affect MSC differentiation[125].
Moreover, a more in-depth understanding of the interaction of PHAs with MSCs in relation to natural functions of these biopolymers can help to develop novel types of medical devices and pharmaceutical formulations based on them: medical devices with selective osteoinductive or chondroinductive activity and desired regenerative activity; prebiotics that promote regenerative activity of the intestinal wall through modulation of microbiota and stimulation of intestine progenitor stem cells; substrates for MSC cultivation in bioreactors; formulations for sustained delivery of drugs with biopolymer-compatible natural bioactivity; experimental model systems for MSC cultivation for drug testing; or therapeutic tissue-engineered systems simultaneously containing probiotic bacteria and MSCs as active ingredients for treatment of gastrointestinal system diseases.
PHAs have integral multifactor effects on MSCs through different mechanisms related to the main characteristics of PHA scaffolds that are difficult to separate from each other: the physicochemical properties of the polymer surface; the morphology of polymer devices; and the possible biological activity of these biopolymers. However, the intrinsic biological activity of natural PHAs plays an important role in the effect of implanted PHA devices on the proliferation and differentiation of MSCs as well as on regeneration of bone, cartilage, loose connective tissue, and other tissues.
In summary, studies that consider the problem of the influence of natural PHAs on the growth and differentiation of MSCs should be carried out in a manner that considers and integrates the functions of these biopolymers in nature. We suggest that these biopolymers of bacteria origin engage in some unexplored mechanism while signaling functions in our body through which microbiota bacteria can “communicate” with cells of the immune system, intestinal mucosa, and other tissues causing them to induce a physiological response. PHAs as bacterial signaling molecules can be involved in regenerative processes of intestinal mucosa regulated by microbiota bacteria through stimulation of intestinal stem cells differentiation into enterocytes and other mucosa epithelial cells and directly (as absorbed or endogenous oPHB) or indirectly effect the proliferation and differentiation of MSCs (Figure 1).
The above data on the participation of intercellular contact molecules such as integrins, microRNAs, the mTOR signaling pathway, antioxidant activity, and piezoelectric properties in the implementation of the biological effects of PHB and data on receptor regulation through the covalent binding of PHB to proteins can indicate the natural signaling function of this biopolymer. Unfortunately, this fascinating problem is studied very poorly. There is only very fragmentary and incomplete information. A broader understanding of the role of PHAs in nature could help to further clarify the unusual or contradictory data in the field of the interactions of PHAs with MSCs and to develop novel medical devices and pharmaceutical formulations based on them.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Russia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Andrukhov O, Bragança J, Li SC, Schmidt NO S-Editor: Ji FF L-Editor: Filipodia E-Editor: Zhou BX
| 1. | Murray IR, West CC, Hardy WR, James AW, Park TS, Nguyen A, Tawonsawatruk T, Lazzari L, Soo C, Péault B. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell Mol Life Sci. 2014;71:1353-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 2. | Liao HT, Chen CT. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J Stem Cells. 2014;6:288-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (16)] |
| 3. | Paschos NK, Brown WE, Eswaramoorthy R, Hu JC, Athanasiou KA. Advances in tissue engineering through stem cell-based co-culture. J Tissue Eng Regen Med. 2015;9:488-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 4. | Oryan A, Alidadi S, Moshiri A, Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res. 2014;9:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 605] [Cited by in RCA: 693] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 5. | Farah S, Anderson DG, Langer R. Physical and mechanical properties of PLA, and their functions in widespread applications - A comprehensive review. Adv Drug Deliv Rev. 2016;107:367-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1317] [Cited by in RCA: 1194] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 6. | Holmes PA, Developments in crystalline polymers, Vol. Biologically produced (R)-3-hydroxyalkanoate polymers and copolymers. Developments in crystalline polymers, Vol . 2. Bassett DC, editor. London: Elsevier 1998; 1-65. |
| 7. | Mokhtarzadeh A, Alibakhshi A, Hejazi M, Omidi Y, Dolatabadi JEN. Bacterial-derived biopolymers: advanced natural nanomaterials for drug delivery and tissue engineering. TrAC-Trend Anal Chem. 2016;82:367-384. [RCA] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Bonartsev AP. Poly(3-hydroxybutyrate): applications. In: Encyclopedia of Polymer Applications. Mishra M, editor. CRC Press, Taylor and Francis Group, Boca Raton 2019; 2061-2076. |
| 9. | Lim J, You M, Li J, Li Z. Emerging bone tissue engineering via Polyhydroxyalkanoate (PHA)-based scaffolds. Mater Sci Eng C Mater Biol Appl. 2017;79:917-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Madison LL, Huisman GW. Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev. 1999;63:21-53. [PubMed] |
| 11. | Artsis MI, Bonartsev AP, Iordanskii AL, Bonartseva GA, Zaikov GE. Biodegradation and Medical Application of Microbial Poly(3-Hydroxybutyrate). Mol Cryst Liq Cryst. 2012;555:232-262. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | de Paula AC, Zonari AA, Martins TM, Novikoff S, da Silva AR, Correlo VM, Reis RL, Gomes DA, Goes AM. Human serum is a suitable supplement for the osteogenic differentiation of human adipose-derived stem cells seeded on poly-3-hydroxibutyrate-co-3-hydroxyvalerate scaffolds. Tissue Eng Part A. 2013;19:277-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Gorodzha SN, Muslimov AR, Syromotina DS, Timin AS, Tcvetkov NY, Lepik KV, Petrova AV, Surmeneva MA, Gorin DA, Sukhorukov GB, Surmenev RA. A comparison study between electrospun polycaprolactone and piezoelectric poly(3-hydroxybutyrate-co-3-hydroxyvalerate) scaffolds for bone tissue engineering. Colloids Surf B Biointerfaces. 2017;160:48-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Bonartsev AP, Zharkova II, Voinova VV, Kuznetsova ES, Zhuikov VA, Makhina TK, Myshkina VL, Potashnikova DM, Chesnokova DV, Khaydapova DD, Bonartseva GA, Shaitan KV. Poly(3-hydroxybutyrate)/poly(ethylene glycol) scaffolds with different microstructure: the effect on growth of mesenchymal stem cells. 3 Biotech. 2018;8:328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Shumilova AA, Myltygashev MP, Kirichenko AK, Nikolaeva ED, Volova TG, Shishatskaya EI. Porous 3D implants of degradable poly-3-hydroxybutyrate used to enhance regeneration of rat cranial defect. J Biomed Mater Res A. 2017;105:566-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Lü LX, Zhang XF, Wang YY, Ortiz L, Mao X, Jiang ZL, Xiao ZD, Huang NP. Effects of hydroxyapatite-containing composite nanofibers on osteogenesis of mesenchymal stem cells in vitro and bone regeneration in vivo. ACS Appl Mater Interfaces. 2013;5:319-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Köse GT, Korkusuz F, Korkusuz P, Purali N, Ozkul A, Hasirci V. Bone generation on PHBV matrices: an in vitro study. Biomaterials. 2003;24:4999-5007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Wang YW, Wu Q, Chen GQ. Attachment, proliferation and differentiation of osteoblasts on random biopolyester poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds. Biomaterials. 2004;25:669-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 170] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Cool SM, Kenny B, Wu A, Nurcombe V, Trau M, Cassady AI, Grøndahl L. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) composite biomaterials for bone tissue regeneration: in vitro performance assessed by osteoblast proliferation, osteoclast adhesion and resorption, and macrophage proinflammatory response. J Biomed Mater Res A. 2007;82:599-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Hosseini FS, Soleimanifar F, Aidun A, Enderami SE, Saburi E, Marzouni HZ, Khani MM, Khojasteh A, Ardeshirylajimi A. Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) improved osteogenic differentiation of the human induced pluripotent stem cells while considered as an artificial extracellular matrix. J Cell Physiol. 2019;234:11537-11544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Lü LX, Wang YY, Mao X, Xiao ZD, Huang NP. The effects of PHBV electrospun fibers with different diameters and orientations on growth behavior of bone-marrow-derived mesenchymal stem cells. Biomed Mater. 2012;7:015002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Köse GT, Ber S, Korkusuz F, Hasirci V. Poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) based tissue engineering matrices. J Mater Sci Mater Med. 2003;14:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Hu YJ, Wei X, Zhao W, Liu YS, Chen GQ. Biocompatibility of poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) with bone marrow mesenchymal stem cells. Acta Biomater. 2009;5:1115-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Yu BY, Chen PY, Sun YM, Lee YT, Young TH. Response of human mesenchymal stem cells (hMSCs) to the topographic variation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) films. J Biomater Sci Polym Ed. 2012;23:1-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Wang Y, Gao R, Wang PP, Jian J, Jiang XL, Yan C, Lin X, Wu L, Chen GQ, Wu Q. The differential effects of aligned electrospun PHBHHx fibers on adipogenic and osteogenic potential of MSCs through the regulation of PPARγ signaling. Biomaterials. 2012;33:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Yan C, Wang Y, Shen XY, Yang G, Jian J, Wang HS, Chen GQ, Wu Q. MicroRNA regulation associated chondrogenesis of mouse MSCs grown on polyhydroxyalkanoates. Biomaterials. 2011;32:6435-6444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Ye C, Hu P, Ma MX, Xiang Y, Liu RG, Shang XW. PHB/PHBHHx scaffolds and human adipose-derived stem cells for cartilage tissue engineering. Biomaterials. 2009;30:4401-4406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Li X, Chang H, Luo H, Wang Z, Zheng G, Lu X, He X, Chen F, Wang T, Liang J, Xu M. Poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds coated with PhaP-RGD fusion protein promotes the proliferation and chondrogenic differentiation of human umbilical cord mesenchymal stem cells in vitro. J Biomed Mater Res A. 2015;103:1169-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Xue K, Zhang X, Gao Z, Xia W, Qi L, Liu K. Cartilage progenitor cells combined with PHBV in cartilage tissue engineering. J Transl Med. 2019;17:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Bretcanu O, Misra S, Roy I, Renghini C, Fiori F, Boccaccini AR, Salih V. In vitro biocompatibility of 45S5 Bioglass-derived glass-ceramic scaffolds coated with poly(3-hydroxybutyrate). J Tissue Eng Regen Med. 2009;3:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Bonartsev AP, Zharkova II, Yakovlev SG, Myshkina VL, Makhina TK, Zernov AL, Kudryashova KS, Feofanov AV, Akulina EA, Ivanova EV, Zhuikov VA, Volkov AV, Andreeva NV, Voinova VV, Bonartseva GA, Shaitan KV, Kirpichnikov MP. 3D-Scaffolds from Poly(3-hydroxybutyrate)Poly(ethylene glycol) Copolymer for Tissue Engineering. J Biomater Tiss Eng. 2016;6:42-52. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Lomas AJ, Chen GG, El Haj AJ, Forsyth NR. Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) supports adhesion and migration of mesenchymal stem cells and tenocytes. World J Stem Cells. 2012;4:94-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Wang Y, Jiang XL, Peng SW, Guo XY, Shang GG, Chen JC, Wu Q, Chen GQ. Induced apoptosis of osteoblasts proliferating on polyhydroxyalkanoates. Biomaterials. 2013;34:3737-3746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Wang L, Wang ZH, Shen CY, You ML, Xiao JF, Chen GQ. Differentiation of human bone marrow mesenchymal stem cells grown in terpolyesters of 3-hydroxyalkanoates scaffolds into nerve cells. Biomaterials. 2010;31:1691-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Lizarraga-Valderrama LR, Nigmatullin R, Taylor C, Haycock JW, Claeyssens F, Knowles JC, Roy I. Nerve tissue engineering using blends of poly(3-hydroxyalkanoates) for peripheral nerve regeneration. Eng Life Sci. 2015;15:612–621. [RCA] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Xie H, Li J, Li L, Dong Y, Chen GQ, Chen KC. Enhanced proliferation and differentiation of neural stem cells grown on PHA films coated with recombinant fusion proteins. Acta Biomater. 2013;9:7845-7854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Xu XY, Li XT, Peng SW, Xiao JF, Liu C, Fang G, Chen KC, Chen GQ. The behaviour of neural stem cells on polyhydroxyalkanoate nanofiber scaffolds. Biomaterials. 2010;31:3967-3975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 38. | Sundaramurthi D, Krishnan UM, Sethuraman S. Epidermal differentiation of stem cells on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) nanofibers. Ann Biomed Eng. 2014;42:2589-2599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Zonari A, Novikoff S, Electo NR, Breyner NM, Gomes DA, Martins A, Neves NM, Reis RL, Goes AM. Endothelial differentiation of human stem cells seeded onto electrospun polyhydroxybutyrate/polyhydroxybutyrate-co-hydroxyvalerate fiber mesh. PLoS One. 2012;7:e35422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Rentsch C, Rentsch B, Breier A, Hofmann A, Manthey S, Scharnweber D, Biewener A, Zwipp H. Evaluation of the osteogenic potential and vascularization of 3D poly(3)hydroxybutyrate scaffolds subcutaneously implanted in nude rats. J Biomed Mater Res A. 2010;92:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Gredes T, Gedrange T, Hinüber C, Gelinsky M, Kunert-Keil C. Histological and molecular-biological analyses of poly(3-hydroxybutyrate) (PHB) patches for enhancement of bone regeneration. Ann Anat. 2015;199:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Bonartsev АP, Muraev АА, Volkov AV. Material-Associated Bone Resorption. Sovremennye Tehnologii v Medicine. 2018;10:26-33. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Löbler M, Sass M, Kunze C, Schmitz KP, Hopt UT. Biomaterial patches sutured onto the rat stomach induce a set of genes encoding pancreatic enzymes. Biomaterials. 2002;23:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Löbler M, Sass M, Kunze C, Schmitz KP, Hopt UT. Biomaterial implants induce the inflammation marker CRP at the site of implantation. J Biomed Mater Res. 2002;61:165-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Nakase Y, Nakamura T, Kin S, Nakashima S, Yoshikawa T, Kuriu Y, Sakakura C, Yamagishi H, Hamuro J, Ikada Y, Otsuji E, Hagiwara A. Intrathoracic esophageal replacement by in situ tissue-engineered esophagus. J Thorac Cardiovasc Surg. 2008;136:850-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Maemura T, Kinoshita M, Shin M, Miyazaki H, Tsujimoto H, Ono S, Hase K, Saitoh D. Assessment of a tissue-engineered gastric wall patch in a rat model. Artif Organs. 2012;36:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Kaihara S, Kim SS, Benvenuto M, Choi R, Kim BS, Mooney D, Tanaka K, Vacanti JP. Successful anastomosis between tissue-engineered intestine and native small bowel. Transplantation. 1999;67:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Takeshita N, Ho KY. Endoscopic Closure for Full-Thickness Gastrointestinal Defects: Available Applications and Emerging Innovations. Clin Endosc. 2016;49:438-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Holmberg FE, Seidelin JB, Yin X, Mead BE, Tong Z, Li Y, Karp JM, Nielsen OH. Culturing human intestinal stem cells for regenerative applications in the treatment of inflammatory bowel disease. EMBO Mol Med. 2017;9:558-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 50. | Trecartin A, Grikscheit T. Tissue Engineering Functional Gastrointestinal Regions: The Importance of Stem and Progenitor Cells. Cold Spring Harb Perspect Med. 2017;7:pii: a025700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Costello CM, Hongpeng J, Shaffiey S, Yu J, Jain NK, Hackam D, March JC. Synthetic small intestinal scaffolds for improved studies of intestinal differentiation. Biotechnol Bioeng. 2014;111:1222-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 52. | Shaffiey SA, Jia H, Keane T, Costello C, Wasserman D, Quidgley M, Dziki J, Badylak S, Sodhi CP, March JC, Hackam DJ. Intestinal stem cell growth and differentiation on a tubular scaffold with evaluation in small and large animals. Regen Med. 2016;11:45-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 53. | Criscenti G, Vasilevich A, Longoni A, De Maria C, van Blitterswijk CA, Truckenmuller R, Vozzi G, De Boer J, Moroni L. 3D screening device for the evaluation of cell response to different electrospun microtopographies. Acta Biomater. 2017;55:310-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Wei G, Ma PX, Tissue Engineering Using Ceramics and Polymers. Polymeric biomaterials for tissue engineering. Tissue Engineering Using Ceramics and Polymers. 2nd ed. Boccaccini AR and Ma PX, editors. Woodhead Publishing Series in Biomaterials: Number 85. Elsewier (Cambridge, UK) 2014; 35-66. [DOI] [Full Text] |
| 55. | Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1029] [Cited by in RCA: 798] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 56. | Bowers KT, Keller JC, Randolph BA, Wick DG, Michaels CM. Optimization of surface micromorphology for enhanced osteoblast responses in vitro. Int J Oral Maxillofac Implants. 1992;7:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 57. | Cochran D, Simpson J, Weber HP, Buser D. Attachment and Growth of Periodontal Cells on Smooth and Rough Titanium. Int J Oral Maxillofac Implants. 1994;9:289-297. [PubMed] |
| 58. | Holzwarth JM, Ma PX. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials. 2011;32:9622-9629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 422] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 59. | Jendrossek D, Pfeiffer D. New insights in the formation of polyhydroxyalkanoate granules (carbonosomes) and novel functions of poly(3-hydroxybutyrate). Environ Microbiol. 2014;16:2357-2373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 60. | Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24:1121-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1793] [Cited by in RCA: 1788] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 61. | Wang YW, Yang F, Wu Q, Cheng YC, Yu PH, Chen J, Chen GQ. Effect of composition of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) on growth of fibroblast and osteoblast. Biomaterials. 2005;26:755-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 62. | Ostwald J, Dommerich S, Nischan C, Kramp B. [In vitro culture of cells from respiratory mucosa on foils of collagen, poly-L-lactide (PLLA) and poly-3-hydroxy-butyrate (PHB)]. Laryngorhinootologie. 2003;82:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Chauvel-Lebret DJ, Pellen-Mussi P, Auroy P, Bonnaure-Mallet M. Evaluation of the in vitro biocompatibility of various elastomers. Biomaterials. 1999;20:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 64. | Wollenweber M, Domaschke H, Hanke T, Boxberger S, Schmack G, Gliesche K, Scharnweber D, Worch H. Mimicked bioartificial matrix containing chondroitin sulphate on a textile scaffold of poly(3-hydroxybutyrate) alters the differentiation of adult human mesenchymal stem cells. Tissue Eng. 2006;12:345-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 65. | Leppik L, Zhihua H, Mobini S, Thottakkattumana Parameswaran V, Eischen-Loges M, Slavici A, Helbing J, Pindur L, Oliveira KMC, Bhavsar MB, Hudak L, Henrich D, Barker JH. Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci Rep. 2018;8:6307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 66. | Bonartsev AP, Voinova VV, Bonartseva GA. Poly(3-hydroxybutyrate) and human microbiota. Appl Biochem Micro+. 2018;54:547–568. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Saad B, Ciardelli G, Matter S, Welti M, Uhlschmid GK, Neuenschwander P, Suter UW. Characterization of the cell response of cultured macrophages and fibroblasts to particles of short-chain poly[(R)-3-hydroxybutyric acid]. J Biomed Mater Res. 1996;30:429-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 68. | Wu AC, Grøndahl L, Jack KS, Foo MX, Trau M, Hume DA, Cassady AI. Reduction of the in vitro pro-inflammatory response by macrophages to poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Biomaterials. 2006;27:4715-4725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 69. | Xiong YC, Yao YC, Zhan XY, Chen GQ. Application of polyhydroxyalkanoates nanoparticles as intracellular sustained drug-release vectors. J Biomater Sci Polym Ed. 2010;21:127-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 70. | Saad B, Ciardelli G, Matter S, Welti M, Uhlschmid GK, Neuenschwander P, Suter UW. Degradable and highly porous polyesterurethane foam as biomaterial: effects and phagocytosis of degradation products in osteoblasts. J Biomed Mater Res. 1998;39:594-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 71. | Ji Y, Li XT, Chen GQ. Interactions between a poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) terpolyester and human keratinocytes. Biomaterials. 2008;29:3807-3814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 72. | Cheng S, Chen GQ, Leski M, Zou B, Wang Y, Wu Q. The effect of D,L-beta-hydroxybutyric acid on cell death and proliferation in L929 cells. Biomaterials. 2006;27:3758-3765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Cheng S, Wu Q, Yang F, Xu M, Leski M, Chen GQ. Influence of DL-beta-hydroxybutyric acid on cell proliferation and calcium influx. Biomacromolecules. 2005;6:593-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Yang XD, Zou XH, Dai ZW, Luo RC, Wei CJ, Chen GQ. Effects of oligo(3-hydroxyalkanoates) on the viability and insulin secretion of murine beta cells. J Biomater Sci Polym Ed. 2009;20:1729-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Sinclair SS, Burg KJ. Effect of osteoclast co-culture on the differentiation of human mesenchymal stem cells grown on bone graft granules. J Biomater Sci Polym Ed. 2011;22:789-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 76. | Nicolaidou V, Wong MM, Redpath AN, Ersek A, Baban DF, Williams LM, Cope AP, Horwood NJ. Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PLoS One. 2012;7:e39871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 77. | Kim JK, Won YJ, Nikoh N, Nakayama H, Han SH, Kikuchi Y, Rhee YH, Park HY, Kwon JY, Kurokawa K, Dohmae N, Fukatsu T, Lee BL. Polyester synthesis genes associated with stress resistance are involved in an insect-bacterium symbiosis. Proc Natl Acad Sci U S A. 2013;110:E2381-E2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 78. | Yamazaki Y, Meirelles PM, Mino S, Suda W, Oshima K, Hattori M, Thompson FL, Sakai Y, Sawabe T, Sawabe T. Individual Apostichopus japonicus fecal microbiome reveals a link with polyhydroxybutyrate producers in host growth gaps. Sci Rep. 2016;6:21631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 79. | Defoirdt T, Halet D, Vervaeren H, Boon N, Van de Wiele T, Sorgeloos P, Bossier P, Verstraete W. The bacterial storage compound poly-beta-hydroxybutyrate protects Artemia franciscana from pathogenic Vibrio campbellii. Environ Microbiol. 2007;9:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 80. | Defoirdt T, Boon N, Sorgeloos P, Verstraete W, Bossier P. Short-chain fatty acids and poly-beta-hydroxyalkanoates: (New) Biocontrol agents for a sustainable animal production. Biotechnol Adv. 2009;27:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 81. | Ma L, Zhang Z, Li J, Yang X, Fei B, Leung PHM, Tao X. A New Antimicrobial Agent: Poly (3-hydroxybutyric acid) Oligomer. Macromol Biosci. 2019;19:e1800432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 82. | Duan Y, Zhang Y, Dong H, Wang Y, Zhang J. Effects of dietary poly-β-hydroxybutyrate (PHB) on microbiota composition and the mTOR signaling pathway in the intestines of litopenaeus vannamei. J Microbiol. 2017;55:946-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 83. | Tanaka Y, Sonoda S, Yamaza H, Murata S, Nishida K, Hama S, Kyumoto-Nakamura Y, Uehara N, Nonaka K, Kukita T, Yamaza T. Suppression of AKT-mTOR signal pathway enhances osteogenic/dentinogenic capacity of stem cells from apical papilla. Stem Cell Res Ther. 2018;9:334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 84. | Wu H, Wu Z, Li P, Cong Q, Chen R, Xu W, Biswas S, Liu H, Xia X, Li S, Hu W, Zhang Z, Habib SL, Zhang L, Zou J, Zhang H, Zhang W, Li B. Bone Size and Quality Regulation: Concerted Actions of mTOR in Mesenchymal Stromal Cells and Osteoclasts. Stem Cell Reports. 2017;8:1600-1616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 85. | Koskimäki JJ, Kajula M, Hokkanen J, Ihantola EL, Kim JH, Hautajärvi H, Hankala E, Suokas M, Pohjanen J, Podolich O, Kozyrovska N, Turpeinen A, Pääkkönen M, Mattila S, Campbell BC, Pirttilä AM. Methyl-esterified 3-hydroxybutyrate oligomers protect bacteria from hydroxyl radicals. Nat Chem Biol. 2016;12:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 86. | de Villiers D, Potgieter M, Ambele MA, Adam L, Durandt C, Pepper MS. The Role of Reactive Oxygen Species in Adipogenic Differentiation. Adv Exp Med Biol. 2018;1083:125-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 87. | Zhou J, Wang F, Ma Y, Wei F. Vitamin D3 contributes to enhanced osteogenic differentiation of MSCs under oxidative stress condition via activating the endogenous antioxidant system. Osteoporos Int. 2018;29:1917-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 88. | Nelson DL, Cox MM, editors. Lehninger Principles of Biochemistry. New-York: W.H. Freeman and Company 2008; 852-860. |
| 89. | Newman JC, Verdin E. β-Hydroxybutyrate: A Signaling Metabolite. Annu Rev Nutr. 2017;37:51-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 551] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 90. | Zhao Y, Zou B, Shi Z, Wu Q, Chen GQ. The effect of 3-hydroxybutyrate on the in vitro differentiation of murine osteoblast MC3T3-E1 and in vivo bone formation in ovariectomized rats. Biomaterials. 2007;28:3063-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 91. | Zou XH, Li HM, Wang S, Leski M, Yao YC, Yang XD, Huang QJ, Chen GQ. The effect of 3-hydroxybutyrate methyl ester on learning and memory in mice. Biomaterials. 2009;30:1532-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 92. | Zhang J, Cao Q, Li S, Lu X, Zhao Y, Guan JS, Chen JC, Wu Q, Chen GQ. 3-Hydroxybutyrate methyl ester as a potential drug against Alzheimer's disease via mitochondria protection mechanism. Biomaterials. 2013;34:7552-7562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 93. | Ji GZ, Wei X, Chen GQ. Growth of human umbilical cord Wharton's Jelly-derived mesenchymal stem cells on the terpolyester poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate). J Biomater Sci Polym Ed. 2009;20:325-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2533] [Cited by in RCA: 4211] [Article Influence: 467.9] [Reference Citation Analysis (0)] |
| 95. | Radivojevic J, Skaro S, Senerovic L, Vasiljevic B, Guzik M, Kenny ST, Maslak V, Nikodinovic-Runic J, O'Connor KE. Polyhydroxyalkanoate-based 3-hydroxyoctanoic acid and its derivatives as a platform of bioactive compounds. Appl Microbiol Biotechnol. 2016;100:161-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 96. | Wang Y, Jiang XL, Yang SC, Lin X, He Y, Yan C, Wu L, Chen GQ, Wang ZY, Wu Q. MicroRNAs in the regulation of interfacial behaviors of MSCs cultured on microgrooved surface pattern. Biomaterials. 2011;32:9207-9217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 97. | Reusch RN. Physiological importance of poly-(R)-3-hydroxybutyrates. Chem Biodivers. 2012;9:2343-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 98. | Cao C, Yudin Y, Bikard Y, Chen W, Liu T, Li H, Jendrossek D, Cohen A, Pavlov E, Rohacs T, Zakharian E. Polyester modification of the mammalian TRPM8 channel protein: implications for structure and function. Cell Rep. 2013;4:302-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 99. | Reusch RN. The role of short-chain conjugated poly-(R)-3-hydroxybutyrate (cPHB) in protein folding. Int J Mol Sci. 2013;14:10727-10748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 100. | Reusch RN, Huang R, Kosk-Kosicka D. Novel components and enzymatic activities of the human erythrocyte plasma membrane calcium pump. FEBS Lett. 1997;412:592-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 101. | O'Connor S, Szwej E, Nikodinovic-Runic J, O'Connor A, Byrne AT, Devocelle M, O'Donovan N, Gallagher WM, Babu R, Kenny ST, Zinn M, Zulian QR, O'Connor KE. The anti-cancer activity of a cationic anti-microbial peptide derived from monomers of polyhydroxyalkanoate. Biomaterials. 2013;34:2710-2718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 102. | Szwej E, Devocelle M, Kenny S, Guzik M, O'Connor S, Nikodinovic-Runic J, Radivojevic J, Maslak V, Byrne AT, Gallagher WM, Zulian QR, Zinn M, O'Connor KE. The chain length of biologically produced (R)-3-hydroxyalkanoic acid affects biological activity and structure of anti-cancer peptides. J Biotechnol. 2015;204:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 103. | Bonartsev AP, Zharkova II, Yakovlev SG, Myshkina VL, Mahina TK, Voinova VV, Zernov AL, Zhuikov VA, Akoulina EA, Ivanova EV, Kuznetsova ES, Shaitan KV, Bonartseva GA. Biosynthesis of poly(3-hydroxybutyrate) copolymers by Azotobacter chroococcum 7B: A precursor feeding strategy. Prep Biochem Biotechnol. 2017;47:173-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 104. | Marriott I, Rati DM, McCall SH, Tranguch SL. Induction of Nod1 and Nod2 intracellular pattern recognition receptors in murine osteoblasts following bacterial challenge. Infect Immun. 2005;73:2967-2973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 105. | Kim HS, Shin TH, Yang SR, Seo MS, Kim DJ, Kang SK, Park JH, Kang KS. Implication of NOD1 and NOD2 for the differentiation of multipotent mesenchymal stem cells derived from human umbilical cord blood. PLoS One. 2010;5:e15369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 106. | Ahn MY, Yoon HE, Park JH, Lee J, Min SK, Ahn SG, Yoon JH. Characterization of NODs and TLRs in innate immune response of human cementoblast cells. Oral Dis. 2013;19:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 107. | Kol A, Foutouhi S, Walker NJ, Kong NT, Weimer BC, Borjesson DL. Gastrointestinal microbes interact with canine adipose-derived mesenchymal stem cells in vitro and enhance immunomodulatory functions. Stem Cells Dev. 2014;23:1831-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 108. | Kim HS, Shin TH, Lee BC, Yu KR, Seo Y, Lee S, Seo MS, Hong IS, Choi SW, Seo KW, Núñez G, Park JH, Kang KS. Human umbilical cord blood mesenchymal stem cells reduce colitis in mice by activating NOD2 signaling to COX2. Gastroenterology. 2013;145:1392-403.e1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 109. | Caruso R, Warner N, Inohara N, Núñez G. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity. 2014;41:898-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 604] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 110. | Kishimoto T, Kaneko T, Ukai T, Yokoyama M, Ayon Haro R, Yoshinaga Y, Yoshimura A, Hara Y. Peptidoglycan and lipopolysaccharide synergistically enhance bone resorption and osteoclastogenesis. J Periodontal Res. 2012;47:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 111. | Prates TP, Taira TM, Holanda MC, Bignardi LA, Salvador SL, Zamboni DS, Cunha FQ, Fukada SY. NOD2 contributes to Porphyromonas gingivalis-induced bone resorption. J Dent Res. 2014;93:1155-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 112. | Marchesan J, Jiao Y, Schaff RA, Hao J, Morelli T, Kinney JS, Gerow E, Sheridan R, Rodrigues V, Paster BJ, Inohara N, Giannobile WV. TLR4, NOD1 and NOD2 mediate immune recognition of putative newly identified periodontal pathogens. Mol Oral Microbiol. 2016;31:243-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 113. | Souza JA, Medeiros MC, Rocha FR, de Aquino SG, Ávila-Campos MJ, Spolidorio LC, Zamboni DS, Graves DT, Rossa C Junior. Role of NOD2 and RIP2 in host-microbe interactions with Gram-negative bacteria: insights from the periodontal disease model. Innate Immun. 2016;22:598-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 114. | Kyriakidis DA, Theodorou MC, Filippou PS, Kyriakidis KD, Tiligada E. Effect of histamine on the signal transduction of the AtoS-AtoC two component system and involvement in poly-(R)-3-hydroxybutyrate biosynthesis in Escherichia coli. Amino Acids. 2008;35:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 115. | Kyriakidis DA, Tiligada E. Signal transduction and adaptive regulation through bacterial two-component systems: the Escherichia coli AtoSC paradigm. Amino Acids. 2009;37:443-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 116. | Liu X, Kumagai G, Wada K, Tanaka T, Fujita T, Sasaki A, Furukawa KI, Ishibashi Y. Suppression of osteogenic differentiation in mesenchymal stem cells from patients with ossification of the posterior longitudinal ligament by a histamine-2-receptor antagonist. Eur J Pharmacol. 2017;810:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 117. | Davoudi Z, Peroutka-Bigus N, Bellaire B, Wannemuehler M, Barrett TA, Narasimhan B, Wang Q. Intestinal organoids containing poly(lactic-co-glycolic acid) nanoparticles for the treatment of inflammatory bowel diseases. J Biomed Mater Res A. 2018;106:876-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 118. | van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1327] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 119. | Aracic S, Semenec L, Franks AE. Investigating microbial activities of electrode-associated microorganisms in real-time. Front Microbiol. 2014;5:663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 120. | Marsili E, Freguia S. Electroactive microorganisms and microbial consortia. Bioelectrochemistry. 2018;120:110-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 121. | Oziat J, Elsen S, Owens RM, Malliaras GG, Mailley P. Electrochemistry provides a simple way to monitor Pseudomonas aeruginosa metabolites. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:7522-7525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 122. | Schulz S, Toft S. Identification of a sex pheromone from a spider. Science. 1993;260:1635-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 123. | Marshall DG, Jackson TA, Unelius CR, Wee SL, Young SD, Townsend RJ, Suckling DM. Morganella morganii bacteria produces phenol as the sex pheromone of the New Zealand grass grub from tyrosine in the colleterial gland. Naturwissenschaften. 2016;103:59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 124. | Quang DN, Hashimoto T, Toyota M, Asakawa Y. Occurrence of a high concentration of spider pheromones in the ascomycete fungus Hypoxylon truncatum. J Nat Prod. 2003;66:1613-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 125. | Li SC, Vu LT, Luo JJ, Zhong JF, Li Z, Dethlefs BA, Loudon WG, Kabeer MH. Tissue elasticity bridges cancer stem cells to the tumor microenvironment through microRNAs: implications for a "watch-and-wait" approach to cancer. Curr Stem Cell Res Ther. 2017;12:455-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |