修回日期: 2023-09-16
接受日期: 2023-09-21
在线出版日期: 2023-10-08
非酒精性脂肪性肝病(non-alcoholic fatty liver disease, NAFLD)是慢性肝病的主要病因, 以肝脏代谢紊乱为主要改变, 体现为脂质异常蓄积、肝细胞氧化应激等, 其病因尚不明确. "肠肝轴"中法尼醇X受体(farnesoid X receptor, FXR)是一种主要的胆汁酸受体, 在"肠-肝轴"的基础上, 通过不同通路调节体内多种物质代谢和病理生理状态, 进而影响NAFLD的发生发展, 是一个具有潜力的治疗靶点. 本文主要对FXR通过"肠-肝轴"调控体内胆汁酸与糖脂代谢进而改善NAFLD的机制进行综述, 以为后续关于FXR靶点药物的研究提供思路.
核心提要: 非酒精性脂肪性肝病(non-alcoholic fatty liver disease, NAFLD)病变机制复杂, 涉及胆汁酸紊乱、胰岛素抵抗、脂质堆积、胆固醇代谢紊乱等多种因素, 而"肠肝轴"中法尼醇X受体则可通过多种机制调控这些物质的代谢稳态, 其效应可对NAFLD起到改善作用.
引文著录: 黄之, 周蓉蓉. FXR调控胆汁酸与糖脂代谢改善NAFLD的机制. 世界华人消化杂志 2023; 31(19): 797-807
Revised: September 16, 2023
Accepted: September 21, 2023
Published online: October 8, 2023
Nonalcoholic fatty liver disease (NAFLD) is the main cause of chronic liver disease, with liver metabolic disorders as major pathological changes, manifested as abnormal lipid accumulation, liver cell oxidative stress, etc., but its etiology is still unclear. The farnesol X receptor (FXR) is a major bile acid receptor in the "gut-liver axis", via which FXR regulates metabolism and affects the pathophysiological status of various substances through different pathways, thus contributing to the occurrence and development of NAFLD. Therefore, FXR has become a potential therapeutic target for NAFLD. This article reviews the relationship between FXR regulation of bile acid, glucose, and lipid metabolism through the "gut-liver axis" and the occurrence and development of NAFLD, to provide new insights and clues for further research about FXR-based pharmaceutical treatments.
- Citation: Huang Z, Zhou RR. Mechanism for FXR to regulate bile acid and glycolipid metabolism to improve NAFLD. Shijie Huaren Xiaohua Zazhi 2023; 31(19): 797-807
- URL: https://www.wjgnet.com/1009-3079/full/v31/i19/797.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v31.i19.797
非酒精性脂肪性肝病(non-alcoholic fatty liver disease, NAFLD)区分于酒精导致的肝病谱, 是一组以细胞代谢紊乱、脂质在肝脏内过多沉积(>5%)为主要病理特征的慢性疾病, 根据肝脏病变程度及临床症状, NAFLD疾病谱包括非酒精性单纯性脂肪肝(non-alcoholic fatty liver, NAFL)、非酒精性脂肪性肝炎(non-alcoholic steatohepatitis, NASH)、肝硬化, 最终发展为肝细胞癌(hepatocellular carcinoma, HCC)[1]. 据估计, 在2018年, 世界上约有25%左右的人群患有NAFLD; 亚洲地区患病率略高于全球均值, 为27.37%[2]. 我国目前尚无全国性的NAFLD流行病学研究, 但根据各地区的大样本调查, 我国NAFLD的发病率正逐年上升, 较富裕地区如北京、上海等地的发病率甚至达到40%左右[3]. 近年研究揭示, NAFLD与血脂异常、中心性肥胖、代谢综合征(metabolic syndrome, MS)、胰岛素抵抗、2型糖尿病、动脉粥样硬化性心血管疾病(atherosclerotic cardiovascular disease, ASCVD)、遗传易感性、肠道菌群、免疫介导等诸多代谢相关的病理生理学变化密切相关[4], 因此, 2020年国际专家小组建议将NAFLD更名为代谢相关性脂肪性肝病(metabolic associated fatty liver disease, MAFLD), 并更新了诊断标准, 对NAFLD的后续研究有重要意义[5]. 然而, 相关研究仍有很多谜团待解开, 人们尚未明确其具体起病机制, 亦未完全理解其病程如何进展, 欧洲药品管理局和美国食品药品监督管理局也尚未批准实有疗效的药物.
早期Day和James提出的"二次打击"学说[6]是阐述NAFLD发病机制的主流理论, 其将胰岛素抵抗为使动因素导致的肝内脂肪堆积视作NAFLD发病的"第一次打击", 进而触发的一系列炎症反应、游离自由基的释放、氧化应激等即为"第二次打击"[7]. 随着研究深入, 研究者们愈发重视遗传易感性、表观遗传学、个人生活方式、肠道菌群、脂肪组织激素分泌等因素在NAFLD发生发展中的作用, 进而, "多重打击"学说[8]逐渐形成, 取代了"二次打击"理论的地位[9]; 其亦揭示了NAFLD发病机制中的多个关键靶点, 为疾病的诊断和靶向治疗的进一步研究提供了更全面的指导与参考[10].
脂肪代谢障碍是NAFLD最直观的病变之一, 而调控肝脏脂肪累积的受体主要有二: 法尼醇X受体(farnesoid X receptor, FXR)和肝X受体(liver X receptor, LXR)[11]. 近年来, FXR作为多种代谢疾病的药物靶点成为了热门研究方向, 大批具有潜在临床应用前景的FXR靶向药物相继出现, 有些已进入临床试验阶段[12]. FXR可介导多种信号通路, 在NAFLD的发生发展中起到关键作用, 本文就对其调控的胆汁酸和糖、脂质代谢进行综述, 以阐明FXR如何作用于这些代谢通路并进一步对NAFLD起到改善作用.
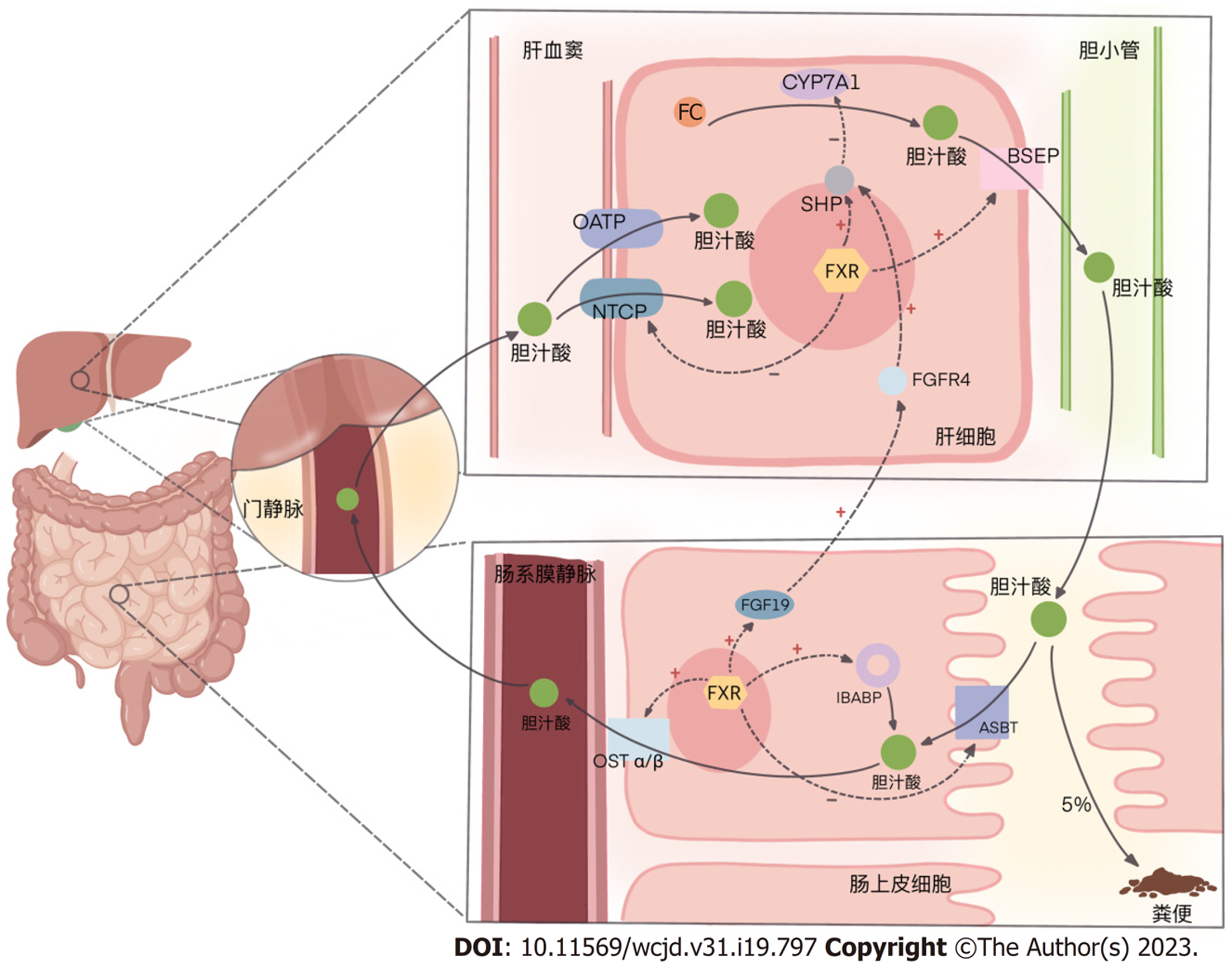
在肝脏与肠道通过肠-肝轴的上下沟通中, 胆汁酸(bile acids, BAs)是发挥主要作用的角色. 胆固醇在肝脏通过关键酶7α-羟化酶(cholesterol 7-alpha-hydroxylase, CYP7A1)[或在替代途径通过27α-羟化酶(cholesterol 27-alpha-hydroxylase, CYP27A1)]生成初级胆汁酸, 形成胆汁通过胆道泌入肠道, 介导脂质的消化吸收; 同时, 各种初级胆汁酸又在肠道菌群的催化下生成次级胆汁酸. 肠道中这些初级和次级胆汁酸约有5%通过粪便排出体外, 其余的则被重吸收.
胆汁酸在肠道由肠上皮细胞游离面的顶端膜钠依赖性胆汁酸转运蛋白(apical sodium-dependent bile acid transporter, ASBT)吸收入肠上皮细胞, 在回肠胆汁酸结合蛋白(ileal bile acid binding protein, IBABP)的帮助下转运, 再由基底面的异二聚体有机溶质转运蛋白α/β(organic solute transporters α/β, OST α/β)将胞内胆汁酸送入肠系膜静脉, 从而经门静脉系统重回肝脏.
回到肝血窦的胆汁酸, 经由肝细胞基底膜上钠离子-牛磺胆酸共转运蛋白(Na+-Taurocholate Co-transporting Polypeptide, NTCP)或有机阴离子转运多肽(organic anion transporting polypeptides, OATP)进入肝细胞, 随肝脏新合成的初级胆汁酸一起泌入肠道, 进入下一轮肠肝循环.
肠道菌群的改变会影响胆汁酸代谢, 不同的次级胆汁酸, 以及各种初级和次级胆汁酸的不同组成比例, 会通过胆汁酸受体对机体、尤其是肝脏产生不同的生物学作用; 而肝脏排出的胆汁酸也可以反过来作用于肠道菌群[13]-如此, 以不断循环的胆汁酸为媒介, 将肠道菌群和肝脏紧密关联, 形成了肠-肝轴中上下沟通的关系[14].
在肠肝循环过程中, 胆汁酸除了发挥消化吸收作用外, 还体现了信号转导作用. FXR是核受体超家族成员(nuclear receptor subfamily 1, group H, member 4, NR1H4)[15], 主要分布在肝脏和肠道, 在肾脏、肾上腺和卵巢等组织中也有少量表达. 胆汁酸是FXR的天然配体[16], 在胆汁酸和糖、脂质等物质的代谢过程中发挥了重要作用. FXR与配体结合后, 可作为单体或继续与类视黄醇X受体(retinoid X receptor, RXR)形成异二聚体形式与下游靶基因的FXR反应元件(FXR response element, FXRE)结合, 进而调控各靶基因的表达[15].
胆汁酸在肠道与肝脏之间循环, 与表达在肠道和肝脏的FXR结合后, 在肝脏和肠道激活多种的通路, 进而对诸多生理生化环节发挥调控作用.
FXR最典型的作用就是其在肝脏和肠道的多个通路减少胆汁酸合成、促进胆汁酸排泄. 加速胆汁酸的肠肝循环, 对胆汁酸代谢实现负反馈调节. 主要涉及如下过程.
肝脏中胆汁酸增多时, 肝FXR激活, 启动下游小分子异源二聚体(small heterodimer partner, SHP), 其一, 抑制CYP7A1(替代途径CYP27A1), 降低肝内胆固醇向初级胆汁酸的转化[17]; 其二, 激活肝细胞中的胆盐输出泵[(bile salt export pump, BSEP), 或称ATP结合盒转运蛋白B11(ATP-binding cassette transporter B11, ABCB11)]并上调其表达, 加快肝细胞内的胆汁酸向胆小管排出[18]; 其三, 抑制肝细胞基底膜上NTCP的功能, 减少其从门静脉摄取胆汁酸[18]. 此外, 2019年一项研究显示, 肝脏胆汁酸增多激活FXR抑制长链脂酰辅酶A合成酶1(long-chain acyl-CoA synthetase, ACSL1)的表达(见后文2.3.1), 还可通过一些更为复杂的机制减少内源性胆固醇的合成, 从原料上抑制胆汁酸的生成, 而以往的研究则未涉及ACSL参与胆汁酸代谢的证据[19].
肠道中胆汁酸增多时, 肠FXR激活, 其一, 诱导成纤维细胞生长因子19[(fibroblast growth factor 19, FGF19); 在啮齿动物为FGF15]的表达与分泌, 通过SHP-CYP7A1(CYP27A1)途径, 抑制胆汁酸合成[20]; 其二, 下调回肠上皮细胞ASBT的表达, 减少其对肠腔内胆汁酸的摄取; 其三, 促进IBABP的表达, 促进其在肠上皮细胞内协助ASBT参与胆汁酸的转运; 其四, 增强OST α/β的表达, 加快肠上皮细胞内胆汁酸向静脉系统排出[21], 以减弱因过量胆汁酸蓄积对肠上皮细胞产生的毒性作用[22]. 见图1.
除此之外, 还包括抑制肠肝循环中关键的肝脏受体类似物1(liver receptor homolog-1, LRH-1)[23]等多个环节复杂的作用, 最终达成对"肠-肝轴"中胆汁酸代谢的调节.
当前大量研究证实了NAFLD与胆汁酸代谢之间的关系, 但具体机制及二者的相互作用关系还有待进一步探究. 在高脂饮食喂养的NAFLD造模大鼠中, 以及NAFLD患者体内, 都能检测到总胆汁酸浓度升高, 以次级胆汁酸浓度升高尤为显著, 且初级胆汁酸和次级胆汁酸也不再维持原有平衡的比例关系[24]. 在NAFLD患者, 可拮抗FXR的次级胆汁酸-去氧胆酸(deoxycholic acid, DCA)含量增加, 而对FXR有激动作用的初级胆汁酸-鹅去氧胆酸(chenodeoxycholic acid, CDCA)含量相对下降[24]; 在NASH患者, 血清和尿液中以CDCA为主要的初级胆汁酸和以DCA、UDCA为主要的次级胆汁酸都检测出含量上升, 且与健康受试者相比, 高脂膳食引起的胆汁酸谱变化在NASH患者中更为显著[25], 这很可能是由于FXR等调控胆汁酸代谢的关键通路功能障碍, 进而引起了胆汁酸稳态失衡. 一项研究发现, 在NAFL和NASH患者, 次级胆汁酸含量的减少与肝纤维化的进展及分期存在反变关系[26], 再结合奥贝胆酸(obeticholic acid, OCA)作为FXR激动剂所具有的抗肝纤维化作用[27], 这也进一步印证了FXR在NAFLD疾病进展中的重要作用, 也进而使得通过激活FXR来干扰BA合成、促进BA代谢以治疗NAFLD成为可能.
生理浓度的胆汁酸可发挥消化及信号传导作用, 当体内胆汁酸浓度升高时, 则将表现出细胞毒性[28]. 高浓度胆汁酸将激活炎症反应和氧化应激介导的细胞死亡途径[29], 且在FXR敲除小鼠, 喂食胆汁酸后发生了显著的肝细胞凋亡及肝损伤[30]. 因此, 当FXR激活后, 其在肝脏和肠道内发挥降胆汁酸作用, 从而对抗了NAFLD病变带来的胆汁酸升高, 对MAFLD病变状态起到改善作用.
NAFLD的另一大特点是胰岛素抵抗, 即机体对胰岛素敏感性减弱, 体细胞糖代谢功能严重失调, 对糖的摄取减弱, 进而导致3-磷酸甘油的耗竭; 激素敏感性脂肪酶(hormone sensitive lipase, HSL)失去抑制, 为能量代谢的需要, 脂肪组织向血中释放大量脂肪酸, 这些脂肪酸在肝内逐渐堆积, 促进肝脏脂肪从头生成的进行, 并诱导脂肪因子和促炎因子的释放, 进而引发NAFLD[31].
近80%的NAFLD患者都存在胰岛素抵抗, 这表明在研究NAFLD的治疗方案时, 不仅追求减少肝脏中的脂肪, 更重要的是了解是否存在胰岛素抵抗, 从根本上解决脂肪蓄积. 已有文献表明FXR激动剂能改善NASH患者的胰岛素敏感性[32].
当前已有大量研究阐述FXR对糖代谢的作用. 研究发现[33], 糖尿病动物模型中肝脏FXR的表达降低, 而FXR缺陷的小鼠则表现为葡萄糖不耐受及胰岛素抵抗[34]. 胆汁酸活化FXR后, 其一方面通过SHP途径抑制肝脏糖异生[35], 另一方面通过FGF19途径促进肝脏糖原合成[36], 从而降低血糖、改善胰岛素敏感性. FXR基因敲除(FXR-/-)小鼠注射表达FXR的腺病毒后, 小鼠血糖明显下降; 2型糖尿病造模的db/db小鼠[瘦素受体(Lepr)基因纯合突变小鼠]在常规饮食喂养后, 血浆中葡萄糖、β-羟丁酸、甘油三酯、游离脂肪酸、胆固醇等均有明显升高, 但口服FXR激动剂GW4064后, 血浆中上述代谢物的含量则显著下降[34]. 这些发现证明了FXR在调控血糖水平中的作用.
碳水化合物反应元件结合蛋白(carbohydrate response element binding protein, ChREBP)调控的靶基因主要是肝脏糖酵解和脂质生成过程中各种酶类, 是促使葡萄糖酵解并向脂质转化的关键转录因子之一[37], 其表达具有促进肝脏脂肪合成的作用. ChREBP基因敲除小鼠在正常饮食时, 丙酮酸生成减少、糖酵解受到抑制, 肝糖原增多, 肝脏中脂质含量增加; 给予高糖饮食时, 糖酵解和糖异生显著下调, 肝脏中由葡萄糖转化的脂质成分明显减少; 而ob/ob小鼠敲除ChREBP基因后, 小鼠体质量明显下降, 同时胰岛素抵抗、脂肪肝及葡萄糖耐受不良等都得到明显改善[38]. 使用GW4064(一种FXR激动剂)激活FXR后, 在野生型小鼠或体外培养的肝细胞中可诱导磷酸烯醇式丙酮酸碳羧化激酶(phosphoenolpyruvate carboxykinase, PEPCK)mRNA的表达, 该酶在糖异生过程中起着重要作用; 但在db/db小鼠, 则在不对葡萄糖转运蛋白2(glucose transporters 2, Glut2)产生影响的情况下抑制了PEPCK的表达和功能, 导致肝细胞糖原合成增加, 进而降低血糖[34]. 这些研究从另一个角度说明了FXR通过ChREBP等通路对糖代谢的调控作用, 往往对胰岛素抵抗有改善作用, 因而对NAFLD的治疗也是有益的.
Fexaramine(FEX)是当前研究较多的肠道FXR激动剂, 与健康人体餐后胆汁酸释放选择性激活肠道FXR的效应类似. 研究发现FEX激活FXR后, 能够改善饮食引起的肥胖和炎症, 抑制肝脏葡萄糖生成[32]; 血清中胰高血糖素样肽1、FGF21水平均增加, 胰岛素抵抗与葡萄糖耐量改善[39], 对NAFLD等代谢紊乱疾病有显著的改善作用.
体内脂质(包括甘油三酯、脂肪酸、胆固醇等物质)代谢的平衡主要由脂质的吸收、生成、氧化、清除等环节共同维持, 这些环节涉及到大量酶促反应, 其中有诸多关键通路受到FXR的调控. FXR-/-小鼠相比野生型小鼠, 可从肠道摄取更多外源性胆固醇, 并可自发形成高脂血症[40], 表明了FXR在维持脂质代谢平衡中的重要作用.
NAFLD最直接的原因是肝脂质代谢紊乱, 肝细胞发生脂质过氧化, 全肝甘油三酯(triglyceride, TG)异常蓄积[41]; 并且, 堆积的脂质还会损伤肝细胞的胰岛素信号转导通路, 导致肝细胞的胰岛素抵抗; 这种效应可通过抑制肝细胞的脂质堆积得到改善[42]. 这表明, 减少肝脏中的脂质蓄积可以作为改善NAFLD的治疗手段, 而FXR作为调控脂质代谢的重要关节点, 也可以借以此成为对抗NAFLD病变的靶点.
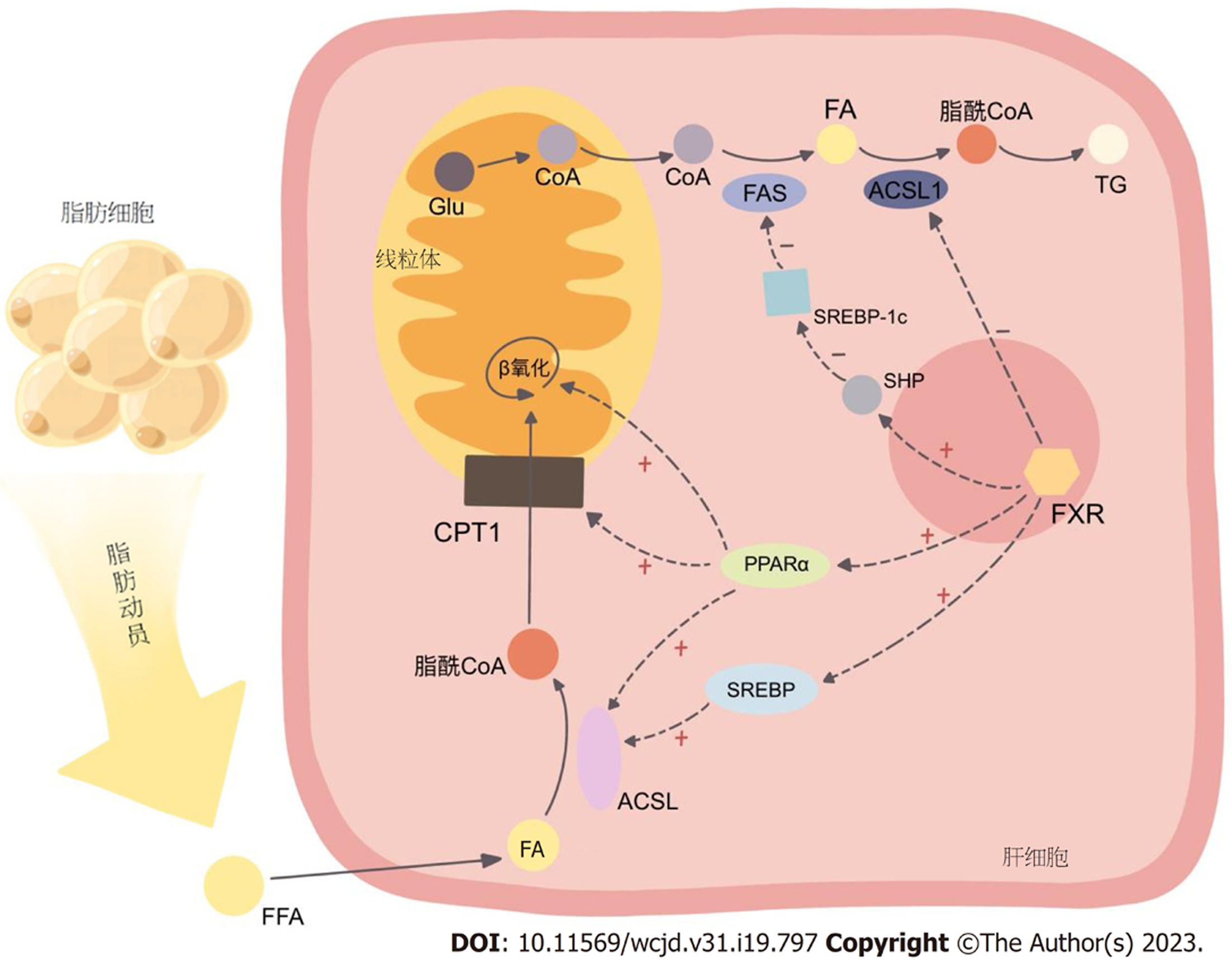
2.3.1 FXR激活后抑制脂肪酸和甘油三酯的合成: 研究表明[43], FXR能通过减少脂肪酸合成和吸收来维持肝脏脂含量, 这对于改善NAFLD肝脂质蓄积有着积极作用. 脂肪酸是人体合成甘油三酯的基本原料, 而体内的脂肪酸则主要有两种来源: 其一, 外源性脂肪酸在胆汁酸的介导下由肠道消化食物吸收而来, 其二, 内源性脂肪酸则利用糖代谢的中间产物乙酰辅酶A合成.
催化脂肪酸合成的酶体系通常称为脂肪酸合酶(fatty acid synthase, FAS)复合体, 主要存在于细胞质中, 在肝、肾、脑、乳腺、脂肪等不同组织均有表达, 以肝中活性最高. 葡萄糖在线粒体内氧化分解产生乙酰辅酶A后, 通过柠檬酸-丙酮酸循环进入胞质, 由乙酰辅酶A羧化酶(acetyl-CoA carboxylase, ACC)转化为丙二酸单酰辅酶A, 继而进入一系列以缩合-加氢-脱水-加氢为基本反应的循环中, 进而合成脂肪酸. FAS体系中, ACC是催化脂肪合成多个步骤中的首个限速酶.
FAS是已知的FXR靶基因, FXR激活后可下调FAS的表达, 以促进脂肪分解过程[44]. 肝脏内的胆汁酸激活FXR后, 通过SHP(在LXR/RXR/LRH-1的协同下)抑制固醇元件结合蛋白-1c(sterol regulatory element-binding protein-1c, SREBP-1c), 降低脂肪酸合酶体系中关键酶的表达, 如乙酰辅酶A羧化酶(acetyl-CoA carboxylase, ACC), 以及催化饱和脂肪酸转化为不饱和脂肪酸的关键酶硬脂酰辅酶A去饱和酶1(stearoyl-CoA desaturase-1, SCD1)[45], 降低脂肪酸的合成, 减少了甘油三酯合成的原料. 但这种作用对于FXR来说可能为间接的, 因而, 也可将ACC和SCD1作为独立的靶点开展研究, 针对此两个酶的抑制剂在改善脂质代谢方面或也具有较好前景[46]. 此外值得一提的是, 研究发现[47], FXR与LXR、PPARα(见下文2.3.2)、SREBP-1c、ChREBP之间存在大量相互重叠的结合位点, 使得其间几种转录因子的激活也会串扰另几种转录因子, 形成了颇为复杂的激活关系网, 这种网络结构可以对物质代谢实现更为精细的调控, 同时, 对其的靶向干预则有可能牵一发而动全身, 更深入的机制还需进一步深入研究去厘清.
脂肪酸需要经过脂酰辅酶A合成酶(acyl-CoA synthase, ACS)活化为脂酰辅酶A, 才能进入各种代谢途径, 如甘油三酯、胆固醇酯、磷脂的合成, 以及脂肪酸β氧化等. 长链脂酰辅酶A合成酶(long-chain acyl-CoA synthetase, ACSL)家族的5个成员(ACSL1、3、4、5和6)中, 已知ACSL1可被PPARα和SREBP-2激活[48], ACSL4可被PPARδ激活[49], ACSL5可被PPARα激活[50], 2019年一项研究显示, ACSL1或为胆汁酸激活FXR后的新作用靶点: OCA作用于野生型小鼠激活FXR后可下调ACSL1的表达, 而FXR敲除小鼠中则无此效应; ACSL家族其它亚型也不会受到FXR激活的影响; 这一调控效应与SHP无关, ACSL1启动子中也并无FXR反应元件(FXRE), 因此这一效应或为转录后机制(如mRNA)的作用[19], 具体仍待进一步研究. FXR将ACSL1抑制后, 脂肪酸活化参与甘油三酯合成的反应就会受阻, 表明了FXR激活对脂质合成的抑制, 起到对抗NAFLD脂质堆积的作用.
然而, 有趣的是, 不仅TG合成需要ACS活化脂肪酸, 脂肪酸的氧化分解同样需要ACS的作用, 而除了FXR通过上述未探明的机制抑制ACSL1(抑制脂肪酸合成)外, FXR还可间接地通过PPARα和SREBP促进ACSL家族部分成员酶的表达(促进脂肪酸分解, 见下文2.3.2), 这两种作用似乎是相互矛盾的, 但其整体上对脂质代谢的调控形成的平衡在FXR激活后改善NAFLD病变的作用依然占据重要地位.
2.3.2 FXR激活后促进甘油三酯的分解与氧化: 甘油三酯的分解从脂肪动员起始, 逐步分解为甘油和游离脂肪酸, 后者与血浆中的清蛋白结合, 从脂肪组织运送至全身, 经ACS活化后, 通过关键酶-肉碱脂酰转移酶1(carnitine palmitoyltransferase Ⅰ, CPT1)即肉碱棕榈酰转移酶1, 介导的限速步骤进入线粒体进行β氧化. 包括CPT1[51]在内的多个参与脂肪酸氧化的酶, 如ACS[48]、脂酰辅酶A脱氢酶1[即脂酰辅酶A氧化酶1(acyl coenzyme a oxidase 1, ACOX1)]等, 均受到过氧化物酶体增殖物激活受体(peroxisome proliferator-activated receptor, PPAR)的调控.
PPAR家族包含三个亚型(α、β/δ、γ), 是一类配体依赖性的转录调节因子, 当其与配体结合激活后, PPARs与RXR异二聚化, 结合至靶基因特定位点的PPAR反应元件(PPARE), 启动目的基因的转录[52]. 脂肪酸是PPARα的内源性配体, 当前已有充分证据表明, PPARα的激活可以促进脂肪酸的β氧化, 其主要涉及PPARα对ACSL1的上调[53]. 研究发现, PPARα[54]和PPARγ[55]启动子区的同向/反向重复序列是FXR的直接结合位点, FXR的启动子中也包含PPARE[56], 因而FXR激活后可在转录水平上诱导PPARα和PPARγ的表达, 进而提高脂肪酸氧化过程中多环节的酶的表达[57], 间接地促进脂肪酸进入线粒体β氧化[58].
此外, ACSL1的表达也可通过SREBP-2的激活而上调[59], 先前研究也表明, SREBP-1c也可在糖尿病小鼠促进肝脏ACSL5的表达[60]; 这两点都是通过促进脂肪酸活化作为脂肪酸氧化前置反应的进行, 以实现对脂肪酸代谢的调控.
另有研究发现, 禁食可诱导肝脏FXR和PPARγ共激活因子-1α(peroxisome proliferator-activated receptor-gamma coactivator 1α, PGC-1α)的表达, 而后者对于FXR发挥其调控糖、脂、胆汁酸物质代谢功能也有着重要作用-PGC-1α可以在cAMP的作用下, 作为协同激活因子[61]显著地分别诱导FXR和SHP的表达, 这种诱导在PPARγ和肝细胞核因子4α(hepatocyte nuclear factor 4alpha, HNF4α)的存在下作用更强, 且诱导后, 可进一步促进FXR靶基因如IBABP、BSEP等的表达, 亦可通过FXR-SHP途径降低SREBP-1c的表达, 发挥降脂作用60. 见图2.
总地来说, FXR激活后, 可抑制内源性脂肪酸及甘油三酯的合成, 促进脂肪酸的氧化分解, 从而起到改善脂质代谢的作用, 该作用能够对抗NAFLD发生发展中典型的脂质沉积, 对NAFLD机体的病理变化起到改善作用.
新的研究表明, 与NAFLD发生发展相关的因素中, 除了常常讨论的胰岛素抵抗、脂质代谢紊乱等, 肝脏游离胆固醇的聚积和肝脏胆固醇稳态的破坏也是重要的一环[62]: 其可导致肝Kupffer细胞氧化应激[63], 通过toll样受体4(TLR4)依赖性信号通路诱导肝脏星状细胞的活化, 促进肝纤维化的发展[64], 还可在肝细胞内引发内质网应激、线粒体功能障碍, 导致细胞凋亡[65].
ATP结合盒转运蛋白A1(ATP-binding cassette transporter A1, ABCA1)在体内广泛分布, 主要介导胞内胆固醇外流; 其也是肝细胞基底膜上重要的游离胆固醇外排蛋白, 低表达会导致肝胆固醇流出受阻, 使NAFLD病情恶化[66]; 研究发现NAFLD患者肝ABCA1的表达则显著低于正常人[67], 证实了上述观点; ABCA1过表达则可增加肝内胆固醇的流出, 减少肝内脂质蓄积, 对高脂饮食(high-fat diet, HFD)诱导的NAFLD有改善作用[68,69]; 他汀类和依折麦布等降脂药也是通过降低肝脏胆固醇水平从而展现出潜在的对NAFLD防治效果[70,71]. 因而, 对肝脏胆固醇代谢的调节, 也可对NAFLD产生一定的有益作用, 或可成为新的治疗靶点, FXR在调节胆固醇代谢方面的作用也成为了其改善NAFLD并发挥潜在治疗作用的重要一环.
2.4.1 FXR通过多个通路调节肝脏胆固醇向胆汁酸的转化: 胆固醇或经肝生化合成向胆汁酸转化, 或直接经胆道肠道通过粪便排出体外, 此为体内胆固醇的两个主要去路.
如前文所述, FXR的激活可由SHP通路减小胆汁酸池, 并改变胆汁酸的组成-其中一条调控途径是以胆汁酸合成的关键酶, 7α-羟化酶(CYP7A1)和/或替代途径的27α-羟化酶(CYP27A1)为靶点的-FXR激活后, 该酶的表达降低, 胆汁酸合成减少; 而胆固醇正是这一系列酶催化生成胆汁酸的合成原料. 从这一方面讲, 是减弱了胆固醇向胆汁酸的转化. 但是另一方面, 胆汁酸在合成后排入肠道, 又可激活肠道FXR, 进而通过RXR-SR-BI通路, 抑制肝细胞对胆固醇的重摄取. 因而, FXR激活后, 可通过多种通路对胆固醇的去向加以调节, 但整体结果是降低肝内胆固醇含量的.
2.4.2 FXR通过上调ABCG5/ABCG8的表达促进肝脏胆固醇排泄: 肝细胞的胆小管面, ATP结合盒转运蛋白G亚家族(ATP-binding cassette, subfamily G, ABCG)的两个半转运蛋白成员ABCG5和ABCG8以异二聚体的形式将肝细胞中的胆固醇泵入胆道[72], 其功能占据了胆汁胆固醇分泌的70%到90%[73].
在肥胖小鼠模型中, 若敲除ABCG5/ABCG8, 会导致胆汁胆固醇排泄减少, 可加剧NAFLD发展[74]; 而ABCG5/ABCG8过表达, 则能改善肝脏的脂肪变性和胰岛素抵抗, 对NAFLD有改善作用[75]. ABCG5/ABCG8是肝细胞X受体(LXR)α和β的直接转录靶点[76], LXR激活可显著提高其表达[77]; 而FXR则可与LXRα共同作用, 调节肝脏ABCG5/ABCG8的表达-由肠道中胆汁酸含量升高引起的肝脏ABCG5/ABCG8的mRNA水平增加似乎是由FXR介导的, 但具体机制目前尚不清楚[78].
2.4.3 FRX与LXR协同诱导SR-BI的表达促进肝对血浆胆固醇的摄取: 通过食物摄入的外源性胆固醇随乳糜微粒(chylomicron, CM)、体内合成的内源性胆固醇随低密度脂蛋白(low density lipoprotein, LDL), 经血液运送至除肝以外的全身组织加以利用, 多余的胆固醇则由高密度脂蛋白(high density lipoprotein, HDL)接收并酯化, 运送回肝脏, 此即胆固醇由体细胞向肝内的逆向转运(reverse cholesterol transport, RCT). 在此运送过程中, HDL中的胆固醇酯(cholesteryl ester, CE)又可在血浆胆固醇酯转运蛋白(cholesteryl ester transfer protein, CETP)的作用下传递至VLDL, 后者又进而转变为LDL. 最后, HDL和LDL中的CE由肝脏B类Ⅰ型清道夫受体[(scavenger receptor class B type I, SR-BI), 也称为高密度脂蛋白受体(high density lipoprotein receptor, HDLR)]和低密度脂蛋白受体(low density lipoprotein receptor, LDLR)介导, 转运入肝细胞.
SR-BI广泛分布于人体众多组织, 但以肝脏中表达最为丰富, 主要存在于肝细胞血窦面和胆小管面[79]; 肝外主要存在于类固醇合成组织如肾上腺、睾丸、卵巢等, 其负责摄取HDL中的CE, 供肝细胞转化为胆汁酸, 或供上述组织合成类固醇激素.
有研究发现, 上调SR-BI表达可改善NAFLD[80]. 使用FXR激动剂治疗NASH仓鼠模型后, 可观察到仓鼠体重减轻, CYP7A1表达降低, SR-BI表达升高, 同时使得NASH的炎症指标也有所好转[81]. 在FXR缺陷小鼠能观察到SR-BI表达减少、血脂水平升高[82], 表明了FXR在调节脂代谢、改善NAFLD方面的作用. 高脂饮食喂食小鼠后可检测到肝脏SR-BI的mRNA和蛋白水平显著升高[83], 可能与NAFLD发生发展起到拮抗作用; 但在血脂正常的仓鼠, 则未观察到SR-BI表达的改变[84], 说明这一机制并不单纯由FXR激活主导.
FXR对SR-BI的调节机制较为复杂, 有研究认为, 与通过SHP-CYP7A1通路抑制胆固醇向胆汁酸的转化类似, FXR同样通过FXR/RXR异二聚体形式诱导SHP表达, 与LRH-1结合, 进而抑制SR-BI的表达; 同时, 在SR-BI的启动子区域并没有发现FXR/RXR的反应元件[85]; 另一项研究发现, FXR的激活继而增加了HNF4α的蛋白质水平, 其可通过小鼠SR-BI启动子区和内含子序列上的HNF4α结合位点激活SR-BI的表达[86].
更多研究表明, SR-BI表达的调节可能与肝X受体/类视黄醇X受体(LXR/RXR)关系更为密切. 在血脂正常的仓鼠, 无论是单独使用FXR激动剂还是LXR激动剂, 都无法提高肝脏SR-BI的基因表达; 而二者联用则可显著提高; 该研究进而发现, FXR可与LXR协同, 促进肝脏SR-BI的转录, 从而诱导其表达、增加肝脏胆固醇外排, 且这种协同作用需在LXR激活状态下由FXR介导[87].
此外, SR-BI还接受肝受体同系物-1(LRH-1)、过氧化物酶体增殖物激活受体γ(PPARγ)、SREBP等的调节, 如HNF4α可上调PPARγ1和PPARγ2, 进而上调SR-BI[88]; 另外, 受SREBP-2调控的肝脏Niemann-Pick C1样1蛋白(Niemann-Pick C1 like 1, NPC1L1)也对胆固醇代谢有重要作用, 但相关研究并不充足[89]. 这些途径则似乎与FXR无关, 具体机制还有待进一步研究.
肥胖和代谢疾病频发的背景下, 国内外的NAFLD发病率都呈现逐年增高的趋势. NAFLD的治疗策略主要在于改善物质代谢、肝脏炎症、脂肪变性、肝纤维化等方面. 针对NAFLD暂未特效药物获批上市, 现临床上使用的药物大多是减轻症状, 并努力抑制病程进展, 但并未根除病因. FXR是一个非常有希望的治疗靶点, 在体内调控着胆汁酸和糖脂相关的重要代谢, 且这些生理生化过程直接或间接地参与了NAFLD的发生与发展. 以FXR为治疗靶点将会是一个行之有效的干预手段. 目前虽然FXR针对性药物层出不穷, 但大都还在临床试验阶段, 要真正应用于临床还需要进行更深入的探索.
学科分类: 胃肠病学和肝病学
手稿来源地: 湖南省
同行评议报告学术质量分类
A级 (优秀): 0
B级 (非常好): 0
C级 (良好): C, C
D级 (一般): D
E级 (差): 0
科学编辑: 张砚梁 制作编辑:张砚梁
| 1. | Pouwels S, Sakran N, Graham Y, Leal A, Pintar T, Yang W, Kassir R, Singhal R, Mahawar K, Ramnarain D. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord. 2022;22:63. [PubMed] [DOI] |
| 2. | Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69:2672-2682. [PubMed] [DOI] |
| 4. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [PubMed] [DOI] |
| 5. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [PubMed] [DOI] |
| 6. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [PubMed] [DOI] |
| 8. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [PubMed] [DOI] |
| 9. | Fang YL, Chen H, Wang CL, Liang L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From "two hit theory" to "multiple hit model". World J Gastroenterol. 2018;24:2974-2983. [PubMed] [DOI] |
| 11. | Khairnar R, Islam MA, Fleishman J, Kumar S. Shedding light on non-alcoholic fatty liver disease: Pathogenesis, molecular mechanisms, models, and emerging therapeutics. Life Sci. 2023;312:121185. [PubMed] [DOI] |
| 12. | Wang H, He Q, Wang G, Xu X, Hao H. FXR modulators for enterohepatic and metabolic diseases. Expert Opin Ther Pat. 2018;28:765-782. [PubMed] [DOI] |
| 13. | Zheng X, Huang F, Zhao A, Lei S, Zhang Y, Xie G, Chen T, Qu C, Rajani C, Dong B, Li D, Jia W. Bile acid is a significant host factor shaping the gut microbiome of diet-induced obese mice. BMC Biol. 2017;15:120. [PubMed] [DOI] |
| 15. | Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, Weinberger C. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687-693. [PubMed] [DOI] |
| 16. | Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365-1368. [PubMed] [DOI] |
| 17. | Chiang JYL, Ferrell JM. Bile Acid Metabolism in Liver Pathobiology. Gene Expr. 2018;18:71-87. [PubMed] [DOI] |
| 18. | Shin DJ, Wang L. Bile Acid-Activated Receptors: A Review on FXR and Other Nuclear Receptors. Handb Exp Pharmacol. 2019;256:51-72. [PubMed] [DOI] |
| 19. | Singh AB, Dong B, Xu Y, Zhang Y, Liu J. Identification of a novel function of hepatic long-chain acyl-CoA synthetase-1 (ACSL1) in bile acid synthesis and its regulation by bile acid-activated farnesoid X receptor. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:358-371. [PubMed] [DOI] |
| 20. | Kir S, Zhang Y, Gerard RD, Kliewer SA, Mangelsdorf DJ. Nuclear receptors HNF4α and LRH-1 cooperate in regulating Cyp7a1 in vivo. J Biol Chem. 2012;287:41334-41341. [PubMed] [DOI] |
| 21. | Kim YC, Byun S, Zhang Y, Seok S, Kemper B, Ma J, Kemper JK. Liver ChIP-seq analysis in FGF19-treated mice reveals SHP as a global transcriptional partner of SREBP-2. Genome Biol. 2015;16:268. [PubMed] [DOI] |
| 22. | Shin DJ, Osborne TF. FGF15/FGFR4 integrates growth factor signaling with hepatic bile acid metabolism and insulin action. J Biol Chem. 2009;284:11110-11120. [PubMed] [DOI] |
| 23. | Moscovitz JE, Kong B, Buckley K, Buckley B, Guo GL, Aleksunes LM. Restoration of enterohepatic bile acid pathways in pregnant mice following short term activation of Fxr by GW4064. Toxicol Appl Pharmacol. 2016;310:60-67. [PubMed] [DOI] |
| 24. | Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, Mastrandrea L, Buck MJ, Baker RD, Genco RJ, Zhu R, Zhu L. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67:1881-1891. [PubMed] [DOI] |
| 25. | Ferslew BC, Xie G, Johnston CK, Su M, Stewart PW, Jia W, Brouwer KL, Barritt AS 4th. Altered Bile Acid Metabolome in Patients with Nonalcoholic Steatohepatitis. Dig Dis Sci. 2015;60:3318-3328. [PubMed] [DOI] |
| 26. | Puri P, Daita K, Joyce A, Mirshahi F, Santhekadur PK, Cazanave S, Luketic VA, Siddiqui MS, Boyett S, Min HK, Kumar DP, Kohli R, Zhou H, Hylemon PB, Contos MJ, Idowu M, Sanyal AJ. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology. 2018;67:534-548. [PubMed] [DOI] |
| 27. | Roy PP, Mahtab MA, Rahim MA, Yesmin SS, Islam SB, Akbar SMF. Treatment of Nonalcoholic Steatohepatitis by Obeticholic Acid: Current Status. Euroasian J Hepatogastroenterol. 2022;12:S46-S50. [PubMed] [DOI] |
| 28. | Zhu S, Yang K, Yang S, Zhang L, Xiong M, Zhang J, Chen B. A high bile acid environment promotes apoptosis and inhibits migration in pancreatic cancer. Bioengineered. 2022;13:6719-6728. [PubMed] [DOI] |
| 29. | Faubion WA, Guicciardi ME, Miyoshi H, Bronk SF, Roberts PJ, Svingen PA, Kaufmann SH, Gores GJ. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest. 1999;103:137-145. [PubMed] [DOI] |
| 30. | Guicciardi ME, Gores GJ. Bile acid-mediated hepatocyte apoptosis and cholestatic liver disease. Dig Liver Dis. 2002;34:387-392. [PubMed] [DOI] |
| 31. | Smith GI, Shankaran M, Yoshino M, Schweitzer GG, Chondronikola M, Beals JW, Okunade AL, Patterson BW, Nyangau E, Field T, Sirlin CB, Talukdar S, Hellerstein MK, Klein S. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest. 2020;130:1453-1460. [PubMed] [DOI] |
| 32. | Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, Atkins AR, Khvat A, Schnabl B, Yu RT, Brenner DA, Coulter S, Liddle C, Schoonjans K, Olefsky JM, Saltiel AR, Downes M, Evans RM. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159-165. [PubMed] [DOI] |
| 33. | Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, Edwards PA. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci USA. 2006;103:1006-1011. [PubMed] [DOI] |
| 34. | Bellafante E, McIlvride S, Nikolova V, Fan HM, Manna LB, Chambers J, Machirori M, Banerjee A, Murphy K, Martineau M, Schoonjans K, Marschall HU, Jones P, Williamson C. Maternal glucose homeostasis is impaired in mouse models of gestational cholestasis. Sci Rep. 2020;10:11523. [PubMed] [DOI] |
| 35. | Yamagata K, Daitoku H, Shimamoto Y, Matsuzaki H, Hirota K, Ishida J, Fukamizu A. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J Biol Chem. 2004;279:23158-23165. [PubMed] [DOI] |
| 36. | Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621-1624. [PubMed] [DOI] |
| 38. | Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci USA. 2004;101:7281-7286. [PubMed] [DOI] |
| 39. | Pathak P, Xie C, Nichols RG, Ferrell JM, Boehme S, Krausz KW, Patterson AD, Gonzalez FJ, Chiang JYL. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology. 2018;68:1574-1588. [PubMed] [DOI] |
| 40. | Kim KH, Choi S, Zhou Y, Kim EY, Lee JM, Saha PK, Anakk S, Moore DD. Hepatic FXR/SHP axis modulates systemic glucose and fatty acid homeostasis in aged mice. Hepatology. 2017;66:498-509. [PubMed] [DOI] |
| 41. | Xiong X, Wang X, Lu Y, Wang E, Zhang Z, Yang J, Zhang H, Li X. Hepatic steatosis exacerbated by endoplasmic reticulum stress-mediated downregulation of FXR in aging mice. J Hepatol. 2014;60:847-854. [PubMed] [DOI] |
| 43. | Clifford BL, Sedgeman LR, Williams KJ, Morand P, Cheng A, Jarrett KE, Chan AP, Brearley-Sholto MC, Wahlström A, Ashby JW, Barshop W, Wohlschlegel J, Calkin AC, Liu Y, Thorell A, Meikle PJ, Drew BG, Mack JJ, Marschall HU, Tarling EJ, Edwards PA, de Aguiar Vallim TQ. FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 2021;33:1671-1684.e4. [PubMed] [DOI] |
| 44. | Shen LL, Liu H, Peng J, Gan L, Lu L, Zhang Q, Li L, He F, Jiang Y. Effects of farnesoid X receptor on the expression of the fatty acid synthetase and hepatic lipase. Mol Biol Rep. 2011;38:553-559. [PubMed] [DOI] |
| 45. | Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408-1418. [PubMed] [DOI] |
| 46. | Samuel VT, Shulman GI. Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metab. 2018;27:22-41. [PubMed] [DOI] |
| 47. | Zhou S, You H, Qiu S, Yu D, Bai Y, He J, Cao H, Che Q, Guo J, Su Z. A new perspective on NAFLD: Focusing on the crosstalk between peroxisome proliferator-activated receptor alpha (PPARα) and farnesoid X receptor (FXR). Biomed Pharmacother. 2022;154:113577. [PubMed] [DOI] |
| 48. | Suzuki H, Watanabe M, Fujino T, Yamamoto T. Multiple promoters in rat acyl-CoA synthetase gene mediate differential expression of multiple transcripts with 5'-end heterogeneity. J Biol Chem. 1995;270:9676-9682. [PubMed] [DOI] |
| 49. | Kan CF, Singh AB, Dong B, Shende VR, Liu J. PPARδ activation induces hepatic long-chain acyl-CoA synthetase 4 expression in vivo and in vitro. Biochim Biophys Acta. 2015;1851:577-587. [PubMed] [DOI] |
| 50. | Janssen AW, Betzel B, Stoopen G, Berends FJ, Janssen IM, Peijnenburg AA, Kersten S. The impact of PPARα activation on whole genome gene expression in human precision cut liver slices. BMC Genomics. 2015;16:760. [PubMed] [DOI] |
| 51. | Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17:259-272. [PubMed] [DOI] |
| 52. | Montaigne D, Butruille L, Staels B. PPAR control of metabolism and cardiovascular functions. Nat Rev Cardiol. 2021;18:809-823. [PubMed] [DOI] |
| 53. | Burri L, Thoresen GH, Berge RK. The Role of PPARα Activation in Liver and Muscle. PPAR Res. 2010;2010. [PubMed] [DOI] |
| 54. | Preidis GA, Kim KH, Moore DD. Nutrient-sensing nuclear receptors PPARα and FXR control liver energy balance. J Clin Invest. 2017;127:1193-1201. [PubMed] [DOI] |
| 55. | Renga B, Mencarelli A, Migliorati M, Cipriani S, D'Amore C, Distrutti E, Fiorucci S. SHP-dependent and -independent induction of peroxisome proliferator-activated receptor-γ by the bile acid sensor farnesoid X receptor counter-regulates the pro-inflammatory phenotype of liver myofibroblasts. Inflamm Res. 2011;60:577-587. [PubMed] [DOI] |
| 56. | Zhang Y, Castellani LW, Sinal CJ, Gonzalez FJ, Edwards PA. Peroxisome proliferator-activated receptor-gamma coactivator 1alpha (PGC-1alpha) regulates triglyceride metabolism by activation of the nuclear receptor FXR. Genes Dev. 2004;18:157-169. [PubMed] [DOI] |
| 57. | Xu S, Jia P, Fang Y, Jin J, Sun Z, Zhou W, Li J, Zhang Y, Wang X, Ren T, Zou Z, Ding X. Nuclear farnesoid X receptor attenuates acute kidney injury through fatty acid oxidation. Kidney Int. 2022;101:987-1002. [PubMed] [DOI] |
| 58. | Xu J, Li Y, Chen WD, Xu Y, Yin L, Ge X, Jadhav K, Adorini L, Zhang Y. Hepatic carboxylesterase 1 is essential for both normal and farnesoid X receptor-controlled lipid homeostasis. Hepatology. 2014;59:1761-1771. [PubMed] [DOI] |
| 59. | Singh AB, Kan CF, Dong B, Liu J. SREBP2 Activation Induces Hepatic Long-chain Acyl-CoA Synthetase 1 (ACSL1) Expression in Vivo and in Vitro through a Sterol Regulatory Element (SRE) Motif of the ACSL1 C-promoter. J Biol Chem. 2016;291:5373-5384. [PubMed] [DOI] |
| 60. | Achouri Y, Hegarty BD, Allanic D, Bécard D, Hainault I, Ferré P, Foufelle F. Long chain fatty acyl-CoA synthetase 5 expression is induced by insulin and glucose: involvement of sterol regulatory element-binding protein-1c. Biochimie. 2005;87:1149-1155. [PubMed] [DOI] |
| 61. | Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O'Malley B, Spiegelman BM. Activation of PPARgamma coactivator-1 through transcription factor docking. Science. 1999;286:1368-1371. [PubMed] [DOI] |
| 62. | Li H, Yu XH, Ou X, Ouyang XP, Tang CK. Hepatic cholesterol transport and its role in non-alcoholic fatty liver disease and atherosclerosis. Prog Lipid Res. 2021;83:101109. [PubMed] [DOI] |
| 63. | Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, Schuppan D, Grønbæk H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145-159. [PubMed] [DOI] |
| 64. | Teratani T, Tomita K, Suzuki T, Oshikawa T, Yokoyama H, Shimamura K, Tominaga S, Hiroi S, Irie R, Okada Y, Kurihara C, Ebinuma H, Saito H, Hokari R, Sugiyama K, Kanai T, Miura S, Hibi T. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology. 2012;142:152-164.e10. [PubMed] [DOI] |
| 65. | Mendez-Sanchez N, Cruz-Ramon VC, Ramirez-Perez OL, Hwang JP, Barranco-Fragoso B, Cordova-Gallardo J. New Aspects of Lipotoxicity in Nonalcoholic Steatohepatitis. Int J Mol Sci. 2018;19. [PubMed] [DOI] |
| 66. | Chen Y, Chen Y, Zhao L, Chen Y, Mei M, Li Q, Huang A, Varghese Z, Moorhead JF, Ruan XZ. Inflammatory stress exacerbates hepatic cholesterol accumulation via disrupting cellular cholesterol export. J Gastroenterol Hepatol. 2012;27:974-984. [PubMed] [DOI] |
| 67. | Vega-Badillo J, Gutiérrez-Vidal R, Hernández-Pérez HA, Villamil-Ramírez H, León-Mimila P, Sánchez-Muñoz F, Morán-Ramos S, Larrieta-Carrasco E, Fernández-Silva I, Méndez-Sánchez N, Tovar AR, Campos-Pérez F, Villarreal-Molina T, Hernández-Pando R, Aguilar-Salinas CA, Canizales-Quinteros S. Hepatic miR-33a/miR-144 and their target gene ABCA1 are associated with steatohepatitis in morbidly obese subjects. Liver Int. 2016;36:1383-1391. [PubMed] [DOI] |
| 68. | Jung JH, Kim HS. The inhibitory effect of black soybean on hepatic cholesterol accumulation in high cholesterol and high fat diet-induced non-alcoholic fatty liver disease. Food Chem Toxicol. 2013;60:404-412. [PubMed] [DOI] |
| 69. | Jeon BH, Lee YH, Yun MR, Kim SH, Lee BW, Kang ES, Lee HC, Cha BS. Increased expression of ATP-binding cassette transporter A1 (ABCA1) as a possible mechanism for the protective effect of cilostazol against hepatic steatosis. Metabolism. 2015;64:1444-1453. [PubMed] [DOI] |
| 70. | Averna M. The effect of ezetimibe on NAFLD. Atheroscler Suppl. 2015;17:27-34. [PubMed] [DOI] |
| 71. | Nascimbeni F, Pellegrini E, Lugari S, Mondelli A, Bursi S, Onfiani G, Carubbi F, Lonardo A. Statins and nonalcoholic fatty liver disease in the era of precision medicine: More friends than foes. Atherosclerosis. 2019;284:66-74. [PubMed] [DOI] |
| 72. | Yu XH, Qian K, Jiang N, Zheng XL, Cayabyab FS, Tang CK. ABCG5/ABCG8 in cholesterol excretion and atherosclerosis. Clin Chim Acta. 2014;428:82-88. [PubMed] [DOI] |
| 73. | Wang Y, Liu X, Pijut SS, Li J, Horn J, Bradford EM, Leggas M, Barrett TA, Graf GA. The combination of ezetimibe and ursodiol promotes fecal sterol excretion and reveals a G5G8-independent pathway for cholesterol elimination. J Lipid Res. 2015;56:810-820. [PubMed] [DOI] |
| 74. | Su K, Sabeva NS, Liu J, Wang Y, Bhatnagar S, van der Westhuyzen DR, Graf GA. The ABCG5 ABCG8 sterol transporter opposes the development of fatty liver disease and loss of glycemic control independently of phytosterol accumulation. J Biol Chem. 2012;287:28564-28575. [PubMed] [DOI] |
| 75. | Su K, Sabeva NS, Wang Y, Liu X, Lester JD, Liu J, Liang S, Graf GA. Acceleration of biliary cholesterol secretion restores glycemic control and alleviates hypertriglyceridemia in obese db/db mice. Arterioscler Thromb Vasc Biol. 2014;34:26-33. [PubMed] [DOI] |
| 76. | Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 2002;277:18793-18800. [PubMed] [DOI] |
| 77. | Back SS, Kim J, Choi D, Lee ES, Choi SY, Han K. Cooperative transcriptional activation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 genes by nuclear receptors including Liver-X-Receptor. BMB Rep. 2013;46:322-327. [PubMed] [DOI] |
| 78. | Wang J, Einarsson C, Murphy C, Parini P, Björkhem I, Gåfvels M, Eggertsen G. Studies on LXR- and FXR-mediated effects on cholesterol homeostasis in normal and cholic acid-depleted mice. J Lipid Res. 2006;47:421-430. [PubMed] [DOI] |
| 79. | Fruhwürth S, Kovacs WJ, Bittman R, Messner S, Röhrl C, Stangl H. Differential basolateral-apical distribution of scavenger receptor, class B, type I in cultured cells and the liver. Histochem Cell Biol. 2014;142:645-655. [PubMed] [DOI] |
| 80. | Xin P, Han H, Gao D, Cui W, Yang X, Ying C, Sun X, Hao L. Alleviative effects of resveratrol on nonalcoholic fatty liver disease are associated with up regulation of hepatic low density lipoprotein receptor and scavenger receptor class B type I gene expressions in rats. Food Chem Toxicol. 2013;52:12-18. [PubMed] [DOI] |
| 81. | Briand F, Brousseau E, Quinsat M, Burcelin R, Sulpice T. Obeticholic acid raises LDL-cholesterol and reduces HDL-cholesterol in the Diet-Induced NASH (DIN) hamster model. Eur. J Pharmacol. 2018;818:449-456. [PubMed] [DOI] |
| 82. | Lambert G, Amar MJ, Guo G, Brewer HB, Gonzalez FJ, Sinal CJ. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem. 2003;278:2563-2570. [PubMed] [DOI] |
| 83. | Gustafsen C, Olsen D, Vilstrup J, Lund S, Reinhardt A, Wellner N, Larsen T, Andersen CBF, Weyer K, Li JP, Seeberger PH, Thirup S, Madsen P, Glerup S. Heparan sulfate proteoglycans present PCSK9 to the LDL receptor. Nat Commun. 2017;8:503. [PubMed] [DOI] |
| 84. | Dong B, Young M, Liu X, Singh AB, Liu J. Regulation of lipid metabolism by obeticholic acid in hyperlipidemic hamsters. J Lipid Res. 2017;58:350-363. [PubMed] [DOI] |
| 85. | Malerød L, Sporstøl M, Juvet LK, Mousavi SA, Gjøen T, Berg T, Roos N, Eskild W. Bile acids reduce SR-BI expression in hepatocytes by a pathway involving FXR/RXR, SHP, and LRH-1. Biochem Biophys Res Commun. 2005;336:1096-1105. [PubMed] [DOI] |
| 86. | Zhang Y, Yin L, Anderson J, Ma H, Gonzalez FJ, Willson TM, Edwards PA. Identification of novel pathways that control farnesoid X receptor-mediated hypocholesterolemia. J Biol Chem. 2010;285:3035-3043. [PubMed] [DOI] |
| 87. | Dong B, Singh AB, Guo GL, Young M, Liu J. Activation of FXR by obeticholic acid induces hepatic gene expression of SR-BI through a novel mechanism of transcriptional synergy with the nuclear receptor LXR. Int J Mol Med. 2019;43:1927-1938. [PubMed] [DOI] |