修回日期: 2023-10-26
接受日期: 2023-11-01
在线出版日期: 2023-11-08
炎症性肠病(inflammatory bowel disease, IBD)包括溃疡性结肠炎和克罗恩病, 其发病机制与肠道菌群紊乱密切相关. 肠道菌群紊乱导致的肠道微环境异常影响氨基酸的代谢. 色氨酸作为人体必需氨基酸, 其代谢产物参与调节免疫、神经元功能和肠道稳态等. IBD疾病的发生与发展伴随色氨酸的缺乏或代谢异常. 本综述主要介绍了肠道菌群代谢物色氨酸及其代谢产物与IBD疾病发生与发展之间的关系, 并为未来预测IBD疾病活动的诊断方法和治疗IBD的方案提供了新思路.
核心提要: 色氨酸(L-Tryptophan, Trp)是人体必须氨基酸, 其代谢产物参与调节免疫、神经元功能和肠道稳态. 本文详细介绍了Trp的三条代谢通路, 以及Trp和其代谢产物与炎症性肠病(inflammatory bowel disease, IBD)发生与发展之间的关系, 为未来IBD的诊断与治疗提供新思路、新靶点.
引文著录: 李梦琳, 孙少鹏, 孙可, 吕宾, 范一宏. 色氨酸代谢在炎症性肠病中的研究进展. 世界华人消化杂志 2023; 31(21): 896-903
Revised: October 26, 2023
Accepted: November 1, 2023
Published online: November 8, 2023
Inflammatory bowel disease (IBD) is comprised of ulcerative colitis and Crohn's disease, the pathogenesis of which is closely related to intestinal flora disorders. Abnormalities in the intestinal microenvironment caused by intestinal flora disorders affect amino acid metabolism. Tryptophan is an essential amino acid, and its metabolites are involved in the regulation of immunity, neuronal function, intestinal homeostasis, etc. The development of IBD disease is accompanied by tryptophan deficiency or metabolic abnormalities. This review focuses on the relationship between the intestinal flora metabolite tryptophan and its metabolites and the occurrence and development of IBD disease, and provides new ideas for future diagnostic methods for predicting IBD disease activity and protocols for treating IBD.
- Citation: Li ML, Sun SP, Sun K, Lv B, Fan YH. Role of tryptophan metabolism in inflammatory bowel disease. Shijie Huaren Xiaohua Zazhi 2023; 31(21): 896-903
- URL: https://www.wjgnet.com/1009-3079/full/v31/i21/896.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v31.i21.896
炎症性肠病(inflammatory bowel disease, IBD)是一组以腹痛、腹泻、便血为主要特征的肠道疾病, 包括溃疡性结肠炎(ulcerative colitis, UC)和克罗恩病(Crohn's disease, CD). 全世界大约有500万人受到IBD的困扰, 且目前尚无完全治愈IBD的方法[1]. 目前认为, IBD的发病机制是遗传易感性、环境影响和微生物组相互作用的结果, 通过削弱肠道屏障导致不适当的肠道免疫激活[2]. 在此过程中, 肠道微生物及其代谢产物的改变对IBD发生与发展起到了重要作用[3].
色氨酸(L-Tryptophan, Trp)是一种人体必需氨基酸, 占总氨基酸的1%, 仅从人类的饮食摄入中获得. 常见的Trp天然食物来源包括燕麦、香蕉、干梅干、牛奶、金枪鱼、奶酪、面包、家禽、花生、巧克力等[4]. 世界卫生组织将Trp的推荐摄入量定为4 mg/kg/d. 迄今为止, 没有报道过膳食中过量Trp的不利影响.
Trp在内源酶或微生物代谢作用下生成许多Trp代谢产物, 其代谢产物在不同的生理过程中发挥关键作用, 包括参与调节免疫、神经元功能和肠道稳态等[5]. 而Trp的缺乏或代谢过程受阻直接导致其代谢产物减少, 从而引起肠道菌群失衡, 甚至诱发或加重IBD[6-8].
Trp参与动物体内血浆蛋白质的更新, 并可促使核黄素发挥作用. 其次, Trp代谢物在从细胞生长和维持到协调有机体对环境和饮食产生反馈的过程中充当了神经递质和信号分子[9]. 这些代谢物在维持肠道稳态和系统免疫中起着至关重要的作用, 也潜在地影响着炎症性肠病、肿瘤、肥胖和代谢综合征、神经系统疾病、感染性疾病、血管炎症和心血管疾病以及肝纤维化等疾病的发生和发展. 它们不仅能促进抗炎巨噬细胞、调节性T细胞、CD4+ CD8αα+调节性细胞、白介素10(interleukin 10, IL-10)和/或白介素35(interleukin 35, IL-35)表达阳性的调节性B细胞的分化和功能, 还能促进产生IL-22的固有淋巴细胞3, 参与维持肠道黏膜稳态[8]. 总之, 这些功能表明了Trp代谢在进化过程中承担了细胞和有机体交流的部分功能, 这些功能使食物供应与人体的生理和行为保持一致.
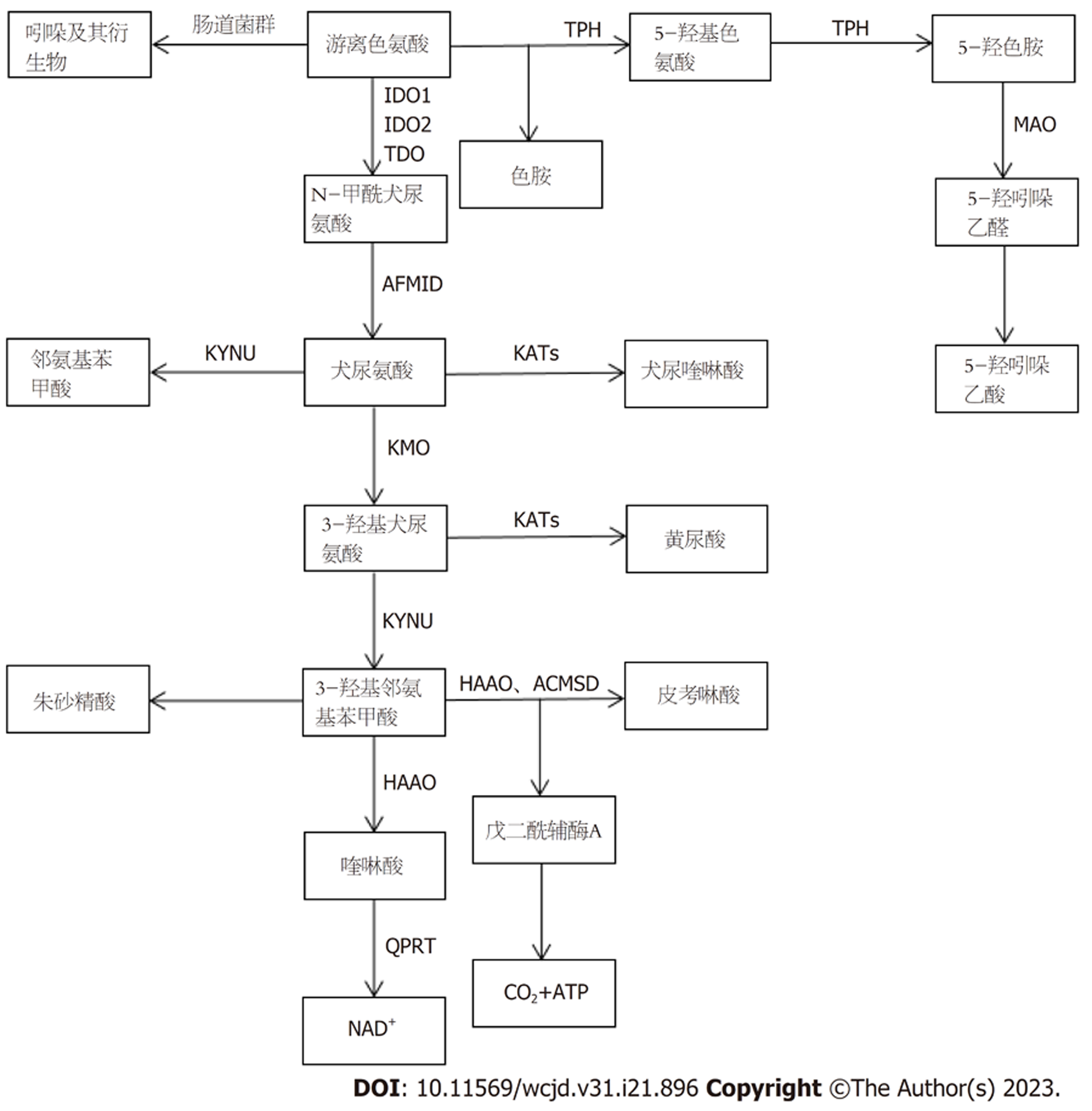
体内游离Trp的水平由食物摄入量和三种Trp代谢途径的活动决定. Trp的代谢途径包括以下三种[4](图1): (1)经肠道菌群的直接代谢途径; (2)通过限速酶吲哚胺2,3-双加氧酶1(indoleamine-2,3-dioxygenase 1, IDO1)在免疫细胞和上皮细胞中的犬尿氨酸(kynurenine, Kyn)途径(kynurenine pathway, KP)代谢; (3)肠嗜铬细胞中通过Trp羟化酶1(TpH1)产生血清素的途径, 即5-羟色胺(5-hydroxytryptamine, 5-HT)途径.
肠道微生物可以将Trp直接转化为吲哚及其衍生物等多种分子. 这些吲哚衍生物都是芳烃受体(aryl hydrocarbon receptor, AhR)的配体[10,11], 如吲哚-3-醛(indole-3-aldehyde, IAld)、吲哚-3-乙酸(indole-3-acid-acetic, IAA)、吲哚-3-丙酸(indole-3-propionic acid, IPA)、吲哚-3-乙醛(indole-3-acetaldehyde, IAAld)、吲哚丙烯酸等. AhR信号被认为是适应性免疫和肠屏障功能的关键组成部分, 对肠道稳态至关重要. AhR直接被食物激活. 它能促进局部IL的产生, 促进上皮细胞的更新和肠道黏膜屏障的完整性, 并且作用于许多类型的免疫细胞, 如上皮内淋巴细胞、辅助性T细胞17、固有淋巴细胞、巨噬细胞、树突状细胞和中性粒细胞[12]. 此外, 许多AhR配体被细胞色素p450家族蛋白加工并失活. 例如Cyp1A1是AhR的直接转录靶标, 构成AhR信号[13]的反馈环路.
微生物代谢在肠道AhR活性中的作用占主导地位. 有研究证明[14], 在无菌或者患病小鼠的肠道中缺乏AhR激动剂. 但是目前被证实能产生AhR配体的肠道共生菌仍是少数, 如鲁塞尔普氏链球菌[15]和乳酸杆菌[14]. 可能还有许多能够产生AhR配体的肠道共生菌有待发现. 在人类肠道微生物群中已经发现了色氨酸代谢途径. Williams的研究证明[16], 生孢梭菌可以使Trp脱羧, 生成神经递质色胺, 并且参与氧化还原反应导致IAA和IPA的产生. IAA和IPA这两种Trp代谢产物已知会影响肠道通透性和宿主免疫[12,17]. 在大肠杆菌中已经鉴定出色氨酸和吲哚活性转运蛋白. 将色氨酸转化为吲哚的色氨酸酶在大肠杆菌和乳酸杆菌中都有表达[18], 但参与吲哚进一步加工的精确微生物酶学途径, 以及它们在其他共生物种中的存在和活性还有待进一步研究描述. 吲哚也是一种种间信号分子, 能够控制细菌生理的各个方面, 如抗生素耐药性、孢子和生物膜的形成. 在不生产吲哚的细菌中, 吲哚及其衍生物显著抑制群体感应并调节毒力因子[19]. 然而, 这些复杂现象在肠道生态系统中的重要性尚未得到明确阐述.
超过95%的游离Trp是KP的底物, 产生多种代谢产物[5]. 其中, 肠道中KP的Trp代谢由IDO1介导, 并导致Kyn和下游产物的产生, 例如喹啉酸(quinolinic acid, QA)、烟酸、烟酰胺腺嘌呤二核苷酸和犬尿酸(kynurenic acid, Kna)[9,20]. 另外还有两种酶将Trp代谢为Kyn, 分别是色氨酸2, 3-双加氧酶(trp 2,3-dioxygenase, TDO)和吲哚胺2,3-双加氧酶2(indoleamine-2,3-dioxy-genase 2, IDO2). KP终产物参与调节多种宿主生物学过程, 涉及神经传递, 炎症和免疫反应. 此外, 一些代谢物似乎在肠道中发挥特定作用. Kna就是这种情况, 其浓度沿着胃肠道逐渐增加, 并表现出黏膜保护和免疫调节作用, 这可能是通过G蛋白偶联受体(G protein-coupled receptor, GPR35)来实现的[21]. GPR35主要在上皮和免疫细胞中表达.
肠道微生物群在刺激IDO1活性方面的关键作用已经得到了明确证明, 特别是在无菌和抗生素治疗的小鼠中[4]. 例如, 约翰逊乳杆菌在大鼠中的定植也降低了回肠IDO mRNA水平和血清犬尿氨酸浓度, 这与约翰逊乳杆菌培养无细胞上清液降低HT-29肠上皮细胞中IDO1活性的作用一致(减少47%)[22,23]. 此外, 有几种肠道细菌编码与真核生物KP同源的酶, 因此也能产生Kyn和下游代谢产物[4].
色氨酸羟化酶(tryptophan hydroxylase , TPH)是血清素生物合成的第一步限速酶, 将Trp转化为5-羟基色胺酸, 又将5-羟基色氨酸转化为5-HT[24,25]. 单胺氧化酶(monoamine oxidase , MAO)可将5-羟色胺转化为5-羟吲哚乙醛, 然后转化为5-羟吲哚乙酸[26], 最终通过尿液排出体外. 芳香族-L-氨基酸脱羧酶可以将色氨酸直接转化为色胺. 褪黑素也可以在血清素的N-乙酰化和O-甲基化后产生[27].
95%的5-HT存在于外周组织中, 而肠嗜铬细胞是肠道中血清素生物合成的主要场所[25]. 它是肠道内分泌细胞中最具特色的细胞, 分散在整个肠道黏膜中. 在生理条件下, 外周5-HT不能通过血脑屏障. 外周5-HT在胃肠道中触发多种功能, 通过激活特异性5-HT受体[28]参与广泛的人体生理功能. 具体来说, 5-HT是一种重要的胃肠道信号分子, 它将信号从肠道传递给内在或外在的神经元, 影响肠道蠕动和运动、分泌、血管舒张以及营养物质的吸收. 此外, 表达于肠上皮细胞顶膜和基底侧膜的5-HT选择性再摄取转运体(SERT; 由SLC6A4基因编码)从间质中清除5-HT.
肠道菌群是肠道5-HT生成的主要参与者. 研究证明[25], 在无菌小鼠中表现出结肠中5-HT生成受损和血液中5-HT浓度低. 肠道菌群调节5-HT生成的机制尚不完全清楚, 但短链脂肪酸在刺激TPH1表达中的作用已被证实. 此外, 一些次级胆汁酸, 如微生物生物转化胆酸盐产生的脱氧胆酸盐, 也可以刺激5-HT生物合成[25].
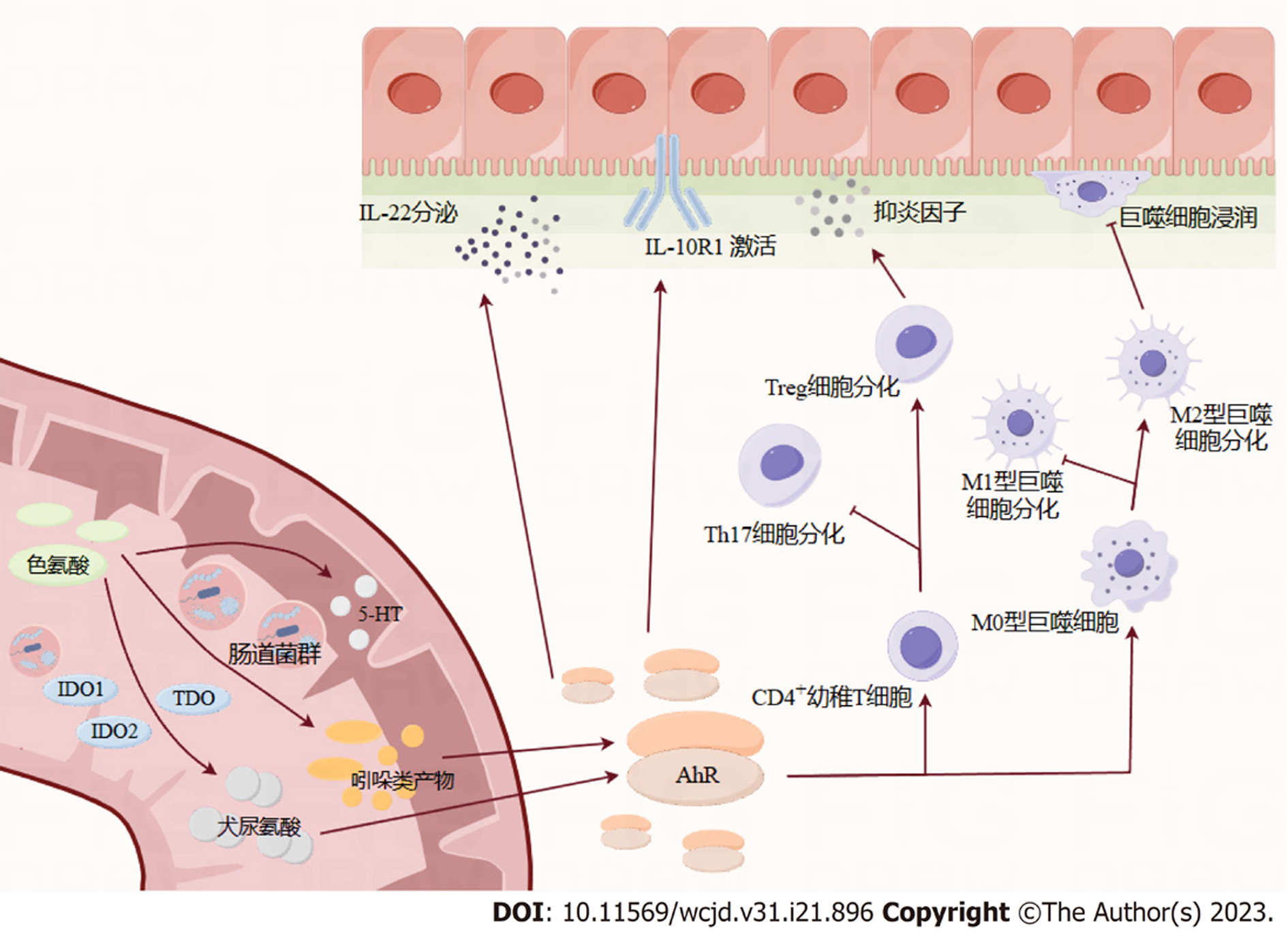
肠道菌群在各种生理功能中起着不可或缺的作用, 如能量代谢[29], 营养物质的消化吸收[30], 宿主免疫稳态[31]. 肠道微生物群的不平衡已被证明与IBD的发展有关, 药物对肠道微生物群的调节和有益细菌的作用可以有效缓解肠道炎症[32]. 除了肠道微生物群组成外, 肠道微生物群代谢物, 包括Trp、胆汁酸和短链脂肪酸, 在维持肠道屏障的完整性和免疫稳态方面也起着重要作用[33]. 表明微生物群的作用至少部分由Trp代谢介导. 一项纳入了535例IBD患者的研究结果显示[34]活动期IBD患者的色氨酸分解代谢增加, 色氨酸代谢产物(尤其是喹啉酸)水平升高, 导致IBD患者的血清色氨酸水平明显低于健康者, 并且CD患者的血清色氨酸水平下降幅度较UC患者更大, IBD患者的血清色氨酸水平与疾病活动度、C反应蛋白及IL-22水平呈负相关. 一种解释可能是与UC相比, CD患者的营养状况更为降低[35]. 因此, 我们将IBD与色氨酸代谢联系起来(图2).
肠道微生物群的改变在IBD中的作用已经得到了明确的证明, 一些重要的文献强调了肠道Trp代谢的改变与肠道微生物的潜在联系. 研究发现[14], 受遗传因素的影响, IBD患者肠道菌群产生的AhR配体与健康受试者相比减少, 肠道组织中AhR表达降低. 还有研究证明, UC患者血清中色氨酸代谢物IPA降低[10].
CARD9是其多态性或突变导致IBD易感性的基因, 通过吲哚类代谢物促进IL-22的产生, 改善结肠炎[14]. 吲哚类代谢物是AHR的强效激动剂. 它们介导了肠道微生物群-上皮细胞互相作用的主要部分. AHR是一种转录因子, 其刺激导致IL-22的产生, 最终有助于黏膜愈合、Foxp3+调节性T细胞的发育和炎症[36]的减弱. 有趣的是, 一些与IBD相关的遗传易感因素, 如白介素23受体(interleukin 23 receptor, IL23R)的多态性与IL-22的功能失调有关[37]. 吲哚代谢物即使在其生理浓度下, 也能增强肠上皮细胞的粘液产生, 并改变炎症反应[38]. 无菌小鼠的结肠上皮屏障中连接蛋白表达减少, 而用吲哚胶囊处理后, 紧密连接和粘附连接相关蛋白增加, 上皮屏障得到保护[39]. 同样, 吲哚胶囊在体内也能对葡聚糖硫酸钠盐(dextran sulfate sodium salt, DSS)诱导的结肠炎产生保护作用[39]. IPA能以AhR依赖的方式增加培养的肠上皮细胞上白细胞介素10受体亚单位1(interleukin-10 receptor subunit 1, IL-10R1)的表达, 并在体内改善结肠炎[10]. 其中IL-10R1的突变和功能无效与幼年时期发病早和难治性IBD有关. AHR拮抗剂逆转了IPA对结肠炎的有益作用[40]. 这些发现表明, 即使在生理浓度下也能强烈激活AHR的吲哚代谢物在肠道的稳态和健康中起着关键作用.
KP的改变可能也参与了IBD的发病机制. IDO1-/-小鼠更易患结肠炎, 证明IDO1是肠道炎症的负调控因子[41]. 与IDO1缺失相关的病理损伤部分是由于促炎细胞因子的激活和结肠中CD4+ Foxp3+调节性T细胞数量的减少[41]. 但是其中涉及的精确机制和代谢物仍然未知. 炎症增加了免疫细胞尤其是树突状细胞中IDO1的表达. IDO1是免疫调节的关键成分, 可防止炎症反应中的过度反应[42]. 较高的IDO1表达诱导树突状细胞耐受, 并同样导致Foxp3+调节性T细胞的分化[43]. 此外, 较高的IDO1表达与M1/M2型巨噬细胞向M2亚型的偏移有关. 巨噬细胞的M2亚型以释放抗炎细胞因子如IL10来缓解炎症而闻名. Kyn是已知的AhR激动剂, 它刺激AhR, 从而促进幼稚T细胞向调节性T细胞而不是辅助性T细胞17分化[43]. 因此, 它可以增加抗炎细胞因子, 减少促炎细胞因子, 减轻炎症[44]. 但在肝癌细胞系中诱导AhR活性所需的浓度使其在生理条件下作为AhR激动剂的相关性受到质疑[18]. 下游代谢通路的改变可能导致抗炎代谢物如Kna的缺乏, 但这仍有待证明. Michaudel的研究证明[45], 回输犬尿喹啉酸可以改善DSS诱导结肠炎小鼠的结肠炎症. 在IBD的背景下, 来自失调微生物群的异常信号可能是KP失衡的驱动因素.
有研究证明[46], 活动期IBD患者的IDO1表达水平升高, 并且犬尿酸水平和犬尿酸/色氨酸比值升高, 这表明在活动期IBD中色氨酸经KP分解增加. Acovic研究发现[47], 血清和粪便犬尿氨酸水平在UC和黏膜愈合患者中升高. 还有研究发现[48], 虽然CD患者的犬尿氨酸水平在与健康者相比无显著差异, 但是犬尿氨酸/色氨酸比值存在差异, 并与CD的疾病活动指数、红细胞沉降率和C反应蛋白水平呈正相关. 有趣的是, 当Gupta等[48]进一步研究同一CD患者在两个不同时间点的犬尿氨酸和疾病活动期的水平, 犬尿氨酸水平的差异变得显著. 出现这种情况可能与该项研究纳入患者例数较少有关, 患者之间的营养状态以及饮食习惯的不同, 存在一定的偏移. 在一项前瞻性随机实验中, 经过饮食诱导缓解的轻中度CD儿童患者粪便中的犬尿氨酸水平也随之下降[49]. 这些发现表明, KP或许是肠道炎症的负调控途径, 未来可以成为治疗IBD的新靶点. 而测定IBD患者血清和粪便样品中的犬尿氨酸可作为预测或监测IBD患者疾病活动度和黏膜愈合的一种新的诊断方法, 但因为存在个体差异性, 未来仍需大数量的研究进一步验证犬尿氨酸的截断值.
IBD患者较普通人群有更高的抑郁患病率, 且这种更高的患病率在疾病活动期患者中尤为明显[50]. 双向肠-脑相互作用已被广泛接受来解释IBD和抑郁症相互作用的机制[51]. 研究证明[52,53], KP代谢产物如血清素、喹啉酸(QUIN)和犬尿喹啉酸(KYNA)的失调与动物模型和人类的抑郁行为有关.
5-HT途径的失调与肠易激综合征(irritable bowel syndrome, IBS)的发病机制密切相关[54]. Minderhoud等[55]报道结肠中较高的黏膜TPH含量与IBD患者出现IBS样症状有关. IBD患者的黏膜5-HT浓度是正常人群的7倍以上[56]. 活动期克罗恩病患者的5-HT水平显著高于缓解期或难治性患者. Manzella等[57]猜测可能是SERT-5-HT-AhR轴的破坏在IBD发病机制中具有额外的作用. 但遗憾的是, 进一步活动期CD与难治性CD之间5-HT差异的机制未有文献阐明. 但5-HT可以起到区分活动期CD与难治性CD的标志物作用, 为难治性CD尽早确诊以及加强用药提供依据. 此外, 血清5-HT在区分克罗恩病严重程度方面优于C反应蛋白(C-reactive protein, CRP)和循环细胞因子, 如肿瘤坏死因子α(tumor necrosis factor α, TNF-α), IL-1β, IL-6, IL-7, IL-17A和IL-22[57].
体外研究发现, 炎症介质如IL-1β和脂多糖(lipopolysaccharides, LPS)可增加克罗恩病患者肠嗜铬细胞分泌5-HT[56]. 在DSS和2,4,6-三硝基苯磺酸(2,4,6Trinitrobenzenesulfonic, TNBS)诱导的结肠炎中, TPH+/+小鼠比TPH-/-小鼠表现出更严重的结肠炎[58]. 一项动物实验证明[59], 微生物从Tph1-/-转移到Tph1+/-小鼠可以降低结肠炎的炎症程度. Tph1-/-小鼠的肠道微生物组成也可以缓解接受DSS治疗的无菌小鼠的结肠炎. 5-HT可减少结肠上皮细胞中防御β素的产生, 并改变肠道微生物组成[59].
似乎黏膜5-HT在炎症过程中增加, 但5-HT受体的每个亚型都有自己的作用. 因此, 对这些受体中的每一个进行选择性的药理学干预可能有助于改善结肠炎的管理. 我们对5-HT受体各亚型的认识有限, 仍存在诸多争议. 未来的研究需要解决这一谜团.
此外, 5-HT被证明与肠道微生物群和肠-脑轴相关[53,60]. 它是Trp最重要的代谢产物之一, 参与中枢神经系统对情绪、行为和认知功能的调节. 选择性5-HT再摄取抑制剂已被临床作为抗抑郁药使用. 而心理治疗不但可以减轻IBD患者焦虑、抑郁等心理症状, 同时对肠道等躯体症状兼具改善效果, 还可以降低IBD活动度, 预防不必要的治疗升级, 是对IBD传统治疗的重要补充.
色氨酸及其代谢产物的缺乏与IBD的疾病发生与发展存在密切关联, 甚至和IBD患者产生抑郁状态有关. 而肠道菌群的失调似乎是引起肠道色氨酸代谢失衡的重要原因. 目前研究证明, 色氨酸及其代谢产物似乎可以作为预测或监测IBD患者疾病活动度和黏膜愈合的一种新的诊断方法, 成为的新的非侵入性的生物标志物应用于临床, 为IBD患者减少不必要的侵入性检查. 从治疗的角度来看, 肠道中的Trp代谢是可以进行操作的, 使用特定途径的分子或利用操纵Trp代谢的微生物作为益生菌. 然而, 微生物-宿主相互作用的复杂性以及所研究疾病和模型的复杂性, 需要进一步的研究来细化靶点和治疗干预. 由于目前的研究数据大多数是来自细胞及动物的基础研究, 未来仍需更多的多中心大样本临床随机试验数据, 色氨酸代谢有望成为IBD治疗的新靶点.
学科分类: 胃肠病学和肝病学
手稿来源地: 浙江省
同行评议报告学术质量分类
A级 (优秀): 0
B级 (非常好): B
C级 (良好): C, C
D级 (一般): D
E级 (差): 0
科学编辑: 张砚梁 制作编辑:张砚梁
| 1. | Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720-727. [PubMed] [DOI] |
| 2. | Ramos GP, Papadakis KA. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin Proc. 2019;94:155-165. [PubMed] [DOI] |
| 3. | Weingarden AR, Vaughn BP. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes. 2017;8:238-252. [PubMed] [DOI] |
| 4. | Agus A, Planchais J, Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23:716-724. [PubMed] [DOI] |
| 5. | Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18:379-401. [PubMed] [DOI] |
| 6. | Li X, Zhang ZH, Zabed HM, Yun J, Zhang G, Qi X. An Insight into the Roles of Dietary Tryptophan and Its Metabolites in Intestinal Inflammation and Inflammatory Bowel Disease. Mol Nutr Food Res. 2021;65:e2000461. [PubMed] [DOI] |
| 7. | Hu Y, Chen Z, Xu C, Kan S, Chen D. Disturbances of the Gut Microbiota and Microbiota-Derived Metabolites in Inflammatory Bowel Disease. Nutrients. 2022;14. [PubMed] [DOI] |
| 8. | Su X, Gao Y, Yang R. Gut Microbiota-Derived Tryptophan Metabolites Maintain Gut and Systemic Homeostasis. Cells. 2022;11. [PubMed] [DOI] |
| 9. | Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: Tryptophan's metabolites in exercise, inflammation, and mental health. Science. 2017;357. [PubMed] [DOI] |
| 10. | Alexeev EE, Lanis JM, Kao DJ, Campbell EL, Kelly CJ, Battista KD, Gerich ME, Jenkins BR, Walk ST, Kominsky DJ, Colgan SP. Microbiota-Derived Indole Metabolites Promote Human and Murine Intestinal Homeostasis through Regulation of Interleukin-10 Receptor. Am J Pathol. 2018;188:1183-1194. [PubMed] [DOI] |
| 11. | Alkhalaf LM, Ryan KS. Biosynthetic manipulation of tryptophan in bacteria: pathways and mechanisms. Chem Biol. 2015;22:317-328. [PubMed] [DOI] |
| 12. | Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, Sonnenburg JL. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551:648-652. [PubMed] [DOI] |
| 13. | Schiering C, Wincent E, Metidji A, Iseppon A, Li Y, Potocnik AJ, Omenetti S, Henderson CJ, Wolf CR, Nebert DW, Stockinger B. Feedback control of AHR signalling regulates intestinal immunity. Nature. 2017;542:242-245. [PubMed] [DOI] |
| 14. | Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, Brot L, Taleb S, Couturier-Maillard A, Nion-Larmurier I, Merabtene F, Seksik P, Bourrier A, Cosnes J, Ryffel B, Beaugerie L, Launay JM, Langella P, Xavier RJ, Sokol H. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22:598-605. [PubMed] [DOI] |
| 15. | Wlodarska M, Luo C, Kolde R, d'Hennezel E, Annand JW, Heim CE, Krastel P, Schmitt EK, Omar AS, Creasey EA, Garner AL, Mohammadi S, O'Connell DJ, Abubucker S, Arthur TD, Franzosa EA, Huttenhower C, Murphy LO, Haiser HJ, Vlamakis H, Porter JA, Xavier RJ. Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe. 2017;22:25-37.e6. [PubMed] [DOI] |
| 16. | Williams BB, Van Benschoten AH, Cimermancic P, Donia MS, Zimmermann M, Taketani M, Ishihara A, Kashyap PC, Fraser JS, Fischbach MA. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16:495-503. [PubMed] [DOI] |
| 17. | Galligan JJ. Beneficial actions of microbiota-derived tryptophan metabolites. Neurogastroenterol Motil. 2018;30. [PubMed] [DOI] |
| 18. | Hubbard TD, Murray IA, Perdew GH. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab Dispos. 2015;43:1522-1535. [PubMed] [DOI] |
| 19. | Lee JH, Wood TK, Lee J. Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol. 2015;23:707-718. [PubMed] [DOI] |
| 20. | Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology. 2017;112:399-412. [PubMed] [DOI] |
| 21. | Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T, Yin Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front Cell Infect Microbiol. 2018;8:13. [PubMed] [DOI] |
| 22. | Valladares R, Bojilova L, Potts AH, Cameron E, Gardner C, Lorca G, Gonzalez CF. Lactobacillus johnsonii inhibits indoleamine 2,3-dioxygenase and alters tryptophan metabolite levels in BioBreeding rats. FASEB J. 2013;27:1711-1720. [PubMed] [DOI] |
| 23. | Freewan M, Rees MD, Plaza TS, Glaros E, Lim YJ, Wang XS, Yeung AW, Witting PK, Terentis AC, Thomas SR. Human indoleamine 2,3-dioxygenase is a catalyst of physiological heme peroxidase reactions: implications for the inhibition of dioxygenase activity by hydrogen peroxide. J Biol Chem. 2013;288:1548-1567. [PubMed] [DOI] |
| 24. | Wang SJ, Sharkey KA, McKay DM. Modulation of the immune response by helminths: a role for serotonin? Biosci Rep. 2018;38. [PubMed] [DOI] |
| 25. | Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264-276. [PubMed] [DOI] |
| 26. | Höglund E, Øverli Ø, Winberg S. Tryptophan Metabolic Pathways and Brain Serotonergic Activity: A Comparative Review. Front Endocrinol (Lausanne). 2019;10:158. [PubMed] [DOI] |
| 27. | Slominski A, Semak I, Pisarchik A, Sweatman T, Szczesniewski A, Wortsman J. Conversion of L-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett. 2002;511:102-106. [PubMed] [DOI] |
| 28. | Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473-486. [PubMed] [DOI] |
| 29. | Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546-1558. [PubMed] [DOI] |
| 30. | Ramakrishna BS. Role of the gut microbiota in human nutrition and metabolism. J Gastroenterol Hepatol. 2013;28 Suppl 4:9-17. [PubMed] [DOI] |
| 31. | Iacob S, Iacob DG, Luminos LM. Intestinal Microbiota as a Host Defense Mechanism to Infectious Threats. Front Microbiol. 2018;9:3328. [PubMed] [DOI] |
| 32. | Sang LX, Chang B, Zhang WL, Wu XM, Li XH, Jiang M. Remission induction and maintenance effect of probiotics on ulcerative colitis: a meta-analysis. World J Gastroenterol. 2010;16:1908-1915. [PubMed] [DOI] |
| 33. | Liu J, Tan Y, Cheng H, Zhang D, Feng W, Peng C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022;13:1106-1126. [PubMed] [DOI] |
| 34. | Nikolaus S, Schulte B, Al-Massad N, Thieme F, Schulte DM, Bethge J, Rehman A, Tran F, Aden K, Häsler R, Moll N, Schütze G, Schwarz MJ, Waetzig GH, Rosenstiel P, Krawczak M, Szymczak S, Schreiber S. Increased Tryptophan Metabolism Is Associated With Activity of Inflammatory Bowel Diseases. Gastroenterology. 2017;153:1504-1516.e2. [PubMed] [DOI] |
| 35. | Filippi J, Al-Jaouni R, Wiroth JB, Hébuterne X, Schneider SM. Nutritional deficiencies in patients with Crohn's disease in remission. Inflamm Bowel Dis. 2006;12:185-191. [PubMed] [DOI] |
| 36. | Gutiérrez-Vázquez C, Quintana FJ. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity. 2018;48:19-33. [PubMed] [DOI] |
| 37. | Mizoguchi A, Yano A, Himuro H, Ezaki Y, Sadanaga T, Mizoguchi E. Clinical importance of IL-22 cascade in IBD. J Gastroenterol. 2018;53:465-474. [PubMed] [DOI] |
| 38. | Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci USA. 2010;107:228-233. [PubMed] [DOI] |
| 39. | Shimada Y, Kinoshita M, Harada K, Mizutani M, Masahata K, Kayama H, Takeda K. Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLoS One. 2013;8:e80604. [PubMed] [DOI] |
| 40. | Aoki R, Aoki-Yoshida A, Suzuki C, Takayama Y. Indole-3-Pyruvic Acid, an Aryl Hydrocarbon Receptor Activator, Suppresses Experimental Colitis in Mice. J Immunol. 2018;201:3683-3693. [PubMed] [DOI] |
| 41. | Takamatsu M, Hirata A, Ohtaki H, Hoshi M, Hatano Y, Tomita H, Kuno T, Saito K, Hara A. IDO1 plays an immunosuppressive role in 2,4,6-trinitrobenzene sulfate-induced colitis in mice. J Immunol. 2013;191:3057-3064. [PubMed] [DOI] |
| 42. | Chung DJ, Rossi M, Romano E, Ghith J, Yuan J, Munn DH, Young JW. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009;114:555-563. [PubMed] [DOI] |
| 43. | Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396-5404. [PubMed] [DOI] |
| 44. | Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci USA. 2010;107:19961-19966. [PubMed] [DOI] |
| 45. | Michaudel C, Danne C, Agus A, Magniez A, Aucouturier A, Spatz M, Lefevre A, Kirchgesner J, Rolhion N, Wang Y, Lavelle A, Galbert C, Da Costa G, Poirier M, Lapière A, Planchais J, Nádvorník P, Illes P, Oeuvray C, Creusot L, Michel ML, Benech N, Bourrier A, Nion-Larmurier I, Landman C, Richard ML, Emond P, Seksik P, Beaugerie L, Arguello RR, Moulin D, Mani S, Dvorák Z, Bermúdez-Humarán LG, Langella P, Sokol H. Rewiring the altered tryptophan metabolism as a novel therapeutic strategy in inflammatory bowel diseases. Gut. 2023;72:1296-1307. [PubMed] [DOI] |
| 46. | Sofia MA, Ciorba MA, Meckel K, Lim CK, Guillemin GJ, Weber CR, Bissonnette M, Pekow JR. Tryptophan Metabolism through the Kynurenine Pathway is Associated with Endoscopic Inflammation in Ulcerative Colitis. Inflamm Bowel Dis. 2018;24:1471-1480. [PubMed] [DOI] |
| 47. | Acovic A, Simovic Markovic B, Gazdic M, Arsenijevic A, Jovicic N, Gajovic N, Jovanovic M, Zdravkovic N, Kanjevac T, Harrell CR, Fellabaum C, Dolicanin Z, Djonov V, Arsenijevic N, Lukic ML, Volarevic V. Indoleamine 2,3-dioxygenase-dependent expansion of T-regulatory cells maintains mucosal healing in ulcerative colitis. Therap Adv Gastroenterol. 2018;11:1756284818793558. [PubMed] [DOI] |
| 48. | Gupta NK, Thaker AI, Kanuri N, Riehl TE, Rowley CW, Stenson WF, Ciorba MA. Serum analysis of tryptophan catabolism pathway: correlation with Crohn's disease activity. Inflamm Bowel Dis. 2012;18:1214-1220. [PubMed] [DOI] |
| 49. | Ghiboub M, Penny S, Verburgt CM, Boneh RS, Wine E, Cohen A, Dunn KA, Pinto DM, Benninga MA, de Jonge WJ, Levine A, Van Limbergen JE. Metabolome Changes With Diet-Induced Remission in Pediatric Crohn's Disease. Gastroenterology. 2022;163:922-936.e15. [PubMed] [DOI] |
| 50. | Panara AJ, Yarur AJ, Rieders B, Proksell S, Deshpande AR, Abreu MT, Sussman DA. The incidence and risk factors for developing depression after being diagnosed with inflammatory bowel disease: a cohort study. Aliment Pharmacol Ther. 2014;39:802-810. [PubMed] [DOI] |
| 51. | Gracie DJ, Hamlin PJ, Ford AC. The influence of the brain-gut axis in inflammatory bowel disease and possible implications for treatment. Lancet Gastroenterol Hepatol. 2019;4:632-642. [PubMed] [DOI] |
| 52. | Chen LM, Bao CH, Wu Y, Liang SH, Wang D, Wu LY, Huang Y, Liu HR, Wu HG. Tryptophan-kynurenine metabolism: a link between the gut and brain for depression in inflammatory bowel disease. J Neuroinflammation. 2021;18:135. [PubMed] [DOI] |
| 53. | Li D, Yu S, Long Y, Shi A, Deng J, Ma Y, Wen J, Li X, Liu S, Zhang Y, Wan J, Li N, Ao R. Tryptophan metabolism: Mechanism-oriented therapy for neurological and psychiatric disorders. Front Immunol. 2022;13:985378. [PubMed] [DOI] |
| 54. | Binienda A, Storr M, Fichna J, Salaga M. Efficacy and Safety of Serotonin Receptor Ligands in the Treatment of Irritable Bowel Syndrome: A Review. Curr Drug Targets. 2018;19:1774-1781. [PubMed] [DOI] |
| 55. | Minderhoud IM, Oldenburg B, Schipper ME, ter Linde JJ, Samsom M. Serotonin synthesis and uptake in symptomatic patients with Crohn's disease in remission. Clin Gastroenterol Hepatol. 2007;5:714-720. [PubMed] [DOI] |
| 56. | Kidd M, Gustafsson BI, Drozdov I, Modlin IM. IL1beta- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn's disease. Neurogastroenterol Motil. 2009;21:439-450. [PubMed] [DOI] |
| 57. | Manzella CR, Jayawardena D, Pagani W, Li Y, Alrefai WA, Bauer J, Jung B, Weber CR, Gill RK. Serum Serotonin Differentiates Between Disease Activity States in Crohn's Patients. Inflamm Bowel Dis. 2020;26:1607-1618. [PubMed] [DOI] |
| 58. | Li N, Ghia JE, Wang H, McClemens J, Cote F, Suehiro Y, Mallet J, Khan WI. Serotonin activates dendritic cell function in the context of gut inflammation. Am J Pathol. 2011;178:662-671. [PubMed] [DOI] |
| 59. | Kwon YH, Wang H, Denou E, Ghia JE, Rossi L, Fontes ME, Bernier SP, Shajib MS, Banskota S, Collins SM, Surette MG, Khan WI. Modulation of Gut Microbiota Composition by Serotonin Signaling Influences Intestinal Immune Response and Susceptibility to Colitis. Cell Mol Gastroenterol Hepatol. 2019;7:709-728. [PubMed] [DOI] |
| 60. | O'Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32-48. [PubMed] [DOI] |