修回日期: 2025-04-09
接受日期: 2025-04-17
在线出版日期: 2025-04-28
结直肠癌是我国最常见的消化道恶性肿瘤之一, 由于早期诊断困难, 其发病率和死亡率逐年增加. 分子影像能监测活体内细胞和分子水平的生物学过程, 具有潜在的临床诊断和治疗价值. 分子影像关键是发展新的成像技术和构建靶向分子探针. 葡萄糖转运蛋白(glucose transporter, Glut)是分布于人体各种细胞膜上的蛋白. 目前研究认为, Glut1的异常表达与结直肠癌密切相关, 其在结直肠癌发生、发展中发挥重要作用, 有望成为结直肠癌分子影像的特异性靶点.
核心提要: 研究表明在肿瘤的发生发展的各个阶段, 肿瘤细胞表面常常高表达某些特异性分子, 其中一些特异性分子已被证明可以作为标志物用于肿瘤的早期诊断. 因此将医学影像技术与探测肿瘤细胞表面的特异性分子标志物相结合, 推动了高度特异的成像技术-肿瘤分子影像. 葡萄糖转运蛋白1是细胞恶变的早期标志, 可以为肿瘤分期、肿瘤侵袭性和组织学分化提供重要依据, 以建立结直肠癌患者的早期诊断和个体化治疗. 在分子影像学研究中, 以葡萄糖转运蛋白为分子成像靶点, 合成荧光、磁共振成像、正电子发射扫描等传统影像技术靶向分子探针, 实现靶向分子成像, 探索到疾病在解剖病理过渡到分子水平的变化, 在疾病早期做出准确诊断, 将极大的提高结直肠癌的诊断和治疗水平. 本文将对葡萄糖转运蛋白在结直肠癌中的表达及其靶向分子影像价值及潜力进行了综述.
引文著录: 邢晓宏, 李晓兵. 葡萄糖转运蛋白在结直肠肿瘤分子影像诊断的研究进展. 世界华人消化杂志 2025; 33(4): 268-275
Revised: April 9, 2025
Accepted: April 17, 2025
Published online: April 28, 2025
Colorectal cancer is one of the most common digestive tract malignant tumors in China. Due to the difficulty in early diagnosis, its morbidity and mortality are increasing year by year. Molecular imaging can monitor biological processes at cellular and molecular levels in vivo, having potential clinical diagnosis and treatment value. The key of molecular imaging is to develop new imaging technology and construct targeted molecular probes. Glucose transporters (Glut) are proteins distributed on various cell membranes in the human body. At present, it is believed that the abnormal expression of Glut1 is closely related to colorectal cancer, playing an important role in the occurrence and development of this malignancy. Therefore, Glut1 is expected to become a specific target for molecular imaging of colorectal cancer.
- Citation: Xing XH, Li XB. Progress in research of glucose transporters in molecular imaging of colorectal tumors. Shijie Huaren Xiaohua Zazhi 2025; 33(4): 268-275
- URL: https://www.wjgnet.com/1009-3079/full/v33/i4/268.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v33.i4.268
结直肠癌(colorectal cancer, CRC)是消化道常见恶性肿瘤, 在疾病早期缺乏明显症状和体征, 发病率和死亡率居高不下[1]. 结直肠癌是男性第三位、女性第二位常见的导致全球癌症相关死亡的肿瘤, 全世界每年有超过120万个新的CRC癌症病例和60.87万人死亡病例[2]. 其中CRC病理分期决定了患者的预后, Ⅰ期患者的5年生存率超过90%和Ⅳ期患者的5年生存率小于10%[3]. 结直肠癌在其发生发展过程中, 可以发生很多基因、蛋白及生物酶等分子标志物的异常, 这些分子标志物包括原癌基因、抑癌基因、凋亡基因激活和失活以及端粒酶逆转录酶(human telomerase reverse transcriptase, hTERT)、尿激酶纤维蛋白溶酶原激活(urokinase-type plasminogen activator receptor, uPAR)和葡萄糖转运蛋白(glucose transporters, Gluts)等异常表达. 在这些分子标志物中, 葡萄糖转运蛋白(Glut)作为细胞膜蛋白被特异性单抗识别而引起关注[4]. 通过特异性单抗标记造影剂结合现代影像技术如磁共振成像(magnetic resonance imaging, MRI)、核医学成像(positron emission tomography, PET)或近红外光学成像(near infrared fluorescent, NIRF),可以实现靶向分子成像, 探索到疾病从解剖病理过渡到分子水平的变化, 在疾病早期做出准确诊断, 能够提升结直肠癌的诊断和治疗水平. 本文对葡萄糖转运蛋白在结直肠癌中的表达及其靶向分子影像诊断价值及潜力作如下综述.
细胞不能通过简单的弥散方式吸收葡萄糖, 它必须借助一种特殊膜蛋白质, 即Glut才能完成吸收过程[5]. Glut是一个蛋白家族, 家族中有14种蛋白已被证实[4]. 它们不但参与葡萄糖、果糖等跨膜转运的正常生理过程, 而且它的过度表达与肿瘤发生发展有关, 许多肿瘤表现为特征性的Glut异常和/或过度表达. 与正常组织相比, 恶性肿瘤对葡萄糖的摄取及代谢处于增高状态, 糖代谢增加是许多恶性肿瘤组织的显著性特征, 恶性肿瘤中糖代谢增强过程由Glut家族所介导. 特异性的Glut在某些肿瘤组织中的表达与肿瘤来源于不同细胞类型有关. 肿瘤细胞通常都有癌基因的表达, Mountjoy等[6]用活化的ras及src癌基因转染啮齿类动物的成纤维细胞, 观察到它们的Glut1 mRNA及蛋白质表达均增加、并进一步认识到葡萄糖转运蛋白在恶性肿瘤细胞或组织的分布和功能可能会促进新的诊断和预后标志物的发展. Gluts家族依其基因的编码产物而分别命名为Glut1-14, 该家族中每个成员都含有12个跨膜区域, C端和N端都在细胞质中, Joost等[7]根据同型异构体不同、组织表达、亚结构、动力学的不同, 将它们分为三大类: Glut Ⅰ(Glut l, Glut2, Glut3, Glut4, Glut14)、Glut Ⅱ(Glut5, Glut7, Glut9, Glut11)、Glut Ⅲ(Glut6, Glut8, Glut10, Glut12, HMIT).
Glut1是家族中起到重要角色作用, 在许多不同组织类型的肿瘤中上调表达[8]. Glut1的过度表达已在肿瘤组织中被广泛地观察到[9]. 以往研究发现在人类正常或良性肿瘤组织中不能或低阳性检测到Glut1表达, 而在恶性肿瘤组织中则普遍高表达, 包括颈部肿瘤、结直肠癌、胃癌、食管癌、肾癌、膀胱癌、肺癌、乳腺癌等[10-15], 且与相应的正常组织相比, Glut1的转录增加, 阳性表达率均达到或超过50%[15,16]. Younes等[17]研究了118例乳腺癌患者Glutl表达, 其中42%有Glut1高表达, 这些高表达组织都伴随着一定程度的高度增殖能力, 认为Glut1是细胞恶变的早期标志之一. 众多研究表明, Glut1的高表达与肿瘤的发生发展甚至预后有一定关系, 可以为肿瘤的诊断、治疗提供新思路[18-20].
Glut2主要表达在肠、肝、肾、及胰腺的B细胞, 对葡萄糖亲和力较低, 似乎仅在血浆葡萄糖水平相对较高时才作为转运体发挥载体功能[21]. Glut3在脑神经元中被发现, 存在于人类所有组织中. 近来研究表明机体处于缺氧等环境下, 氧化磷酸化途径在受到抑制时, Glut3的表达可明显升高,并在肿瘤的发生、发展、转移、侵袭等生物学特性中发挥重要作用[22]. Glut4主要表达在胰岛素敏感组织中如心脏、骨骼肌、脂肪组织等[23]. 此外Glut4是肌肉和脂肪细胞主要的转运蛋白, 一般情况下, 不能起转运葡萄糖的作用, 仅在胰岛素的信号刺激下能促进饭后葡萄糖进入上述组织中储存起来. Glut14主要表达于是睾丸, 它的同源异构体没有在小鼠中发现. Glut5主要主要作为果糖转运体存在于小肠、肾脏和睾丸[24]. Glut7的发现较晚, 目前对其了解甚少, 主要表达于肠上皮细胞的刷状缘[25]. Glut9高表达肾脏和肝脏[26]. Glut11高表达在胰腺、肾和胎盘, 同时也弱表达于心脏和骨骼肌中[27]. Glut6主要表达在脑、脾脏和外周血白细胞[28,29]. Glut8可能是一个多功能转运蛋白, 研究表明Glut8的作用是在脂肪细胞中摄取葡萄糖并且由脂肪细胞调节[30,31]. Glut10主要表达在肝脏和胰腺, 其SLC2A10基因在染色体(20q12-q13.1)之间, 它的异常与2型糖尿病有关[29]. Glut12的高表达前列腺癌和乳腺癌中[19,32]. H +肌醇协同转运体(H+/myo-inositol transporter, HMIT)高表达在缺乏糖转运活动的大脑[32].
研究显示Glut1在正常或良性病变中不表达, 而在恶性肿瘤组织中高水平表达, 提示可能与肿瘤细胞糖的利用和吸收增加有关. 研究表明, 一些恶性肿瘤如结直肠恶性肿瘤高表达Glut1[33]. 周玉玲等[34]采用免疫组织化学方法检测到Gluts在大肠腺癌组织中的表达阳性率分别为58.3%, 而在正常大肠组织中无表达,与正常大肠组织表达比较有显著性差异(P<0.0l). Younes[35]发现Glut1在结直肠癌中的表达与淋巴结转移呈正相关性. Haber等[36]对112例结直肠癌患者的回顾性研究中发现Glut1的表达与肿瘤的转移有明显统计学意义; 并通过COX生存期分析得出结论, 认为Glut1的表达量可作为结直肠癌患者预后的一项有意义指标. Sakashita[37]调查显示Glut1表达存在于18%低度不典型增生的腺瘤, 63%高度不典型增生腺瘤, 但这种病变的阳性通常是较弱. 对于癌组织能显示Glut1的积极性和浸润深度之间的相关性(T1与T2分别是45%和74%, P<0.01), 阳性表达被认为是一个潜在的恶变标记. Glut1在动物的肿瘤模型中也有表达, Nelson等[38]发现Glut1在肺癌(LX-1)、乳腺癌(MX-1)和结肠癌(DLD-1)的肿瘤移植模型中都有表达. 周玉玲团队研究提示Glut1可以作为结直肠癌恶行程度及预后判断的指标之一[34]. 在体外实验同样证实, 文献显示在人类结肠癌细胞系Caco-2, 其转染ras基因后表达Glut1和Glut3, 但抑制了Glut5和Glut2的表达[39]. 结果显示转染ras基因的Caco-2细胞葡萄糖代谢明显增加, 这个结果表明Caco-2癌细胞主要是通过Glut1和Glut3为胞膜转运葡萄糖. 这表明上调Glut1和Glut3的表达是在转化过程中发生, 该蛋白的表达增加可能是一个肿瘤进程的基本一部分. Glut1的肿瘤基因表达增加和激活可能有两种机制, 一是ras或src等癌基因和生长因子调节相关, 转染ras或src, 可能导致糖吸收和糖转运蛋白的增加; 另外, 肿瘤微环境局部缺氧也可能诱导糖代谢适应性吸收增加Glut1表达增加[36]. 综合文献, Glut1的高强度表达提示癌前病变可能存在潜在恶变, 且与肿瘤进展、分化以及患者预后情况甚至生存率有着一定关系. 以上这些研究表明, Glut1是细胞恶性变的早期标志之一, 可以为肿瘤分期、肿瘤侵袭性和组织学分化提供重要依据, 有利于CRC早期诊断和个体化治疗.
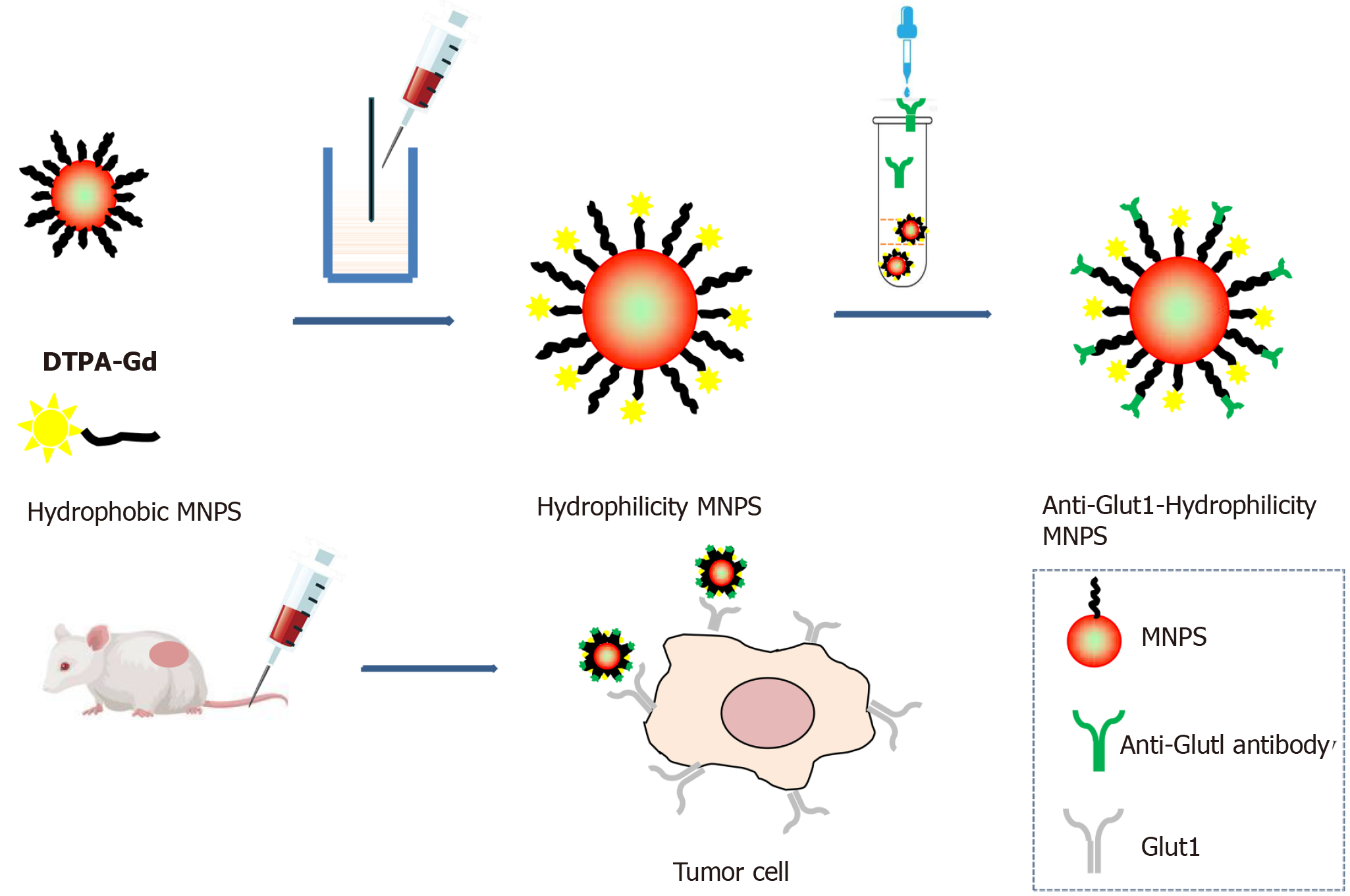
肿瘤分子影像研究范围广泛, 其中纳米颗粒对比剂研究是现今分子影像学热门课题. 纳米材料通过提供传统影像增强的对比度、改进的靶向能力和多功能成像模式, 建立了肿瘤分子成像的创新. 例如, 量子点(qualitative description, QD)表现出强烈的荧光特性, 使其适用于光学成像[40]. 金纳米粒子可以增强影像的对比度[41,42]. 此外, 人工智能(artificial intelligence, AI)和机器学习方法通过增强图像重建、自动分割和提高诊断准确性, 也正在改变肿瘤影像诊断[43]. 这篇综述介绍了基于纳米材料的癌症成像的最新进展, 强调了纳米技术和人工智能驱动创新在分子影像诊断领域的进展. 图1清晰展示了功能纳米颗粒合成过程示意图和体内靶向肿瘤成像应用流程图. 功能化纳米粒子旨在识别特定的肿瘤标志物, 其通过结合抗体或配体偶联, 与肿瘤受体相互作用, 提高成像特异性并降低全身对比剂的毒性[45]. 研究人员可以合理地设计纳米粒子, 使其具有最佳的物理化学性质, 增强其作为成像对比剂的功效, 同时尽量减少不良反应.
特异性分子探针多基于两类对比剂构成. 一类对比剂是超顺磁性氧化铁(superparamagnetic iron oxide nanoparticles, SPIONs), 它是一种新型的磁共振阴性对比剂. 磁性纳米颗粒容易被网状内皮系统吞噬细胞摄取[46,47], 为了达到靶向分子成像, 需要在磁性纳米颗粒表面修饰靶向小分子, 多肽、抗体、叶酸等, 通过靶向分子与肿瘤细胞表面受体特异地结合在肿瘤区域富集, 达到对肿瘤(magnetic resonance, MR)造影增强效果[48]. 高明远团队[49,50]率先开展SPIONs的肿瘤诊断研究, Hu等[51]通过对Fe3O4纳米颗粒与抗CEA单抗偶联, 得MR分子影像探针(Fe3O4-rch24), 并在活体动物模型上开展了磁共振成像, 研究结果显示Fe3O4-rch24探针能特异性识别皮下种植的结直肠癌肿瘤病灶, 产生T2成像对比增强信号, 增强信号于注射探针后24 h达到峰值, 与未注射前相比, T2值下降10%, 而对照组(未偶联抗体的Fe3O4纳米颗粒)则没有显示明显的信号下降. 与单抗相比, 单链抗体具有分子量小、通透性强、免疫原性低等优点. Yang等[52]借鉴这些优点, 采用化学交联法构建表皮生长因子受体(epidermal growth factor receptor, EGFR)抗体MR靶向超顺磁性分子探针, 并证明该探针可增强体内原位移植胰腺癌模型的磁共振成像. 除此之外, 多肽、叶酸也因分子量小而备受青睐[53-55]. 如王小会采用多肽与Fe3O4-BSA偶联, 体外细胞实验证实该分子探针可特异性的识别胰腺癌细胞[56].
另一类对比剂为顺磁性物质钆(Gd)的有机金属配合物, 如(e.g. Gd -DTPA, Gd-DOTA, Gd-HPDO3A), 产生T1加权像的对比增强. Gd(Ⅲ)类特异性分子探针制备常用各式微粒转运体等包裹Gd(Ⅲ)后, 与靶向性配体连接, 靶向性配体与靶结构上的受体结合后, 靶结构即可显像. 刘峰君[57]采用RGD多肽与Gd(Ⅲ)、荧光基团构建了双模态分子探针, 实验表明该探针有较高弛豫率, 结合多肽分子实现荧光-MR双模态分子成像. 宋歌等构建了QDs@Gd3+-RGD[一类含有精氨酸-甘氨酸-天冬氨酸(Arg.Gly.Asp)的短肽]双模态显像纳米探针成功实现体外结直肠癌LOVO细胞的MRI显像[58]. Xing等[44]设计了一种细胞靶向顺磁-荧光双信号分子探针GdDTPA•BSA@QDs-PcAb, 可以用于小鼠皮下结直肠癌体内MRI和荧光成像. 综上, 特异性分子探针制备的方法是基于对比剂与靶向性配体连接在一起, 靶向性配体与靶结构上的受体结合后, 靶结构即可显像. 从理论上讲, Glut1可作为直肠癌受体的重要表面标志物实现直肠癌靶向分子成像.
18氟-脱氧葡萄糖(18F-fluorodeoxyglucose, 18F-FDG)PET显像可反映组织的功能代谢情况, 已被广泛应用于临床. FDG是一种用氟原子标记的葡萄糖的类似物, FDG的一个重要特性是经过磷酸化后, 生成的FDG-6-P不再继续分解, 而保留在肿瘤细胞中, 对FDG进行核标记后即可对糖代谢旺盛、已糖激酶活跃及Glutl上调的细胞进行分子显像. 其次, 结直肠癌通常表现为18F-FDG PET高摄取, 摄取程度取决于18F-FDG PET跨膜转运, 糖酵解集血管生成等因素. Tateishi等[59]通过对骨与软组织中Glut1的含量检测及FDG-PET的检杏, 发现随肿瘤恶性的程度增加, Glutl的含量也相应增加, FDG-PET检查的阳性率也随之增加. Higashi等[60]在胰腺癌中、Yen[61]在鼻咽癌中也有同样的结论. 李迎辞探讨Glutl在结直肠癌中的表达及其与18F-FDG PET/CT显像最大标准摄取值(maximum standard uptake value, SUVmax)的关系, 结果显示CRC的SUV与Glutl的表达存在相关性(r = 0.63, P<0.05)[62]. 刘松涛对20例临床可疑直肠癌术后局部复发的患者行FDG PET显像, 将显像结果与病理组织学及临床随访结果对比发现直肠癌术后局部复发的灵敏性和特异性分别为100%、60%; 并且采用半定量法显示恶性肿瘤的标准摄取值(SUV, 范围为2.7-17.2, 平均9.9)明显高于良性病变者(SUV为1.3-4.0, 平均2.6), 诊断直肠癌术后局部复发的灵敏性和特异性分别为100%、80%[63]. 申景涛对20例结直肠癌患者行术前18F-FDG PET检查, 结果显示20例患者均为高摄取, SUV值表达呈正相关[64]. 综上所述, 提示18F-FDG的跨膜转运过程影响结直肠癌组织的18F-FDG摄取过程, 因此从理论上讲, Glut1-FDG-PET分子成像可以提高对结直肠肿瘤良恶性鉴别的早期诊断、分期、肿瘤化疗或放疗前后的评估和术后复发或远处转移的灵敏性和特异性的检测方法, 对临床治疗肿瘤意义重大.
目前磁共振多模态分子影像技术在肿瘤早期诊断中的应用主要以荧光分子探针为基础, 合成多功能靶向探针, 结合光学成像与MRI以实现肿瘤及癌前病变的早期诊断. Zhang等[65]以钆离子、近红外低毒量子点、牛血清蛋白等为原料, 构建出MRI弛豫率/荧光效率高和生物相容性好的Gd3+/量子点多模态纳米探针, T1加权MRI成像证实了Gd3+/量子点多模态纳米探针具有很好的阳性对比功效. 此外构建多靶点分子探针, 针对某一类特定的肿瘤, 选择其特异表达的几种标志物, 在合成的探针上同时耦联针对这些靶点的靶向分子, 使制备的探针可以完成多个靶点的同时识别, 能够进一步提高多模态分子影像检测的特异性及准确度. Qiao等[49,66]采用EGFR和成纤维细胞激活蛋白, 前者在结直肠癌中高表达, 后者专一性高表达于肿瘤间质, 可更好地用于描绘肿瘤边界, 适用于微小肿瘤的早期诊断, 以化学键合的方式通过修饰在磁性纳米颗粒表面聚乙烯二醇末端的羧基与上述靶点相关的抗体进行耦联, 获得肿瘤分子影像探针, 其结果已显示出该分子探针直肠癌细胞具有良好的靶向性. 因此, 以Gluts(e.g. Glut1)为成像靶点, 通过特异性单抗标记造影剂构建基于Glut1蛋白为靶点的NIRF/MRI/PET等多模态靶向分子探针, 实现直肠癌活体水平显像理论上是可行的.
但是随着纳米颗粒对比剂合成技术研究进展, 纳米颗粒对环境和人类健康的潜在危害逐渐引起了研究人员的关注. 许多研究团队研究了某些纳米颗粒如量子点的毒性, 表明纳米颗粒会对生物系统有一定的影响或损伤[67]. 其毒理机制主要源于对比剂中重金属离子的释放和活性氧的产生[68]. 目前的研究结果表明, 纳米颗粒对比剂的毒性不仅与其制备方法、表面性质和粒径等物理和化学性质有关, 还受到细胞类型、治疗环境和给药途径的影响, 研究方法不统一、不系统, 使得评价和比较纳米颗粒生物安全性的工作极其复杂和困难. 本文综述旨在提示绿色、生物安全、环境友好的分子探针是未来合成技术首先需要考虑的问题, 通过不同的合成类型及化学修饰, 进一步为生物医学和环境应用开发更安全的分子探针.
近年来, 越来越多地使用基于AI的放射组学来帮助检测癌症患者的病变、病理诊断、放射治疗靶点划定和疗效预测. 人工智能深度学习技术通过实现自动降噪、分割和特征提取来进行图像分析显著提高了医疗的效率和准确性. 此外在分子影像诊断领域, 人工智能能促进描绘出杂交瘤中肿瘤和纳米粒子精确分布[43], 如PET/MRI, 能够增强对恶性病变的检测特异性和敏感性[69]. 目前人工智能加上近红外成像[目前基于吲哚青绿(ICG荧光探针)]的广泛可用性, 可以与动态放射灌注成像类似的方式对息肉/癌前病变进行结肠镜组织分类和预后分层[42]. 下一阶段的进展可能包括基于特定肿瘤内荧光信号模式的术中评估, 对小的直肠恶性肿瘤进行详细的数字影像诊断表征[70]. 这可能包括T分期和瘤内分子过程分析(例如关于血管生成、分化、炎症成分和肿瘤与基质比率), 有可能准确预测对非手术治疗的微观局部反应, 从而实现个性化治疗. 这些进步也适用于目前从临床试验中出现的下一代荧光成像剂. 此外, 随着临床研究中新的荧光分子探针的出现, 并且协同人工智能, 这种新技术发展提高自动检测和量化对比度, 提高了研究人员对分子探针体内循环的准确性模拟, 优化靶向成像纳米颗粒对比剂, 提高分子影像诊断可靠性.
随着分子影像的快速发展, 寻找和探索一些特异性强、灵敏度高的靶向分子探针是影像学者们的目标之一, 同时发展新的影像技术, 只有这样才能将目前结直肠癌的影像学诊断从基于大体形态学的非特异性影像学诊断, 推进到基于病变分子生物学水平的特异性早期诊断, 使临床早期干预诊断治疗结直肠癌成为可能. 本文旨在强调最近的分子影像创新和癌症成像的未来方向, 探讨纳米技术驱动的潜力来提高影像诊断准确性.
学科分类: 胃肠病学和肝病学
手稿来源地: 上海市
同行评议报告学术质量分类
A级 (优秀): A
B级 (非常好): B
C级 (良好): C
D级 (一般): D
E级 (差): 0
科学编辑: 刘继红 制作编辑:郑晓梅
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [PubMed] [DOI] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] |
| 3. | Edge SB, Sobin LH, Page DL, Gospodarowicz MK, Greene FL, Winchester DP. Re: Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2005;97:463-4; author reply 464-5. [PubMed] [DOI] |
| 4. | Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298:E141-E145. [PubMed] [DOI] |
| 5. | Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654-662. [PubMed] [DOI] |
| 6. | Mountjoy KG, Flier JS. Vanadate regulates glucose transporter (Glut-1) expression in NIH3T3 mouse fibroblasts. Endocrinology. 1990;127:2025-2034. [PubMed] [DOI] |
| 7. | Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review). Mol Membr Biol. 2001;18:247-256. [PubMed] [DOI] |
| 8. | Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29-40. [PubMed] [DOI] |
| 9. | Mobasheri A. Glucose: an energy currency and structural precursor in articular cartilage and bone with emerging roles as an extracellular signaling molecule and metabolic regulator. Front Endocrinol (Lausanne). 2012;3:153. [PubMed] [DOI] |
| 10. | Endo M, Tateishi U, Seki K, Yamaguchi U, Nakatani F, Kawai A, Chuman H, Beppu Y. Prognostic implications of glucose transporter protein-1 (glut-1) overexpression in bone and soft-tissue sarcomas. Jpn J Clin Oncol. 2007;37:955-960. [PubMed] [DOI] |
| 11. | Ma X, Hui Y, Lin L, Wu Y, Zhang X, Liu P. Clinical significance of COX-2, GLUT-1 and VEGF expressions in endometrial cancer tissues. Pak J Med Sci. 2015;31. [DOI] |
| 12. | Koh YW, Lee SJ, Park SY. Differential expression and prognostic significance of GLUT1 according to histologic type of non-small-cell lung cancer and its association with volume-dependent parameters. Lung Cancer. 2017;104:31-37. [PubMed] [DOI] |
| 13. | Huang H, Song S, Liu W, Ye S, Bao Y, Mirza M, Li B, Huang J, Zhu R, Lian H. Expressions of glucose transporter genes are diversely attenuated and significantly associated with prostate cancer progression. Am J Clin Exp Urol. 2023;11:578-593. [PubMed] |
| 14. | Lu K, Yang J, Li DC, He SB, Zhu DM, Zhang LF, Zhang XU, Chen XC, Zhang B, Zhou J. Expression and clinical significance of glucose transporter-1 in pancreatic cancer. Oncol Lett. 2016;12:243-249. [PubMed] [DOI] |
| 15. | Boström PJ, Thoms J, Sykes J, Ahmed O, Evans A, van Rhijn BW, Mirtti T, Stakhovskyi O, Laato M, Margel D, Pintilie M, Kuk C, Milosevic M, Zlotta AR, Bristow RG. Hypoxia Marker GLUT-1 (Glucose Transporter 1) is an Independent Prognostic Factor for Survival in Bladder Cancer Patients Treated with Radical Cystectomy. Bladder Cancer. 2016;2:101-109. [PubMed] [DOI] |
| 16. | Cho H, Lee YS, Kim J, Chung JY, Kim JH. Overexpression of glucose transporter-1 (GLUT-1) predicts poor prognosis in epithelial ovarian cancer. Cancer Invest. 2013;31:607-615. [PubMed] [DOI] |
| 17. | Younes M, Lechago LV, Lechago J. Overexpression of the human erythrocyte glucose transporter occurs as a late event in human colorectal carcinogenesis and is associated with an increased incidence of lymph node metastases. Clin Cancer Res. 1996;2:1151-1154. [DOI] |
| 19. | Chandler JD, Williams ED, Slavin JL, Best JD, Rogers S. Expression and localization of GLUT1 and GLUT12 in prostate carcinoma. Cancer. 2003;97:2035-2042. [PubMed] [DOI] |
| 20. | Jun YJ, Jang SM, Han HL, Lee KH, Jang KS, Paik SS. Clinicopathologic significance of GLUT1 expression and its correlation with Apaf-1 in colorectal adenocarcinomas. World J Gastroenterol. 2011;17:1866-1873. [PubMed] [DOI] |
| 21. | Burcelin R, Dolci W, Thorens B. Glucose sensing by the hepatoportal sensor is GLUT2-dependent: in vivo analysis in GLUT2-null mice. Diabetes. 2000;49:1643-1648. [PubMed] [DOI] |
| 22. | Maher F, Simpson IA. Modulation of expression of glucose transporters GLUT3 and GLUT1 by potassium and N-methyl-D-aspartate in cultured cerebellar granule neurons. Mol Cell Neurosci. 1994;5:369-375. [PubMed] [DOI] |
| 23. | Bruno J, Brumfield A, Chaudhary N, Iaea D, McGraw TE. SEC16A is a RAB10 effector required for insulin-stimulated GLUT4 trafficking in adipocytes. J Cell Biol. 2016;214:61-76. [PubMed] [DOI] |
| 24. | Matosin-Matekalo M, Mesonero JE, Laroche TJ, Lacasa M, Brot-Laroche E. Glucose and thyroid hormone co-regulate the expression of the intestinal fructose transporter GLUT5. Biochem J. 1999;339:233-239. [PubMed] [DOI] |
| 25. | Paolini R, Moore C, Matthyssen T, Cirillo N, McCullough M, Farah CS, Botha H, Yap T, Celentano A. Transcriptional regulation of glucose transporters in human oral squamous cell carcinoma cells. J Oral Pathol Med. 2022;51:679-683. [PubMed] [DOI] |
| 26. | Doege H, Bocianski A, Joost HG, Schürmann A. Activity and genomic organization of human glucose transporter 9 (GLUT9), a novel member of the family of sugar-transport facilitators predominantly expressed in brain and leucocytes. Biochem J. 2000;350 Pt 3:771-776. [PubMed] [DOI] |
| 27. | Doege H, Bocianski A, Scheepers A, Axer H, Eckel J, Joost HG, Schürmann A. Characterization of human glucose transporter (GLUT) 11 (encoded by SLC2A11), a novel sugar-transport facilitator specifically expressed in heart and skeletal muscle. Biochem J. 2001;359:443-449. [PubMed] [DOI] |
| 28. | McMillin SL, Schmidt DL, Kahn BB, Witczak CA. GLUT4 Is Not Necessary for Overload-Induced Glucose Uptake or Hypertrophic Growth in Mouse Skeletal Muscle. Diabetes. 2017;66:1491-1500. [PubMed] [DOI] |
| 29. | Valberg SJ, Velez-Irizarry D, Williams ZJ, Pagan JD, Mesquita V, Waldridge B, Maresca-Fichter H. Novel Expression of GLUT3, GLUT6 and GLUT10 in Equine Gluteal Muscle Following Glycogen-Depleting Exercise: Impact of Dietary Starch and Fat. Metabolites. 2023;13:718. [PubMed] [DOI] |
| 30. | Aerni-Flessner L, Abi-Jaoude M, Koenig A, Payne M, Hruz PW. GLUT4, GLUT1, and GLUT8 are the dominant GLUT transcripts expressed in the murine left ventricle. Cardiovasc Diabetol. 2012;11:63. [PubMed] [DOI] |
| 31. | Carayannopoulos MO, Chi MM, Cui Y, Pingsterhaus JM, McKnight RA, Mueckler M, Devaskar SU, Moley KH. GLUT8 is a glucose transporter responsible for insulin-stimulated glucose uptake in the blastocyst. Proc Natl Acad Sci USA. 2000;97:7313-7318. [PubMed] [DOI] |
| 32. | Pujol-Gimenez J, de Heredia FP, Idoate MA, Airley R, Lostao MP, Evans AR. Could GLUT12 be a Potential Therapeutic Target in Cancer Treatment? A Preliminary Report. J Cancer. 2015;6:139-143. [PubMed] [DOI] |
| 33. | Vasudevan S, Mehta A, Sharma SK, Sharma A. Expression of glucose transporter 1 (SLC2A1) - Clinicopathological associations and survival in an Indian cohort of colorectal cancer patients. J Cancer Res Ther. 2022;18:650-655. [PubMed] [DOI] |
| 35. | Younes M, Lechago LV, Lechago J. Overexpression of the human erythrocyte glucose transporter occurs as a late event in human colorectal carcinogenesis and is associated with an increased incidence of lymph node metastases. Clin Cancer Res. 1996;2:1151-1154. [PubMed] |
| 36. | Haber RS. GLUT1 Glucose Transporter Expression in Colorectal Carcinoma. Cancer. 1998;83:34-40. [DOI] |
| 37. | Sakashita M, Aoyama N, Minami R, Maekawa S, Kuroda K, Shirasaka D, Ichihara T, Kuroda Y, Maeda S, Kasuga M. Glut1 expression in T1 and T2 stage colorectal carcinomas: its relationship to clinicopathological features. Eur J Cancer. 2001;37:204-209. [PubMed] [DOI] |
| 38. | Nelson CA, Wang QJ, Bourque JP, Crane PD. Targeting of Glucose Transport Proteins for Tumor Imaging: Is It Feasible? JNM. 1996;37:1031-1037. [DOI] |
| 39. | Airley R, Evans A, Mobasheri A, Hewitt SM. Glucose transporter Glut-1 is detectable in peri-necrotic regions in many human tumor types but not normal tissues: Study using tissue microarrays. Ann Anat. 2010;192:133-138. [PubMed] [DOI] |
| 40. | Liang Z, Khawar MB, Liang J, Sun H. Bio-Conjugated Quantum Dots for Cancer Research: Detection and Imaging. Front Oncol. 2021;11:749970. [PubMed] [DOI] |
| 41. | Sobol NB, Korsen JA, Younes A, Edwards KJ, Lewis JS. ImmunoPET Imaging of Pancreatic Tumors with (89)Zr-Labeled Gold Nanoparticle-Antibody Conjugates. Mol Imaging Biol. 2021;23:84-94. [PubMed] [DOI] |
| 42. | Cardellini J, Dallari C, De Santis I, Riccio L, Ceni C, Morrone A, Calamai M, Pavone FS, Credi C, Montis C, Berti D. Hybrid lipid-AuNP clusters as highly efficient SERS substrates for biomedical applications. Nat Commun. 2024;15:7975. [PubMed] [DOI] |
| 43. | Chow JCL. Nanomaterial-Based Molecular Imaging in Cancer: Advances in Simulation and AI Integration. Biomolecules. 2025;15:444. [PubMed] [DOI] |
| 44. | Xing X, Zhang B, Wang X, Liu F, Shi D, Cheng Y. An "imaging-biopsy" strategy for colorectal tumor reconfirmation by multipurpose paramagnetic quantum dots. Biomaterials. 2015;48:16-25. [PubMed] [DOI] |
| 45. | Liu F, Ye W, Wang J, Song F, Cheng Y, Zhang B. Parallel comparative studies on toxicity of quantum dots synthesized and surface engineered with different methods in vitro and in vivo. Int J Nanomedicine. 2017;12:5135-5148. [PubMed] [DOI] |
| 46. | Wei H, Hu Y, Wang J, Gao X, Qian X, Tang M. Superparamagnetic Iron Oxide Nanoparticles: Cytotoxicity, Metabolism, and Cellular Behavior in Biomedicine Applications. Int J Nanomedicine. 2021;16:6097-6113. [PubMed] [DOI] |
| 47. | Shilo M, Sharon A, Baranes K, Motiei M, Lellouche JP, Popovtzer R. The effect of nanoparticle size on the probability to cross the blood-brain barrier: an in-vitro endothelial cell model. J Nanobiotechnology. 2015;13:19. [PubMed] [DOI] |
| 48. | Louie A. Multimodality imaging probes: design and challenges. Chem Rev. 2010;110:3146-3195. [PubMed] [DOI] |
| 49. | Qiao R, Jia Q, Zeng J. Magnetic Iron Oxide Nanoparticles and Their Applications in Magnetic Resonance Imaging. ABBS. 2011;27:272-288. [DOI] |
| 50. | Qiao RR, Zeng JF, Jia QJ, Du J, Shen L, Gao MY. Magnetic Iron Oxide Nanoparticle--an Important Cornerstone of MR Molecular Imaging of Tumors. Acta Phys-Chim Sin. 2012;28:993-1011. [DOI] |
| 51. | Hu FQ, Wei L, Zhou Z, Ran YL, Li Z, Gao MY. Preparation of biocompatible magnetite nanocrystals for in vivo magnetic resonance detection of cancer. Adv Mater. 2006;18:2553. [DOI] |
| 52. | Yang L, Mao H, Wang YA, Cao Z, Peng X, Wang X, Duan H, Ni C, Yuan Q, Adams G, Smith MQ, Wood WC, Gao X, Nie S. Single chain epidermal growth factor receptor antibody conjugated nanoparticles for in vivo tumor targeting and imaging. Small. 2009;5:235-243. [PubMed] [DOI] |
| 53. | Xie J, Chen K, Lee HY, Xu C, Hsu AR, Peng S, Chen X, Sun S. Ultrasmall c(RGDyK)-coated Fe3O4 nanoparticles and their specific targeting to integrin alpha(v)beta3-rich tumor cells. J Am Chem Soc. 2008;130:7542-7543. [PubMed] [DOI] |
| 54. | Lee HY, Li Z, Chen K, Hsu AR, Xu C, Xie J, Sun S, Chen X. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)-conjugated radiolabeled iron oxide nanoparticles. J Nucl Med. 2008;49:1371-1379. [PubMed] [DOI] |
| 55. | Shi XY, Wang SH, Swanson SD, Ge S, Cao ZY, Van Antwerp ME, Landmark KJ, Baker Jr JR. Dendrimer-functionalized shell-crosslinked iron oxide nanoparticles for in-vivo magnetic resonance imaging of tumors. Adv Mater. 2008;20:1671. [DOI] |
| 56. | Wang X, Xing X, Zhang B, Liu F, Cheng Y, Shi D. Surface engineered antifouling optomagnetic SPIONs for bimodal targeted imaging of pancreatic cancer cells. Int J Nanomedicine. 2014;9:1601-1615. [PubMed] [DOI] |
| 59. | Tateishi U, Yamaguchi U, Seki K, Terauchi T, Arai Y, Hasegawa T. Glut-1 expression and enhanced glucose metabolism are associated with tumour grade in bone and soft tissue sarcomas: a prospective evaluation by [18F]fluorodeoxyglucose positron emission tomography. Eur J Nucl Med Mol Imaging. 2006;33:683-691. [PubMed] [DOI] |
| 60. | Higashi T, Saga T, Nakamoto Y, Ishimori T, Konishi J. Relationship Between Retention Index in Dual-Phase 18F-FDG PET, and Hexokinase-II and Glucose Transporter-1 Expression in Pancreatic Cancer. J Nucl Med. 2002;43:173-180. |
| 61. | Yen RF, Hong RL, Tzen KY, Pan MH, Chen HH. Whole-Body 18F-FDG PET in Recurrent or Metastatic Nasopharyngeal Carcinoma. J Nucl Med. 2005;46:770-774. |
| 62. | 李 迎辞, 于 丽娟, 田 墨涵, 王 大龙, 王 文志. Glut1与Ki-67在结直肠癌中的表达及其与18F-FDG PET/CT SUVmax的相关性. 中国医学影像技术. 2011;27:1855-1858. |
| 65. | Zhang J, Hao G, Yao C, Yu J, Wang J, Yang W, Hu C, Zhang B. Albumin-Mediated Biomineralization of Paramagnetic NIR Ag2S QDs for Tiny Tumor Bimodal Targeted Imaging in Vivo. ACS Appl Mater Interfaces. 2016;8:16612-16621. [PubMed] [DOI] |
| 66. | Qiao R, Yang C, Gao M. Superparamagnetic iron oxide nanoparticles: from preparations to in vivo MRI applications. J Mater Chem. 2009;19:6274. [DOI] |
| 67. | Lin X, Chen T. A Review of in vivo Toxicity of Quantum Dots in Animal Models. Int J Nanomedicine. 2023;18:8143-8168. [PubMed] [DOI] |
| 68. | Huong LTT, Hung NP, Ha NT, Luyen NT, Hien NT, Ca NX, Thuy NTM. Chemically synthesized CdSe quantum dots induce apoptosis in AGS gastric cancer cells via ROS generation. Nanoscale Adv. 2025;7:572-582. [PubMed] [DOI] |
| 69. | Romeo V, Clauser P, Rasul S, Kapetas P, Gibbs P, Baltzer PAT, Hacker M, Woitek R, Helbich TH, Pinker K. AI-enhanced simultaneous multiparametric (18)F-FDG PET/MRI for accurate breast cancer diagnosis. Eur J Nucl Med Mol Imaging. 2022;49:596-608. [PubMed] [DOI] |
| 70. | Boland PA, Hardy NP, Moynihan A, McEntee PD, Loo C, Fenlon H, Cahill RA. Intraoperative near infrared functional imaging of rectal cancer using artificial intelligence methods - now and near future state of the art. Eur J Nucl Med Mol Imaging. 2024;51:3135-3148. [PubMed] [DOI] |