修回日期: 2023-05-20
接受日期: 2023-06-29
在线出版日期: 2023-07-08
铁死亡是一种以细胞内铁超载、脂质过氧化物堆积为特征的新型程序性细胞死亡方式. 近年来, 研究已证实干预铁死亡可有效预防或治疗包括结直肠癌在内的肿瘤疾病. 然而铁死亡在不同类型结直肠癌中所扮演的角色不尽相同. 本文总结了参与铁死亡发生发展过程中的三大效应途径即铁代谢、脂质代谢及氨基酸代谢, 并在此基础上探讨了靶向铁死亡疗法在结直肠癌共识分子分型各亚型中的具体机制与临床应用. 现有研究表明, 靶向铁死亡疗法在优化结直肠癌综合治疗方面具有广阔的应用前景. 本文对铁死亡机制及其在肿瘤防治中的研究进行综述, 对探索靶向铁死亡疗法治疗不同类型结直肠癌中的应用具有指导意义.
核心提要: 在众多结直肠癌(colorectal cancer, CRC)分型中, 具明确生物学可解释性的CRC共识分子分型(consensus molecular subtype, CMS)最具影响力. 研究发现, 铁死亡可有效防治包括CRC在内的肿瘤疾病. 本文综述了铁死亡在CMS不同亚型中的功能, 为进一步开发精准的靶向治疗药物提供理论支撑.
引文著录: 张馨瑞, 罗依婷, 朱方圆, 叶露, 倪思忆, 刘英超, 沈雁. 结直肠癌治疗新靶标: 铁死亡代谢及调控机制. 世界华人消化杂志 2023; 31(13): 528-536
Revised: May 20, 2023
Accepted: June 29, 2023
Published online: July 8, 2023
Ferroptosis is a new type of programmed cell death charac-terized by intracellular iron overload and lipid peroxidation accumulation. In the past 10 years, research has demonstrated that intervention of ferroptosis can effectively prevent or treat cancer diseases, including colorectal cancer. However, the role of ferroptosis in different types of colorectal cancer is not the same. This article summarizes the three major pathways involved in the occurrence and development of ferroptosis, namely, iron metabolism, lipid metabolism, and amino acid metabolism, and discusses the specific mechanisms and clinical applications of ferroptosis targeted therapy in colorectal cancer of various consensus molecular subtypes. Existing studies have shown that ferroptosis targeted therapy has broad application prospects in optimizing the comprehensive treatment of colorectal cancer. This article will provide an important framework for studying the mechanism of ferroptosis in tumor prevention and treatment and have guiding significance in exploring the application of ferroptosis targeted therapy in the treatment of different types of colorectal cancer.
- Citation: Zhang XR, Luo YT, Zhu FY, Ye L, Ni SY, Liu YC, Shen Y. Novel target for treatment of colorectal cancer: Metabolism and regulatory mechanisms of ferroptosis. Shijie Huaren Xiaohua Zazhi 2023; 31(13): 528-536
- URL: https://www.wjgnet.com/1009-3079/full/v31/i13/528.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v31.i13.528
结直肠癌(colorectal cancer, CRC)是常见的消化道恶性肿瘤. 根据WTO国际癌症研究机构最新发布的流行病统计数据(GLOBOCAN2020), CRC的发病率(10.0%)和死亡率(9.4%)分别位居全球恶性肿瘤的第3位和第2位[1]. 近年来, CRC发病呈年轻化趋势, 预计到2030年将有约11%的结肠癌和23%的直肠癌逐渐扩展到50岁以下成年人中[2]. 由于传统放化疗的毒副作用及耐药性等问题, CRC患者的生存预后并不理想. 因此, CRC的防治仍是世界公共卫生的重要课题之一.
铁死亡是一种新型细胞死亡方式, 研究证实铁死亡与多种疾病如神经退行性变、心脑血管疾病、免疫系统疾病、炎症性疾病和缺血/再灌注损伤等的预后密切相关, 其在包括CRC在内的恶性肿瘤防治方面所扮演的角色和具体机制备受关注[3-6]. 本文总结了铁死亡的发生机制和三大效应途径, 在此基础上初步探讨铁死亡在CRC发生发展中的作用和临床应用.
铁死亡是一类铁离子依赖的、活性氧(reactive oxygen species, ROS)积累驱动的、由非酶反应(Fenton反应)和酶机制介导的调节性细胞死亡(regulated cell death, RCD)方式[7,8]. 细胞内脂质过氧化物(lipide peroxide, LPO)蓄积并破坏生物膜结构功能稳定性所导致的细胞死亡是铁死亡的实质.
2003年, Dolma等[9]在探索肿瘤治疗药物的筛选试验中发现了一种新型小分子化合物Erastin, 能特异性诱导Ras基因突变的人包皮成纤维细胞死亡. 随后, 一系列Ras选择性致死化合物(ras selective lethal compound, RSL)相继被发现并被证实能以Erastin相同方式诱导肿瘤细胞死亡[10]. 不同于其他RCD, 这类特殊的死亡方式不依赖于caspase级联反应, 但依赖于铁离子水平, 不能被坏死、凋亡、焦亡、自噬等抑制剂逆转, 却能被抗氧化剂维生素E或铁螯合剂去铁胺(deferoxamine, DFO)逆转[10]. 2012年, Dixon等[7]正式将这类具有铁依赖性和氧化性, 并呈现独特的遗传学、形态学和生物化学特征的细胞死亡方式命名为铁死亡.
细胞铁死亡的标志性形态表现主要包括: 细胞膜断裂、出泡; 线粒体皱缩、体积减少, 双层膜密度增加, 内嵴模糊不清、减少或消失; 核膜完整、胞核大小正常、无DNA片段化[11,12]; 线粒体和内质网中可见大量铁离子分布[7]. 生化表现方面, 可见二价铁离子(Fe2+)浓度升高、ROS和LPO大量蓄积、胱氨酸/谷氨酸反向转运体(cystine/glutamate antiporter, System Xc-)功能受抑、谷胱甘肽合成障碍、谷胱甘肽过氧化物酶4(glutathione peroxidase 4, GPX4)活性下降以及腺嘌呤二核苷酸磷酸氧化水平提高等[12,14].
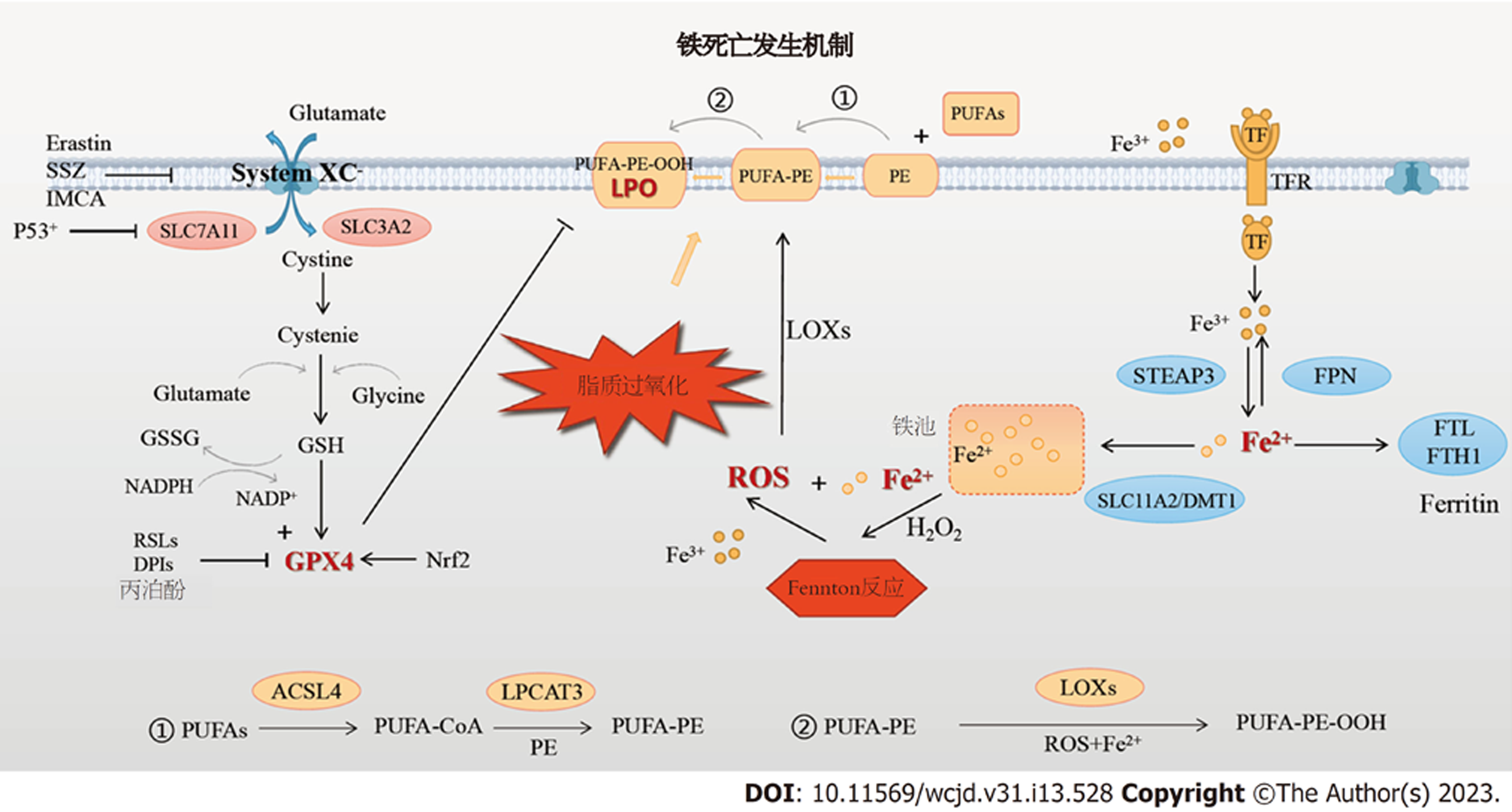
现有研究表明, 多种代谢途径和机制参与了铁死亡的启动和推进, 其中最为核心的是铁代谢途径、脂质代谢途径和氨基酸代谢途径(见图1). 各种途径的多个功能分子相互影响, 共同决定了铁死亡的发生与否和效应水平.
铁是人体中含量最丰富的必需微量元素. 约3/4的铁作为"功能性铁"分布于外周循环中, 参与循环中氧的运输、细胞内DNA、ATP的合成氧化等重要生理过程; 约1/4的铁作为"贮存铁", 以铁蛋白、含铁血黄素等形式贮存于肝、脾与骨髓[3]. 循环中的三价铁离子(Fe3+)与转铁蛋白(transferrin, TF)结合后被运输至细胞膜, 与膜上的转铁蛋白受体(TFRC/TFR1/CD71)结合成复合物后被转运入胞内的内涵体. 随后, 在氧化铁还原酶前列腺六跨膜上皮抗原(six-transmembrane epithelial antigen of prostate3, STEAP3)的催化下, Fe3+被还原成Fe2+, 再经溶质载体家族11成员2/二价金属离子转运体1(solute carrier family 7member 11, SLC11A2/divalent metal transporter-1, DMT1)的介导, Fe2+从内涵体解体并释放入胞质的不稳定铁池中[15,16]. Fe2+过量时, 或存储于铁蛋白轻链多肽和铁蛋白重链多肽1(ferritin heavy chain polypeptide1, FTH1)组成的储铁蛋白复合物Ferritin中, 或被膜铁转运蛋白1氧化成Fe3+后泵出细胞, 参与外周铁的再循环.
Fenton反应是铁死亡的启动环节. 其核心过程即Fe2+与过氧化氢相互作用后在形成Fe3+和大量ROS[17]. 低浓度ROS是细胞存活和增殖不可或缺的成分; 而高浓度ROS则可通过氧化蛋白质、DNA及生物膜结构上的各种脂类, 改变膜结构和功能活性, 引发细胞死亡[18,19]. 在上述铁代谢过程中, 任一效应蛋白的功能异常都将破坏胞内铁稳态, 引起不同程度的铁超载和ROS蓄积. 研究发现, 使用DFO、环吡酮等铁螯合剂, 或敲除TFRC基因, 或上调胞质中Ferritin水平等手段, 均可抑制细胞内铁超载以降低铁死亡水平[15,16], 而Ferritin选择性自噬降解则能释放储存铁、促进细胞铁死亡[20,21], 此外, 敲除溶质载体家族11成员3基因阻碍铁转运亦会加剧Erastin诱导的铁死亡[22]. 综上可知, 铁代谢紊乱可通过调控Fenton反应, 诱导或抑制细胞发生铁死亡.
脂质是细胞膜磷脂的主要成分, 脂质代谢在维持细胞膜稳态中具有重要意义. 酰基辅酶A合成酶长链家族成员4(acyl-CoA synthetase long-chain family member 4, ACSL4)和溶血磷脂酰胆碱酰转移酶3(recombinant lysophosphatidylcholine acyltransferase 3, LPCAT3)是膜磷脂合成的限速酶, 二者共同影响多不饱和脂肪酸(polyunsaturated fatty acids, PUFAs)的酯化重构[13]. 生理情况下, PUFAs首先被ACSL4催化为PUFA-CoA, 随后在LPCAT3的介导下酯化并结合至细胞膜的磷脂酰乙醇胺(phosphatidylethanolamines, PE)上形成PUFA-PE, 引起膜结构不饱和度增加. PUFA-PE是诱导铁死亡的关键底物, 其水平高低对细胞的铁死亡敏感性具有重要影响[23].
脂质过氧化是铁死亡的中心环节. 细胞经Fenton反应输出过量ROS, 后者可在Fe2+辅助、脂氧合酶(lipoxygenase, LOXs)催化下, 将细胞膜上的PUFA-PE过氧化为PUFA-PE-OOH, 即LPO[17,24]. LPO作为铁死亡的核心介质, 主要以三种方式破坏生物膜: (1)促进膜磷脂转向, 通过其氧化的脂肪酸与亲水头部的结合, 减少膜厚度; (2)改变脂类间的相互作用和细胞膜的理化性质; (3)代谢产物4-羟基壬烯醛和丙二醛可使细胞膜中的蛋白质和核酸发生异常共价修饰, DNA断裂, 分子结构改变[23,25-28]. 通过以上机制, 细胞膜、线粒体及溶酶体等细胞器膜变薄、曲率增加, 液态性、流动性降低, 膜通透性升高, Na+和Ca2+内流增加, 引起细胞水肿及钙超载, 进一步引起膜穿孔解体及细胞内容物释放, 最终导致细胞死亡. 此外, LPO尚可激活磷脂酶分解膜磷脂催化花生四烯酸代谢反应, 生成前列腺素、血栓素A等多种生物活性物质, 促进再灌注损伤; 线粒体膜上的LPO可抑制线粒体功能, 减少ATP生成, 加重细胞能量代谢障碍[29-31]. 研究发现, 与单不饱和脂肪酸相比, 含有更多不饱和键的PUFA更易受到ROS攻击、产生LPO[22]. PUFA的丰度和定位决定了细胞的脂质过氧化程度和铁死亡水平[4].
谷胱甘肽(glutathione, r-glutamyl cysteingl +glycine, GSH)是机体抗氧化体系的重要组分之一, 包括还原型和氧化型两种形式, 在减轻氧化应激、脂质过氧化损伤和保护组织细胞等方面发挥至关重要的作用[32-34]. 作为GSH合成关键蛋白的System Xc-, 其本质上是一种广泛分布于细胞膜磷脂双分子层中的Na+依赖性氨基酸反向转运体, 由轻链溶质载体家族7成员11(solute carrier family 7, member 11, SLC7A11)和重链溶质载体家族3成员2(solute carrier family 3, member 2, SLC3A2)经共价二硫键连接组成. 其中, SLC7A11对胱氨酸和谷氨酸具有高度特异性, 主要负责氨基酸的双向转运; 而SLC3A2作为伴侣蛋白, 帮助增强SLC7A11功能的稳定发挥[35,36]. 生理情况下, System Xc-以1:1的比例外排谷氨酸同时摄取胱氨酸[37], 为GSH的生物合成提供原料. GPX4是细胞内主要的抗氧化酶. GPX4一方面可催化GSH由还原型转化为氧化型, 减少ROS的生成, 另一方面可将有毒的LPO还原成无毒性的脂质醇, 从而保护生物膜的脂质双分子层发生过氧化损伤[38,39].
研究表明[40], 沉默 SLC7A11基因表达会增加HT-1080细胞对Erastin诱导铁死亡的敏感性, 而过表达SLC7A11后, 细胞对铁死亡的耐受性明显增强. Yang等[41]研究发现, GPX4表达减少可增加细胞对铁死亡的敏感性, 而增加GPX4表达则能有效降低铁死亡的发生率. 以上研究表明: 由System Xc-、GSH和GPX4组成的协调有序的抗氧化效应结构体, 即System Xc-/GSH/GPX4轴是铁死亡的主要保护体系. 各种可引起System Xc-功能受抑、GSH合成障碍和/或GPX4活性下降的病理状况均可导致细胞抗氧化功能受损, 氧化还原平衡失调, 直接或间接地促使脂质过氧化和铁死亡发生[42].
近来, 铁死亡已被广泛认为是抑癌基因p53的内源性抗癌机制之一, 其在骨肉瘤、肺癌细胞中的表现尤为明显. p53主要通过抑制其下游靶点System Xc-/SLC7A11的活性而激活铁死亡, p53乙酰化对该过程具有重要的调控作用[43]. 2015年, 一篇发表在Nature上的研究报告显示, 乙酰化修饰的突变型p53尽管丧失了细胞周期阻滞、衰老和凋亡等功能, 但其能通过催化SLC7A11亚基的启动子区减少其表达、抑制System Xc-的功能、降低GPX4的抗氧化活性而提高细胞对铁死亡的敏感性, 突变型p53基因小鼠同野生型小鼠一样, 仍保持着一定的抑癌表观, 抑制了自发性胸腺淋巴瘤的早期发生[44].
目前, 靶向System Xc-的铁死亡诱导剂主要分为两类: 一类是包括Erastin、柳氮磺胺吡啶(sulfasalazine, SSZ)、丁硫氨酸亚砜亚胺、索拉非尼、青蒿素及其衍生物[7,45-47]等为代表的直接诱导剂, 通过直接抑制System Xc-活性, 减少GSH合成, 引发细胞氧化还原失衡, 诱导铁死亡; 另一类是以苯并吡喃衍生物 [48]为代表的间接诱导剂, 通过激活AMPK/mTOR通路, 诱导 SLC7A11表达下调, 间接抑制System Xc-活性.
谷氨酰胺酶2(recombinant glutaminase 2, GLS2)和LOXs可能也是p53调节铁死亡的潜在下游靶点. 上调GLS2可催化谷氨酸大量合成, 胞内高浓度的谷氨酸抑制胱氨酸入胞, 从而减少谷胱甘肽合成, 诱发p53依赖性铁死亡[49,50]. 也有研究报道p53通过增强亚精胺/精胺N1-乙酰转移酶1的活化水平而增强LOXs的活性, 促进细胞发生脂质过氧化和铁死亡[51].
转录因子(nuclear factor erythroid 2-related factor 2, Nrf2)具有亮氨酸拉链结构, 能通过调节机体抗氧化蛋白的表达, 有效对抗机体内外源性氧化损伤, 维持细胞内稳态平衡. 作为铁死亡负性调控因子之一, Nrf2具有抑制细胞铁摄取、限制ROS产生和上调SLC7A11功能[52]. Nrf2调控铁死亡的下游主要靶点是GPX4. 研究发现, 上调Nrf2基因水平可促进GPX4的编码转录, 而敲除Nrf2或GPX4基因可增强Erastin或索拉菲尼诱导的肝癌细胞铁死亡[53]. 文献报道, GPX4基因敲除小鼠[54]或细胞[55]内超氧阴离子、羟基自由基等ROS大量增加都会加剧脂质过氧化, 进而诱导高水平铁死亡的发生; 此外, 胚胎小鼠可在全身敲除GPX4基因后7.5 d左右死亡[56].
目前, 一系列靶向Nrf2-GPX4信号转导过程死亡诱导剂正被逐步开发或发现. 实验表明, 全反式视黄酸、葫芦巴碱、鸦胆子苦醇等Nrf2抑制剂可通过抑制金属硫蛋白1的表达, 加速GSH耗竭和脂质过氧化, 从而促进索拉非尼诱导的铁死亡[57]. RSLs(RSL3、RSL5)、DPIs(DPI7、DPI10、DPI12)、丙泊酚等则是针对GPX4催化活性位点的特异性抑制剂. 有学者采用微小RNA等手段从基因层面进行封闭, 发现阻断GPX4活性可降低其对ROS、LPO毒性的保护能力, 进而促进脂质过氧化进程和铁死[44].
CRC是一种高度异质性疾病, 依据发生部位、遗传学和表观遗传学特征对CRC进行分型, 将有助于指导临床分层和精准性治疗, 改善患者预后. 众多分型中, 2015年由国际结直肠癌分型联盟提出的共识分子分型(consensus molecular subtype, CMS)系统因其明确的生物学可解释性而最具影响力. 研究发现, 铁死亡对CRC的发生发展具有重要影响, 铁死亡在CMS不同亚型中的功能不尽相同, 可为CRC临床诊疗提供靶向性参考依据.
约14%的CRC为CMS1型. 该型好发于右半结肠, 与肿瘤微环境(tumor microenvironment, TME)密切相关, 以免疫抑制细胞高度浸润、免疫调节分子高表达和免疫逃逸增强为主要特征. CMS1肿瘤显示出广泛的高甲基化状态, 且涵盖多数错配修复缺陷(different mismatch repair, dMMR)与高度微卫星不稳定性(high microsatellite instability, MSI-H)[58].
众多研究报道[59,60], TME中浸润的免疫抑制细胞类型及其数量与肿瘤的临床预后有关. 免疫抑制细胞介导铁死亡发生将阻遏肿瘤的发展进程. 郑勇斌等系统研究了165个铁死亡相关基因对TME的调控机制及其对CRC预后的影响, 发现铁死亡高风险组内免疫抑制细胞浸润减少, 而低风险组却存在相反的免疫景观[61]. TME中主要的抗肿瘤效应执行者CD8+T细胞可产生干扰素(IFNγ)等多种细胞毒介质, 后者既能通过下调System Xc-亚基SLC3A2和SLC7A11的表达、促进ACSL4信号转导而促进铁死亡, 增加肿瘤细胞的放疗敏感性, 又可通过释放损伤相关分子模式(damage associated molecular patterns, DAMPs)进一步激活免疫系统, 加重CD8+T细胞浸润[60-62].
除免疫异常外, 炎症反应是构成TME的另一大核心要素. 慢性炎症病灶中的ROS、RNS等氧化应激因子以及各种促炎、促肿瘤分子, 促进了TME形成以及"炎-癌"转化. 溃疡性结肠炎是公认的CRC主要癌前病变之一, 其发生发展已被阐明与铁死亡机制介导的慢性炎症密切相关[63,64]. Chen等[65]通过动物研究发现, 多种铁死亡抑制剂如铁他汀-1、利蒲他汀-1和Deferprone均能够有效改善结肠炎小鼠的症状、减轻结肠大体形态学和组织学炎症, 而这种保护作用是通过阻断Nrf2/HO-1信号通路, 进而调节COX2、ACSL4、GPX4、FTH1等铁死亡效应蛋白表达、降低结肠黏膜氧化应激水平而实现的.
尽管铁死亡在TME中的具体作用机制仍待进一步明确, 但现有研究结果已表明, 铁死亡对CMS1型CRC发挥双重作用: 一方面, 异常激活的免疫反应推动肿瘤细胞铁死亡而发挥抗癌效应; 另一方面, 正常组织细胞铁死亡引起的炎症损伤又使得免疫反应过度激活, 大大促进了细胞"炎-癌"转化, 发挥促癌效应.
约37%的CRC为CMS2型. 该型好发于左半结肠, 以上皮细胞功能缺陷为特征, 其发生多遵循Fearon[66]经典的多步骤理论. 与其他亚型相比, CMS2型主要由染色体不稳定途径介导, 包括DNA 甲基化、抑癌基因(APC、P53等)突变等.
在CMS2型CRC中, APC基因突变率最高. 高频突变的APC基因通过Wnt-β-catenin信号途径调控铁死亡, 从而参与CMS2型肿瘤的发展进程[58]. 正常情况下, β-catenin通过结合以APC复合物为组分的构架蛋白轴蛋白Axin而发生泛素化降解, APC基因突变导致β-catenin与Axin的结合序列缺失、降解减少, 胞质内游离β-catenin过量累积入核进而结合转录因子TCF/LEF, 促进靶基因的转录表达[67]. Wang[68]等研究揭示: Wnt-β-catenin信号途径下游靶基因可结合并诱导GPX4启动子的表达, 从而抑制铁死亡. Luo等[69]研究发现, Wnt抑制剂C59通过增加胞内Fe2+、LPO及线粒体超氧化物而诱导CRC细胞铁死亡, 而Wnt激动剂BML-284则可逆转这一现象. 上述结果表明, APC基因介导的Wnt-β-catenin信号途径异常激活可抑制细胞铁死亡, 促进CRC癌变进程.
P53基因突变率仅次于APC. P53主要通过两种不同方式诱导细胞铁死亡. 一种是转录依赖性方式, 即抑制SLC7A11的转录激活. 研究发现, 诱导P53基因R273H和R175H位点突变后, 其下游靶点System Xc-/SLC7A11活性下调, 促进肿瘤细胞铁死亡[44,70]. 研究发现, P53基因的杂合性缺失上调了二肽基-肽酶-4(dipeptidyl peptidase-4, DPP4)活性, 促进了后者介导的脂质过氧化和铁死亡. 另一种是非转录依赖性方式. 即干扰P53与DPP4的结合并抑制其活性[71,72]. 研究发现, P53基因的杂合性缺失上调了DPP4活性, 促进了后者介导的脂质过氧化和铁死亡.
约13%的CRC为CMS3型. 该型左右半结肠发生率无显著差别, 以显著的代谢异常和KRAS等基因高频突变为特征. 细胞代谢重编程是肿瘤的重要标志, 有助于肿瘤的进展转移[73]. 一系列研究表明, 铁死亡通过影响物质能量代谢影响CMS3型CRC, 而在传统放化疗基础上联用铁死亡诱导策略, 可实现协同增效的有益结果[69,74,75]. 牛爽等[76]通过对比实验发现, Erastin联合顺铂方案可介导氨基酸代谢, 通过增加耐顺铂人结肠腺癌细胞株LOVO/DDP中的ROS水平, 使细胞活力显著下降, 而该效应可被铁死亡抑制剂逆转. 同样地, 放疗可提高肿瘤细胞内ROS水平和脂质过氧化水平, 在一定限度内诱导铁死亡. Lang等[77]在人纤维肉瘤移植小鼠实验中发现, 将包括Erastin、RSL3、SSZ等的铁死亡诱导剂与放疗联合运用, 可调节细胞内脂质、氨基酸代谢, 提高铁死亡水平, 小鼠对放疗的敏感性较单独放疗明显增强.
KRAS基因作为肿瘤进展的"门控基因", 可调控肿瘤细胞生长和血管生成. CMS3型中频发突变的KRAS基因诱导细胞各类代谢重编程, 包括氨基酸代谢、糖代谢、脂肪代谢等, 并通过System Xc-GSH-GPX4途径抑制铁死亡[78]. 众多临床数据表明, KRAS突变对靶向治疗似乎并不敏感, 尤其是表皮生长因子受体抑制剂. 或许靶向物质能量代谢等铁死亡相关环节, 可改善KRAS耐药性难题, 实现优化CRC综合治疗.
约23%CRC为CMS4型. 该型左半结肠较右半多发, 易发生远处转移, 以转化生长因子β(transforming growth factor-β, TGF-β)信号通路异常激活、上皮间充质转化(epithelial-mesenchymal transition, EMT)上调、血管生成、间质浸润等为主要特征. 其中, TGF-β可通过依赖(促进间充质细胞特性蛋白表达)或非依赖(减少钙粘蛋白表达)Smad方式诱导EMT发生[79]; 而EMT是CRC发生、浸润和转移的关键驱动因素[80]. 以EMT为特征的CMS4型CRC极易发生铁死亡, 且对铁死亡诱导剂反应更敏感. Sun等[81]的细胞实验发现, TGF-β诱导EMT过程中可出现System Xc-受抑、GSH耗竭和氧化应激增加等铁死亡特征表现, 而该过程可被铁死亡抑制剂(ferrostatin-1, Fer-1)抑制. Chen等[82]研究发现, 源自中药姜黄的生物活性化合物β-榄香烯和西妥昔单抗联合应用, 可通过诱导铁死亡和调节EMT等机制抑制KRAS突变型CRC的生长和淋巴转移, 发挥协同抗癌效应. 上述研究表明, 靶向抑制TGF-β信号通路或许可从源头抑制CRC转移灶形成, 提高CMS4型肿瘤预后.
铁死亡各效应途径中的信号分子和/或调控因子或可作为该病的潜在治疗靶点, 诱导铁死亡并加速CRC细胞死亡可能是潜在有效的抗癌策略.
作为一种全新的调节性细胞死亡方式, 铁死亡已受到广泛关注并吸引着众多学科开展广泛研究. 现已阐明, 铁死亡的发生主要由铁代谢、脂质代谢及氨基酸代谢三大效应途径推进, 并受Nrf2、GPX4、P53、SystemXc-、SLC7A11等诸多因子调控. 基于肿瘤细胞逃避凋亡等传统程序性死亡的行为特性, 铁死亡的发现为肿瘤的综合防治拓展了广阔的研究方向和前景, 厘清铁死亡机制内部的交互关系并探寻靶向性药物实施调控是从临床角度出发对基础研究提出的新挑战. 当聚焦于CRC时, 现有研究证据也使我们认识到: 针对CMS各个亚型中的不同靶点. 铁死亡在CRC进程中所扮演的角色亦不同, 探索靶向铁死亡的疗法对CRC分型治疗具有指导意义: 当其充当促癌推手时, 我们需设法减缓或停滞正常细胞的铁死亡; 而当其作为抗癌卫士时, 则要激活并提高肿瘤细胞的铁死亡水平. 然而, 然而, 在一个复杂的机体内环境中, 铁死亡与其他细胞病理生理机制间存在怎样的相互影响?铁死亡靶向疗法对CRC的特异性如何?怎样在该类患者的临床前研究和临床试验中控制铁死亡靶向疗法的潜在不良反应?答案仍未可知, 需要带着上述问题开展更多更深入的研究, 为进一步开发精准安全的靶向抗癌药物提供支撑依据.
学科分类: 胃肠病学和肝病学
手稿来源地: 浙江省
同行评议报告学术质量分类
A级 (优秀): 0
B级 (非常好): B
C级 (良好): C, C
D级 (一般): 0
E级 (差): 0
科学编辑: 张砚梁 制作编辑:张砚梁
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [PubMed] [DOI] |
| 2. | Stoffel EM, Murphy CC. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology. 2020;158:341-353. [PubMed] [DOI] |
| 3. | Saint-Germain E, Mignacca L, Vernier M, Bobbala D, Ilangumaran S, Ferbeyre G. SOCS1 regulates senescence and ferroptosis by modulating the expression of p53 target genes. Aging (Albany NY). 2017;9:2137-2162. [PubMed] [DOI] |
| 4. | Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317-331. [PubMed] [DOI] |
| 5. | Qiu Y, Cao Y, Cao W, Jia Y, Lu N. The Application of Ferroptosis in Diseases. Pharmacol Res. 2020;159:104919. [PubMed] [DOI] |
| 6. | Devos D, Moreau C, Kyheng M, Garçon G, Rolland AS, Blasco H, Gelé P, Lenglet TT, Veyrat-Durebex C, Corcia P, Dutheil M, Bede P, Jeromin A, Oeckl P, Otto M, Meininger V, Danel-Brunaud V, Devedjian JC, Duce JA, Pradat PF. Author Correction: A ferroptosis-based panel of prognostic biomarkers for Amyotrophic Lateral Sclerosis. Sci Rep. 2020;10:3312. [PubMed] [DOI] |
| 7. | Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-1072. [PubMed] [DOI] |
| 8. | Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius E, Scheel CH, Mourão A, Buday K, Sato M, Wanninger J, Vignane T, Mohana V, Rehberg M, Flatley A, Schepers A, Kurz A, White D, Sauer M, Sattler M, Tate EW, Schmitz W, Schulze A, O'Donnell V, Proneth B, Popowicz GM, Pratt DA, Angeli JPF, Conrad M. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693-698. [PubMed] [DOI] |
| 9. | Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285-296. [PubMed] [DOI] |
| 10. | Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15:234-245. [PubMed] [DOI] |
| 11. | Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Rådmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Förster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O'Donnell VB, Kagan VE, Schick JA, Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180-1191. [PubMed] [DOI] |
| 12. | Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88. [PubMed] [DOI] |
| 13. | Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, Prokisch H, Trümbach D, Mao G, Qu F, Bayir H, Füllekrug J, Scheel CH, Wurst W, Schick JA, Kagan VE, Angeli JP, Conrad M. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91-98. [PubMed] [DOI] |
| 14. | Wang H, Liu C, Zhao Y, Gao G. Mitochondria regulation in ferroptosis. Eur J Cell Biol. 2020;99:151058. [PubMed] [DOI] |
| 15. | McComb S, Chan PK, Guinot A, Hartmannsdottir H, Jenni S, Dobay MP, Bourquin JP, Bornhauser BC. Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or -7. Sci Adv. 2019;5:eaau9433. [PubMed] [DOI] |
| 16. | El Hout M, Dos Santos L, Hamaï A, Mehrpour M. A promising new approach to cancer therapy: Targeting iron metabolism in cancer stem cells. Semin Cancer Biol. 2018;53:125-138. [PubMed] [DOI] |
| 17. | Cao X, Wen P, Fu Y, Gao Y, Qi X, Chen B, Tao Y, Wu L, Xu A, Lu H, Zhao G. Radiation induces apoptosis primarily through the intrinsic pathway in mammalian cells. Cell Signal. 2019;62:109337. [PubMed] [DOI] |
| 18. | Li L, Thakur K, Cao YY, Liao BY, Zhang JG, Wei ZJ. Anticance-rous potential of polysaccharides sequentially extracted from Polygonatum cyrtonema Hua in Human cervical cancer Hela cells. Int J Biol Macromol. 2020;148:843-850. [PubMed] [DOI] |
| 19. | Laubach V, Kaufmann R, Bernd A, Kippenberger S, Zöller N. Extrinsic or Intrinsic Apoptosis by Curcumin and Light: Still a Mystery. Int J Mol Sci. 2019;20. [PubMed] [DOI] |
| 20. | Du J, Wang T, Li Y, Zhou Y, Wang X, Yu X, Ren X, An Y, Wu Y, Sun W, Fan W, Zhu Q, Wang Y, Tong X. DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic Biol Med. 2019;131:356-369. [PubMed] [DOI] |
| 21. | Wang YQ, Chang SY, Wu Q, Gou YJ, Jia L, Cui YM, Yu P, Shi ZH, Wu WS, Gao G, Chang YZ. The Protective Role of Mitochondrial Ferritin on Erastin-Induced Ferroptosis. Front Aging Neurosci. 2016;8:308. [PubMed] [DOI] |
| 22. | Geng N, Shi BJ, Li SL, Zhong ZY, Li YC, Xua WL, Zhou H, Cai JH. Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur Rev Med Pharmacol Sci. 2018;22:3826-3836. [PubMed] [DOI] |
| 23. | Conrad M, Pratt DA. The chemical basis of ferroptosis. Nat Chem Biol. 2019;15:1137-1147. [PubMed] [DOI] |
| 24. | Hassan W, Noreen H, Khalil S, Hussain A, Rehman S, Sajjad S, Rahman A, da Rocha JB. Ethanolic extract of Nigella sativa protects Fe(II) induced lipid peroxidation in rat's brain, kidney and liver homogenates. Pak J Pharm Sci. 2016;29:231-237. [PubMed] |
| 25. | Barrera G, Pizzimenti S, Ciamporcero ES, Daga M, Ullio C, Arcaro A, Cetrangolo GP, Ferretti C, Dianzani C, Lepore A, Gentile F. Role of 4-hydroxynonenal-protein adducts in human diseases. Antioxid Redox Signal. 2015;22:1681-1702. [PubMed] [DOI] |
| 26. | Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Bayır H. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81-90. [PubMed] [DOI] |
| 27. | Weinlich R, Oberst A, Beere HM, Green DR. Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol. 2017;18:127-136. [PubMed] [DOI] |
| 28. | Liu X, Li Y, Peng S, Yu X, Li W, Shi F, Luo X, Tang M, Tan Z, Bode AM, Cao Y. Epstein-Barr virus encoded latent membrane protein 1 suppresses necroptosis through targeting RIPK1/3 ubiquitination. Cell Death Dis. 2018;9:53. [PubMed] [DOI] |
| 29. | Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347-364. [PubMed] [DOI] |
| 30. | Petrie EJ, Czabotar PE, Murphy JM. The Structural Basis of Necroptotic Cell Death Signaling. Trends Biochem Sci. 2019;44:53-63. [PubMed] [DOI] |
| 31. | Zhang T, Yin C, Boyd DF, Quarato G, Ingram JP, Shubina M, Ragan KB, Ishizuka T, Crawford JC, Tummers B, Rodriguez DA, Xue J, Peri S, Kaiser WJ, López CB, Xu Y, Upton JW, Thomas PG, Green DR, Balachandran S. Influenza Virus Z-RNAs Induce ZBP1-Mediated Necroptosis. Cell. 2020;180:1115-1129.e13. [PubMed] [DOI] |
| 32. | Zhao Y, Li Y, Zhang R, Wang F, Wang T, Jiao Y. The Role of Erastin in Ferroptosis and Its Prospects in Cancer Therapy. Onco Targets Ther. 2020;13:5429-5441. [PubMed] [DOI] |
| 33. | Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17:2054-2081. [PubMed] [DOI] |
| 34. | Wei X, Yi X, Zhu XH, Jiang DS. Posttranslational Modifications in Ferroptosis. Oxid Med Cell Longev. 2020;2020:8832043. [PubMed] [DOI] |
| 35. | Doran AC, Yurdagul A, Tabas I. Efferocytosis in health and disease. Nat Rev Immunol. 2020;20:254-267. [PubMed] [DOI] |
| 36. | Schwabe RF, Luedde T. Apoptosis and necroptosis in the liver: a matter of life and death. Nat Rev Gastroenterol Hepatol. 2018;15:738-752. [PubMed] [DOI] |
| 37. | Pitman KE, Alluri SR, Kristian A, Aarnes EK, Lyng H, Riss PJ, Malinen E. Influx rate of (18)F-fluoroaminosuberic acid reflects cystine/glutamate antiporter expression in tumour xenografts. Eur J Nucl Med Mol Imaging. 2019;46:2190-2198. [PubMed] [DOI] |
| 38. | Chang TK, Shravage BV, Hayes SD, Powers CM, Simin RT, Wade Harper J, Baehrecke EH. Uba1 functions in Atg7- and Atg3-independent autophagy. Nat Cell Biol. 2013;15:1067-1078. [PubMed] [DOI] |
| 39. | Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, Roveri A, Peng X, Porto Freitas F, Seibt T, Mehr L, Aichler M, Walch A, Lamp D, Jastroch M, Miyamoto S, Wurst W, Ursini F, Arnér ESJ, Fradejas-Villar N, Schweizer U, Zischka H, Friedmann Angeli JP, Conrad M. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell. 2018;172:409-422.e21. [PubMed] [DOI] |
| 40. | Chang LC, Chiang SK, Chen SE, Yu YL, Chou RH, Chang WC. Heme oxygenase-1 mediates BAY 11-7085 induced ferroptosis. Cancer Lett. 2018;416:124-137. [PubMed] [DOI] |
| 41. | Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA. 2016;113:E4966-E4975. [PubMed] [DOI] |
| 42. | Sato M, Kusumi R, Hamashima S, Kobayashi S, Sasaki S, Komiyama Y, Izumikawa T, Conrad M, Bannai S, Sato H. The ferroptosis inducer erastin irreversibly inhibits system x(c)- and synergizes with cisplatin to increase cisplatin's cytotoxicity in cancer cells. Sci Rep. 2018;8:968. [PubMed] [DOI] |
| 43. | Wang SJ, Li D, Ou Y, Jiang L, Chen Y, Zhao Y, Gu W. Acetylation Is Crucial for p53-Mediated Ferroptosis and Tumor Suppression. Cell Rep. 2016;17:366-373. [PubMed] [DOI] |
| 44. | Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57-62. [PubMed] [DOI] |
| 45. | Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS, Stockwell BR. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. [PubMed] [DOI] |
| 46. | Ooko E, Saeed ME, Kadioglu O, Sarvi S, Colak M, Elmasaoudi K, Janah R, Greten HJ, Efferth T. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine. 2015;22:1045-1054. [PubMed] [DOI] |
| 47. | Imai H, Matsuoka M, Kumagai T, Sakamoto T, Koumura T. Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis. Curr Top Microbiol Immunol. 2017;403:143-170. [PubMed] [DOI] |
| 48. | Zhang L, Liu W, Liu F, Wang Q, Song M, Yu Q, Tang K, Teng T, Wu D, Wang X, Han W, Li Y. IMCA Induces Ferroptosis Mediated by SLC7A11 through the AMPK/mTOR Pathway in Colorectal Cancer. Oxid Med Cell Longev. 2020;2020:1675613. [PubMed] [DOI] |
| 49. | Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol Cell. 2015;59:298-308. [PubMed] [DOI] |
| 50. | Jennis M, Kung CP, Basu S, Budina-Kolomets A, Leu JI, Khaku S, Scott JP, Cai KQ, Campbell MR, Porter DK, Wang X, Bell DA, Li X, Garlick DS, Liu Q, Hollstein M, George DL, Murphy ME. An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model. Genes Dev. 2016;30:918-930. [PubMed] [DOI] |
| 51. | Ou Y, Wang SJ, Li D, Chu B, Gu W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci USA. 2016;113:E6806-E6812. [PubMed] [DOI] |
| 52. | Wang Y, Wei Z, Pan K, Li J, Chen Q. The function and mechanism of ferroptosis in cancer. Apoptosis. 2020;25:786-798. [PubMed] [DOI] |
| 53. | Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273-285. [PubMed] [DOI] |
| 54. | Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338-1343. [PubMed] [DOI] |
| 55. | Gong Y, Wang N, Liu N, Dong H. Lipid Peroxidation and GPX4 Inhibition Are Common Causes for Myofibroblast Differentiation and Ferroptosis. DNA Cell Biol. 2019;38:725-733. [PubMed] [DOI] |
| 56. | Yant LJ, Ran Q, Rao L, Van Remmen H, Shibatani T, Belter JG, Motta L, Richardson A, Prolla TA. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med. 2003;34:496-502. [PubMed] [DOI] |
| 57. | Sun X, Niu X, Chen R, He W, Chen D, Kang R, Tang D. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology. 2016;64:488-500. [PubMed] [DOI] |
| 58. | Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, Bot BM, Morris JS, Simon IM, Gerster S, Fessler E, De Sousa E Melo F, Missiaglia E, Ramay H, Barras D, Homicsko K, Maru D, Manyam GC, Broom B, Boige V, Perez-Villamil B, Laderas T, Salazar R, Gray JW, Hanahan D, Tabernero J, Bernards R, Friend SH, Laurent-Puig P, Medema JP, Sadanandam A, Wessels L, Delorenzi M, Kopetz S, Vermeulen L, Tejpar S. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350-1356. [PubMed] [DOI] |
| 59. | Stockwell BR, Jiang X. A Physiological Function for Ferroptosis in Tumor Suppression by the Immune System. Cell Metab. 2019;30:14-15. [PubMed] [DOI] |
| 60. | Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, Xia H, Zhou J, Li G, Li J, Li W, Wei S, Vatan L, Zhang H, Szeliga W, Gu W, Liu R, Lawrence TS, Lamb C, Tanno Y, Cieslik M, Stone E, Georgiou G, Chan TA, Chinnaiyan A, Zou W. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270-274. [PubMed] [DOI] |
| 61. | Yang C, Huang S, Cao F, Zheng Y. Role of ferroptosis-related genes in prognostic prediction and tumor immune microenvironment in colorectal carcinoma. PeerJ. 2021;9:e11745. [PubMed] [DOI] |
| 62. | Liao P, Wang W, Wang W, Kryczek I, Li X, Bian Y, Sell A, Wei S, Grove S, Johnson JK, Kennedy PD, Gijón M, Shah YM, Zou W. CD8(+) T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell. 2022;40:365-378.e6. [PubMed] [DOI] |
| 63. | Xu M, Tao J, Yang Y, Tan S, Liu H, Jiang J, Zheng F, Wu B. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis. 2020;11:86. [PubMed] [DOI] |
| 64. | Wang S, Liu W, Wang J, Bai X. Curculigoside inhibits ferroptosis in ulcerative colitis through the induction of GPX4. Life Sci. 2020;259:118356. [PubMed] [DOI] |
| 65. | Chen Y, Zhang P, Chen W, Chen G. Ferroptosis mediated DSS-induced ulcerative colitis associated with Nrf2/HO-1 signaling pathway. Immunol Lett. 2020;225:9-15. [PubMed] [DOI] |
| 66. | Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479-507. [PubMed] [DOI] |
| 67. | Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13:165. [PubMed] [DOI] |
| 68. | Wang Y, Zheng L, Shang W, Yang Z, Li T, Liu F, Shao W, Lv L, Chai L, Qu L, Xu Q, Du J, Liang X, Zeng J, Jia J. Wnt/beta-catenin signaling confers ferroptosis resistance by targeting GPX4 in gastric cancer. Cell Death Differ. 2022;29:2190-2202. [PubMed] [DOI] |
| 69. | Luo Y, Huang S, Wei J, Zhou H, Wang W, Yang J, Deng Q, Wang H, Fu Z. Long noncoding RNA LINC01606 protects colon cancer cells from ferroptotic cell death and promotes stemness by SCD1-Wnt/β-catenin-TFE3 feedback loop signalling. Clin Transl Med. 2022;12:e752. [PubMed] [DOI] |
| 70. | Liu DS, Duong CP, Haupt S, Montgomery KG, House CM, Azar WJ, Pearson HB, Fisher OM, Read M, Guerra GR, Haupt Y, Cullinane C, Wiman KG, Abrahmsen L, Phillips WA, Clemons NJ. Inhibiting the system x(C)(-)/glutathione axis selectively targets cancers with mutant-p53 accumulation. Nat Commun. 2017;8:14844. [PubMed] [DOI] |
| 71. | Tarangelo A, Magtanong L, Bieging-Rolett KT, Li Y, Ye J, Attardi LD, Dixon SJ. p53 Suppresses Metabolic Stress-Induced Ferroptosis in Cancer Cells. Cell Rep. 2018;22:569-575. [PubMed] [DOI] |
| 72. | Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J, Zhong M, Yuan H, Zhang L, Billiar TR, Lotze MT, Zeh HJ, Kang R, Kroemer G, Tang D. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 2017;20:1692-1704. [PubMed] [DOI] |
| 73. | La Vecchia S, Sebastián C. Metabolic pathways regulating colorectal cancer initiation and progression. Semin Cell Dev Biol. 2020;98:63-70. [PubMed] [DOI] |
| 74. | Han L, Yan Y, Fan M, Gao S, Zhang L, Xiong X, Li R, Xiao X, Wang X, Ni L, Tong D, Huang C, Cao Y, Yang J. Pt3R5G inhibits colon cancer cell proliferation through inducing ferroptosis by down-regulating SLC7A11. Life Sci. 2022;306:120859. [PubMed] [DOI] |
| 75. | Sui X, Zhang R, Liu S, Duan T, Zhai L, Zhang M, Han X, Xiang Y, Huang X, Lin H, Xie T. RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer. Front Pharmacol. 2018;9:1371. [PubMed] [DOI] |
| 77. | Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L, Liao P, Zhou J, Zhang Q, Dow A, Saripalli AL, Kryczek I, Wei S, Szeliga W, Vatan L, Stone EM, Georgiou G, Cieslik M, Wahl DR, Morgan MA, Chinnaiyan AM, Lawrence TS, Zou W. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov. 2019;9:1673-1685. [PubMed] [DOI] |
| 78. | Yan H, Talty R, Jain A, Cai Y, Zheng J, Shen X, Muca E, Paty PB, Bosenberg MW, Khan SA, Johnson CH. Discovery of decreased ferroptosis in male colorectal cancer patients with KRAS mutations. Redox Biol. 2023;62:102699. [PubMed] [DOI] |
| 79. | Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors. 2011;29:196-202. [PubMed] [DOI] |
| 80. | Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018;18:128-134. [PubMed] [DOI] |
| 81. | Sun L, Dong H, Zhang W, Wang N, Ni N, Bai X, Liu N. Lipid Peroxidation, GSH Depletion, and SLC7A11 Inhibition Are Common Causes of EMT and Ferroptosis in A549 Cells, but Different in Specific Mechanisms. DNA Cell Biol. 2021;40:172-183. [PubMed] [DOI] |
| 82. | Chen P, Li X, Zhang R, Liu S, Xiang Y, Zhang M, Chen X, Pan T, Yan L, Feng J, Duan T, Wang D, Chen B, Jin T, Wang W, Chen L, Huang X, Zhang W, Sun Y, Li G, Kong L, Chen X, Li Y, Yang Z, Zhang Q, Zhuo L, Sui X, Xie T. Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics. 2020;10:5107-5119. [PubMed] [DOI] |