修回日期: 2020-08-13
接受日期: 2020-09-21
在线出版日期: 2020-10-28
生物节律起源于生物钟, 是机体为适应外界环境的昼夜变化而进化成的一种内在变化节律. 在其影响下, 哺乳动物具有了明显的进食和禁食周期, 产生了营养物质供需的节律性变化. 近年来, 大量研究表明生物节律与机体代谢有着紧密联系, 而肝脏作为机体的代谢中枢, 其功能必然受到昼夜节律的影响. 随着现代社会节奏的加快, 熬夜、轮班、快餐等生活方式打乱了机体原本节律, 导致肝脏相关的代谢性疾病发病率大大增加, 而昼夜节律的紊乱又可促进这些疾病的发生、发展, 并影响其预后和转归. 本文就肝脏生物钟基因的功能与糖、脂质、胆汁酸、蛋白质等代谢物质间的关系展开综述.
核心提要: 肝脏作为全身新陈代谢的枢纽, 其功能包括调节碳水化合物、脂肪、蛋白质的代谢,胆汁合成, 造血凝血及解毒等多个方面. 近年来研究证实, 肝脏内进行的各色生理活动几乎都受到生物钟的控制, 而生物节律的紊乱则会引起肝脏新陈代谢及相应功能的紊乱, 严重时可发展为代谢性疾病. 随着现代生活节奏加快, 工作休息节律与生理节律的去同步化已成常态, 相关代谢疾病的发病率逐年攀升并呈年轻化趋势, 故深入研究生物节律与肝脏代谢显得十分必要. 本文结合近几年的相关科学报道, 主要介绍了肝脏生物节律的来源和调控机制, 总结了生理和病理条件下肝脏生物节律的变化, 重点阐述了他们在调节葡萄糖、脂质、胆汁酸等代谢过程中所发挥的作用以及对某些疾病发生发展的潜在影响.
引文著录: 高文康, 舒艳芸, 叶进, 潘晓莉. 生物节律与肝脏能量代谢. 世界华人消化杂志 2020; 28(20): 1025-1035
Revised: August 13, 2020
Accepted: September 21, 2020
Published online: October 28, 2020
Circadian rhythm, generated by the circadian clock, is an internal rhythm that the body evolved to adapt to the diurnal changes in the external environment. Under its influence, mammals have distinct feeding and fasting cycles, which cause rhythmic changes in nutrient supply and demand. In recent years, many studies have shown that biorhythms are closely related to body metabolism. The liver, as the metabolism center of the body, is affected by circadian rhythm. However, with the acceleration of the pace of modern life and the change of life styles, the body's original rhythm is disrupted, resulting in a significant increase in the incidence of liver related metabolic diseases. Meanwhile, the disorder of circadian rhythm can also promote the occurrence and development of these diseases, and affect their prognosis and outcome. This paper reviews the relationship between the function of liver clock genes and the metabolism of liver glucose, lipids, bile acids, protein, etc.
- Citation: Gao WK, Shu YY, Ye J, Pan XL. Circadian clock and liver energy metabolism. Shijie Huaren Xiaohua Zazhi 2020; 28(20): 1025-1035
- URL: https://www.wjgnet.com/1009-3079/full/v28/i20/1025.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v28.i20.1025
自生命诞生以来, 生物体便开始不断进化以适应地球自转导致的环境改变, 尤其是阳光和温度的日变化[1] .而生命体的功能也表现出以24 h为周期的节律性波动, 比如清晨心率血压升高, 夜晚逐渐下降, 我们把这些生理活动的改变称为昼夜节律, 它起源于一种内源性计时系统(即生物钟)[2]. 机体内诸如睡眠-觉醒周期、体温、血压、激素和代谢等都受到昼夜节律调节[3,4].
肝脏作为全身新陈代谢的枢纽, 其功能包括调节碳水化合物、脂肪、蛋白质的代谢, 胆汁合成, 造血凝血及解毒等多个方面[5]. 研究证实, 肝脏内进行的各色生理活动几乎都受到生物钟的控制, 而生物节律的紊乱则会引起肝脏新陈代谢及相应功能的紊乱, 严重时可发展为代谢性疾病[6-9]. 随着现代生活节奏加快, 工作休息节律与生理节律的去同步化已成常态, 相关代谢疾病的发病率逐年攀升并呈年轻化趋势[9], 故深入研究生物节律与肝脏代谢显得十分必要. 本文结合近几年的相关科学报道, 主要介绍了肝脏生物节律的来源和调控机制, 总结了生理和病理条件下肝脏生物节律的变化, 重点阐述了他们在调节葡萄糖、脂质、胆汁酸等代谢过程中所发挥的作用以及对某些疾病发生发展的潜在影响.
哺乳动物的生活几乎无时不刻地受到生物节律的调控, 譬如睡眠觉醒周期[10,11]. 它使机体适应白天和黑夜, 让不同的组织器官在不同的时间段上发挥着各自的功能, 那么机体是如何被赋予时间特性的呢?
早在20世纪60年代, 时间生物学创始人之一Colin Pittendrigh就曾假设昼夜节律系统是由光敏感的"起搏器"时钟和外周从属的振荡器所组成[12], 后来的研究也证实这一观点. 哺乳动物视网膜感光器(视杆细胞和视锥细胞)将光能转化为电脉冲, 并通过视网膜神经节细胞将其传递至大脑, 表达光色素黑素的视网膜神经节细胞的子细胞对可见光谱具有特定的敏感性, 并直接将光信号传递到下丘脑区域, 即视交叉上核(suprachiasmatic nucleus, SCN). 之后通过体液和神经调节, SCN将"时间信息"(也称为"给时器")传递给其他组织器官以及细胞, 从而实现外周组织时间上的同步化[13]. 因此, 从解剖学上讲, 哺乳动物产生昼夜节律的系统是分级的, 通常将受光照限制的SCN称为核心生物钟, 它为机体内所有其他外围时钟设定了基线时间. 最近几年, SCN的网络拓扑结构和其功能已成为人们日益关注的话题. 已知人类的SCN大约由100000个神经元组成, 密布在SCN的中心和外周区域, 它们具有不同的联系方式, 神经递质谱和昼夜节律相位, 但是, 不同类型的神经元在SCN中如何分布, 相互之间如何识别以及对昼夜节律产生中所发挥的功能作用尚未可知, 有待研究[14].
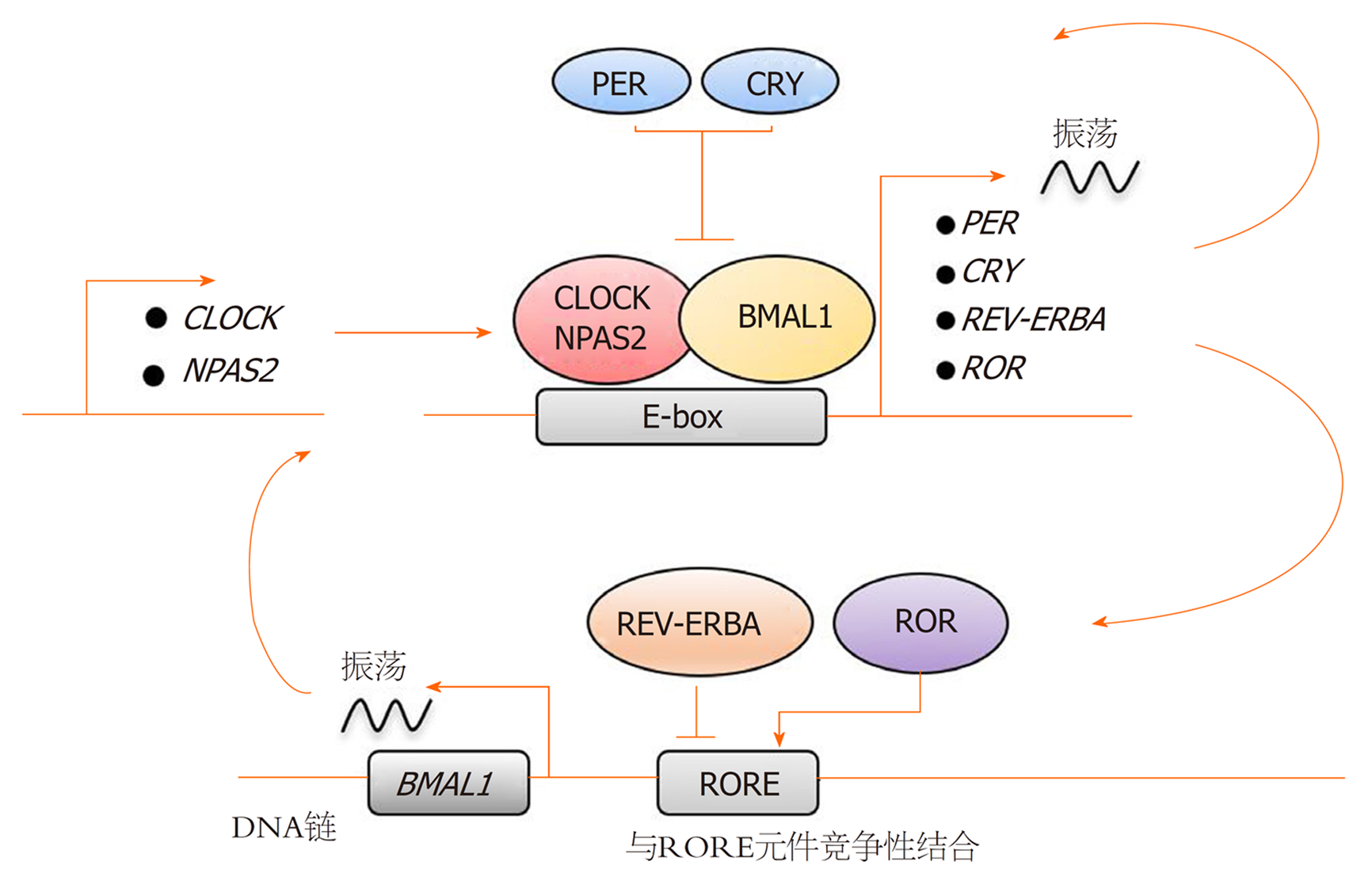
生物钟是一种细胞自主的分子机制, 在分子水平上也是有层次的运行. 细胞节律震荡使大量基因有节律地表达, 导致机体生理和行为的明显改变[15]. 研究发现人体所有细胞中的昼夜节律振荡依赖由生物钟基因组成的转录-翻译反馈环[16]的调控(图1). 其中一条研究较为清楚的反馈环路涉及时钟基因(Period, Per)和隐花色素基因(Cryptochrome, Cry), 他们由转录激活因子CLOCK (circadian locomotor output cycles kaput), NPAS2 (neuronal Per-Arnt-Sim domain protein 2)和ARNT1 (aryl hydrocarbon receptor nuclear translocatorlike protein 1, 也称为BMAL1)激活, 形成CLOCK-BMAL1和NPAS2-BMAL1异二聚体, 这些复合物与启动子区的E-box元件(E-box elements)结合, 激活Per和Cry基因的转录, 其翻译得到的Per和Cry蛋白又被导入细胞核并抑制自身基因的转录[16-18], 随后新的昼夜节律周期开始. 第二个反馈环路则使得振荡机制更加稳固, 它由核受体亚家族1D (nuclear receptor subfamily 1 group D member, NR1D, 亦称为REV-ERBA)与RAR相关的孤儿受体(RAR related orphan receptor, ROR)家族组成, 它们同样由核心时钟激活, 并竞争性结合ROR反应元件结合位点(ROR response element binding sites, RORE), 以调控BMAL1基因的节律性表达. 其中, REV-ERBA的结合抑制BMAL1转录, 属于负调控因子; 而ROR结合启动子区域可促进BMAL1的转录[19]. 此外, 最近研究还发现DECs的表达发挥了时钟基因的功能, 并且DECs可以通过与BMAL1结合或与CLOCK-BMAL1竞争结合E-box位点来抑制自身转录[20], 这形成了第三条自主反馈环路. 总之, 这些复杂的反馈回路产生了大约24 h的周期节律[21], 我们称之为昼夜节律.
核心钟基因可控制器官特异性的转录因子的节律性表达, 再通过这些特异性转录因子控制主要代谢调节因子及相关酶的表达[5]. 肝脏在维持全身代谢机能中发挥着不可替代的作用, 于是生物钟与肝脏功能的关系受到广泛关注. 研究人员通过对小鼠进行一些高通量的生物节律过程研究, 以明确生物钟是否参与调节肝脏生理功能. 这些研究涉及了顺反组[22-25]、转录组[26,27]、蛋白质组[28-30]和脂质组[31,32]等多个方面. 其中, 顺反组分析[22-25]显示这两个不同的mRNA谱系来源于昼夜节律振荡器, 可以定期募集或移除转录因子和共调节因子, 通过表观遗传修饰改变生物钟钟控基因(clock-controlled gene, CCG)的染色质状态, 从而调控基因转录以控制生物体节律. 转录组分析揭示了肝脏中两个明显的转录峰, 它们分别与休息期和活动期相对应[33-37], 这很可能反应机体高度不同的生理需求, 比如肝脏介导的能量代谢和解毒活动. DNA修复, 核糖体生物发生, 自噬和内质网应激等细胞过程也受到昼夜调节, 一般集中在翻译后水平[28-30]. 总而言之, 这些研究大体揭示了生物钟对肝脏功能生理水平上的调控, 而调节失控则表现出有助于非酒精性脂肪性肝病(non-alcoholic fatty liver disease, NAFLD)及其他代谢疾病发展的势头.
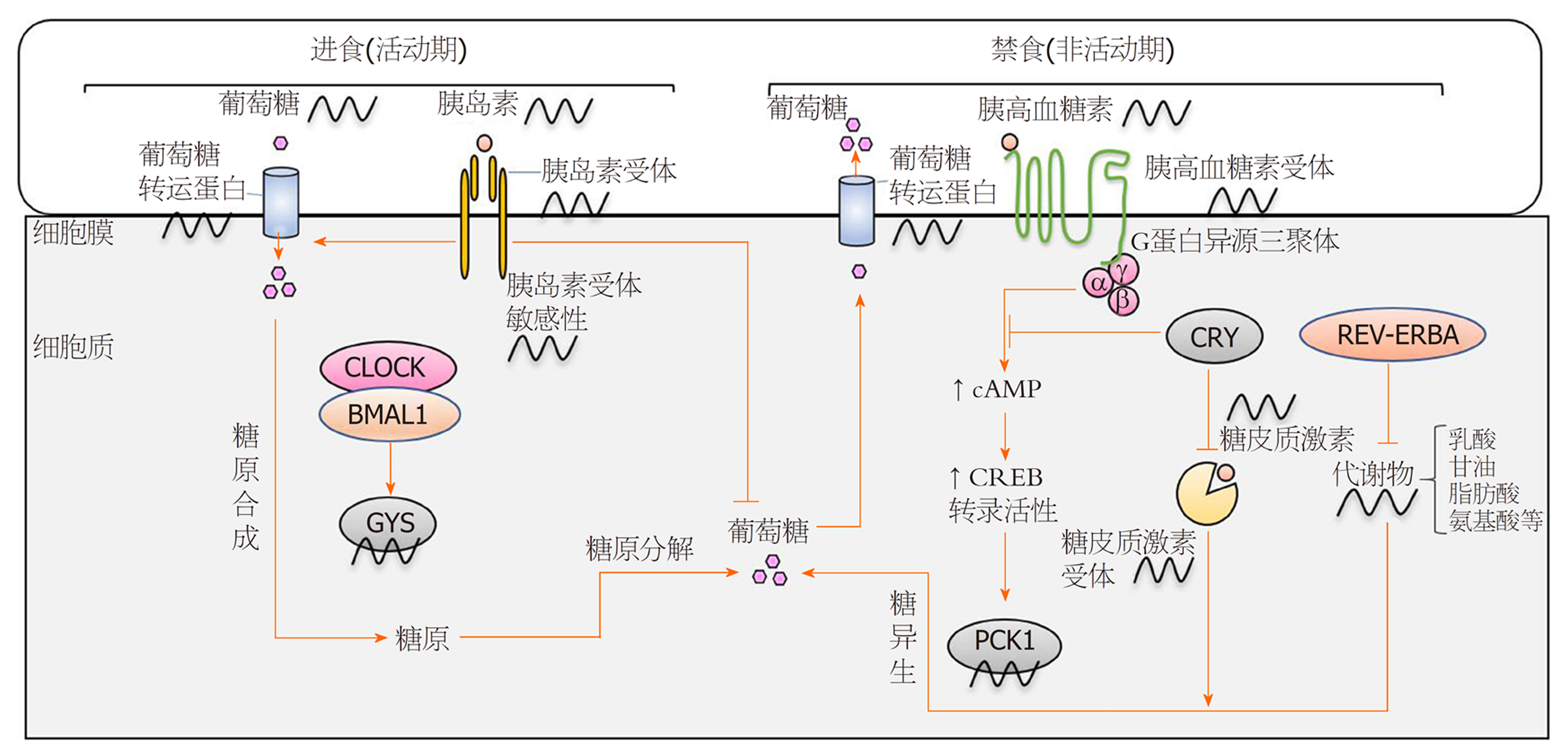
肝脏是哺乳动物调节糖代谢的主要器官, 并与脑、胰腺、骨骼肌等器官协同维持体内血糖水平稳定[5]. 虽然直接的葡萄糖信号是机体适应血糖快速变化的关键, 但是生物钟似乎为此提供了有节奏的基线调节. 譬如, 夜间饥饿后的食物摄取[38]. 研究表明, 生物钟通过同步各组织相关的糖代谢活动来维持葡萄糖稳态, 其中大脑中的中枢生物钟控制着休息-活动及饮食-禁食的节律, 产生节律性的营养吸收和信号传递, 而肝脏外周生物钟的作用可能是缓冲由这些节律性行为所引起血糖水平的周期性波动[39].
在机体活动期, 葡萄糖转运蛋白(glucose transporter, Glut)和胰岛素受体的表达水平达到峰值[27,40], 以应对摄食引起的血糖升高. 在葡萄糖进入肝细胞后, 即被磷酸化生成葡萄糖-6-磷酸(glucose-6-Phosphate, G6P), 该产物可通过糖酵解或磷酸戊糖旁路被消耗掉, 当葡萄糖摄入过量时也可以通过糖原复合物的形式储存起来, 生物钟可调控上述所有过程[34,41]. 进入休息期, 体内葡萄糖水平下降, 通常糖皮质激素可以刺激糖异生以产生葡萄糖维持机体所需, 而Cry可通过蛋白质间的相互作用抑制糖皮质激素受体来调控这一过程[42,43]. 此外, 隐花色素进一步调控糖异生以及胰高血糖素受体的信号转导, 主要机制如下: 胰高血糖素受体激活后通过G蛋白异源三聚体传递信号, 刺激环磷酸腺苷(cyclic adenosine monophosphate, cAMP)的产生, cAMP可介导环磷腺苷效应元件结合蛋白(cAMP-response element binding protein, CREB)的节律性磷酸化及转录活性[44], 进而CREB调节糖异生的关键酶--磷酸烯醇式丙酮酸羧激酶1 (phosphoenolpyruvate carboxykinase, PCK1)的表达[45]. 隐花色素因负调控G蛋白异源三聚体从而抑制cAMP的积累, 间接降低了PCK1的表达(图2).
另外, 葡萄糖代谢还受到核受体REV-ERBA的调控[46]. REV-ERBA是核心生物钟的组分之一, 并且是肝生物钟及机体代谢的中央调节器[47]. REV-ERBA通过CLOCK-BMAL1依赖的转录激活而有节奏的表达, 它与RORα和RORγ竞争结合时钟基因启动子区域中的RORE和DevDR2元件, 以反馈调控核心钟基因的表达[48]. 此外, REV-ERBA在靶基因启动子区的结合位点与肝特异性转录因子--肝细胞核因子(hepatocyte nuclear factor, HNF) 4α或HNF6结合, 通过组蛋白去乙酰基酶3 (histone deacetylase 3, HDAC3)及其他共阻遏物调节转录基因代谢, 例如, 长链脂肪酸延长酶5 (Elongation of very long chain fatty acids protein 5, ELOVL5)或酰基辅酶A合成酶短链家族成员3 (acyl-CoA synthetase short chain family member 3, ACSS3)[49]. 由此可见, 肝脏生物钟通过多种机制协同维持生理状态下的葡萄糖水平.
我们知道, 机体内葡萄糖水平与胰岛素密切相关, 而胰岛素抵抗是2型糖尿病、非酒精性脂肪性肝病等代谢性疾病中关键的病理生理过程, 所以不难得到这样的假设, 即生物节律的紊乱可能是导致胰岛素抵抗的重要因素. 其中, 最早的证据出现在上个世纪60年代, 人们发现2型糖尿病患者糖耐量日节律的改变[50]. 随后的研究表明时钟基因突变小鼠会患代谢综合征[51], 非正常时间段(睡眠阶段)摄食会导致小鼠肥胖[52], 昼夜节律失调导致人糖耐量下降[53]等, 这些结论促生了昼夜节律假说的提出, 尤其对小鼠组织(如肝脏[38,54]、胰腺[55-57]、肌肉[58,59]等)特异性基因敲除模型的研究进一步支持该假说. 最近几年实验还观察到夜间光线暴露对葡萄糖代谢的影响. 比如夜间昏暗的光线可以扰乱小鼠日常进食和行为活动节律, 导致其肥胖和糖耐量降低, 但在大鼠中尚未发现[60-62]; 2017年一项对大鼠的研究报告发现环境光的波长会对葡萄糖耐量产生影响: 白光, 绿光可以降低糖耐量而非蓝光, 红光[63]; 人类的观察结果与啮齿类动物实验一致, 如明亮的环境光可直接降低健康个体胰岛素敏感性[64]; 健康受试者在夜间保持清醒时, 明亮的光线会增加其血浆葡萄糖水平[65]; 另外, 睡眠障碍, 社交时差及轮班工作等常见的可引起人类节律紊乱的原因也被证实会增加代谢性疾病的风险. 如节律性睡眠障碍患者的睡眠不足可导致其糖耐量降低和高血糖[66]; 轮班工人罹患2型糖尿病的风险增加, 增加程度与上夜班的次数相关[67]; 昼夜节律失调降低了非轮班工人和慢性轮班工人的葡萄糖耐量和胰岛素敏感性[68-70]. 综上可见, 无论何种原因导致的时钟节律紊乱均在组织水平上促进了胰岛素抵抗, 也促进了相关代谢性疾病的发生发展. 对于胰岛素抵抗的治疗,一方面人们试图通过改善光照, 调节睡眠时间等恢复机体正常的节律, 另一方面也期待可以找到提高生物钟基因表达量的分子来降低血糖水平并减轻饮食诱导的肥胖[71]. 就目前而言, REV-ERBα激动剂SR9011及REV-ERBβ激动剂SR9009是具有希望的药物, 此外ROR激动剂和CRY稳定剂也被证明对治疗代谢性疾病有一定作用[72-74], 它们在人体I期药物研究值得期待.
肝脏是胆固醇转化为胆汁酸(bile acids, BAs)的主要场所, 肝细胞合成的胆汁酸主要促进肠道营养吸收, 此外它还是一种重要的信号分子[75,76], 具有旁分泌和内分泌功能[77]. 譬如它是法尼醇受体(farnesoid X receptor, FXR)以及G蛋白偶联受体TGR5 (G-protein-coupled receptor TGR5)的生理性配体, 能激活一些信号通路(如丝裂原活化蛋白激酶通路[75,76]). 通过调节这些不同的信号网络, 胆汁酸不但能调节自身水平, 也能调控甘油三酯, 胆固醇和葡萄糖水平[75,76].
研究发现, 胆汁酸的合成, 调节胆汁酸的关键酶以及核受体均随昼夜节律而发生明显改变[78-80]. 其中, 胆汁酸合成主要受转录反馈环的调控, 该环路主要组分包括核受体FXR, SHP (small heterodimer partner)[81]以及肠成纤维细胞生长因子15 (intestinal fibroblast growth factor 15, FGF15, 在人类中是FGF19)[82,83], 生物钟均参与调控上述组分[84], 并共同驱使胆汁酸经典合成通路中的限速酶--胆固醇7α羟化酶(cholesterol 7-alpha -hydroxylase, Cyp7a1)的节律性转录. 但FXR的节律对调节肝脏胆汁酸稳态贡献度大小仍然存在争议, 譬如在FXR缺失的小鼠和肠道特异性FXR-/-小鼠中, 无法检测到FGF15和有机溶质转运体α (organic solute transporter alpha, OSTα)[84,85], 但是Cyp7a1表达的昼夜节律在这两种模型中并没有改变. 猜测除FXR外, 可能存在其他途径参与胆汁酸稳态的调节. 此外, Cyp7a1的表达还受到生物钟基因的调控. 如肝脏CLOCK调控的, 富含脯氨酸和酸性氨基酸(proline and acidic amino acid rich, PAR)的碱性亮氨酸拉链转录因子DBP控制着Cyp7a1的转录, 在时间上限制其表达[80]. REV-ERBα也是调节胆固醇和胆汁酸代谢的关键因子, 在REV-ERBα缺乏的小鼠中, Cyp7a1的节律表达受到抑制[86,87], 其机制可能是通过调节E4bp4和Shp基因的转录, 来调控Cyp7a1的表达[86]. 最近的研究发现胆汁酸还是潜在的时间生物学信号, 可以反过来影响生物钟分子. 如2016年Govindarajan等[88]人发现非结合胆汁酸显著改变了回结肠以及肝脏生物钟基因的表达水平, 其中肝脏的昼夜节律调节器(如DBP)和相关基因(如Per2, Per3和Cry2)的表达发生了显著改变. 随后人们又发现进食可诱导胆汁酸池组分的变化进而影响生物钟分子[89]. 总之, 这些机制共同作用使得机体在胆汁酸水平上产生了昼夜节律[90].
昼夜节律调节胆汁酸稳态对于实现机体生理平衡至关重要, 一旦失调(如限制进食、睡眠剥夺)就很可能导致胆汁淤积和代谢性疾病的发生[91]. 有研究发现, 限时喂养(time-restricted feeding, TRF)可以扰乱肝脏Cyp7a1 mRNA的节律表达, 诱导血浆胆汁酸水平和组成的改变, 影响脂质代谢, 并导致代谢紊乱的发生[89,92]. 而睡眠中断会诱导脂质蓄积和动员的失调, 破坏胆汁酸稳态, 增加诸如肥胖、胰岛素抵抗等代谢障碍的风险[93]. 尽管越来越多的证据证实胆汁酸水平具有昼夜节律, 但究其运输节律及诱导代谢性疾病的分子机理上(尤其是时间病理学)仍然需要更多研究.
肝脏主要通过调节脂肪酸从头合成及氧化、脂蛋白合成、脂质吸收转化来参与脂质代谢. 由Turek等[51]人开创性研究显示: 生物钟基因突变的小鼠, 血液中胆固醇和甘油三酯含量升高. 此后, 人们对小鼠模型进行了大量有关生物钟的实验.
针对小鼠CLOCK基因的研究发现其可以影响肝脏脂质的代谢. CLOCK突变(Clockmt/mt)小鼠高表达微粒体甘油三酯转移蛋白(microsomal-triglycerid transfer protein, MTP), 其机制主要是由于CLOCK蛋白能够上调小异源二聚体伴侣SHP以负向调控MTP的表达, 其中SHP通过与MTP启动子上的HNF4α/肝核受体同源物1 (liver nuclear receptor homolog 1, LRH-1)结合以抑制MTP的表达[94]. 此外, 在Clockmt/mt小鼠中参与脂质吸收的基因其表达并未显示昼夜节律, 对限制喂养也没有反应[94,95], 而表现出与甘油三酯合成和脂解相关基因节律性表达的改变[96-98].
针对小鼠体内BMAL1的研究发现其也可调节脂肪的合成、分解、储存及利用. 缺乏BMAL1的小鼠表现如下: (1)血浆甘油三酯的日节律紊乱[99]; (2)几种关键的成脂因子表达降低[100], 如PPARγ、脂肪细胞脂肪酸结合蛋白2 (adipocyte fatty acid-binding protein 2, aP2)、CCAAT/增强子结合蛋白α [CCAAT/enhancer-binding protein α, (C/EBP)α]、SREBP-1a和FAS; (3)脂肪组织未表现出脂解基因相关的节律振荡, 如Hsl和Atgl, 表明BMAL1参与脂解过程[97]; (4)脂肪储存和利用方面的损害, 如循环中游离脂肪酸水平增加, 诱导肝脏和骨骼肌中异位脂肪的形成. 此外, 缺乏BMAL1的小鼠呼吸商很高, 意味着BMAL1在利用脂肪参与能量代谢上面发挥了作用[101].
针对小鼠体内BMAL1主要抑制因子REV-ERBα的研究发现, 缺乏REV-ERBα的小鼠表现出脂质代谢和胆汁酸代谢受损[102,103]. 其中, REV-ERBα的调节功能受核受体辅助抑制因子1 (nuclear receptor co-repressor 1, NCoR1)的控制, 它通过激活HDAC3的一个亚基来抑制目标基因(如BMAL1等)的转录[104]. REV-ERBα和NCoR1募集与HDAC3募集同步, 其中HDAC3的募集在明暗时期分别具有高低效率[100]: 在暗期, 低浓度的REV-ERBα降低了HDAC3与肝脏代谢基因的关联, 有利于脂质的生物合成和储存. 在光期, 高水平的REV-ERBα增加了HDAC3与肝脏代谢基因的关联, 从而减少了脂质的生物合成. 一旦小鼠肝脏中REV-ERBα或HDAC3的缺失, 则会导致高甘油三酯血症和肝脏脂肪变性[105].
Per和Cry基因也参与调节脂质代谢, 如缺乏Per1和/或Per2的小鼠血浆甘油三酯水平降低[32,106]. 对Per2敲除小鼠的研究表明, Per2可以阻断PPARγ招募启动子而对其发挥抑制作用[106]. Cry基因缺乏会增加饮食诱导肥胖的易感性, 如高脂饮食的Cry1/2-/-小鼠与野生型小鼠相比更快且更容易出现肥胖, 且其白色脂肪组织中与脂质摄取和脂肪生成相关基因的表达上调, 如Fas, Acc1, Acsl4, Dgat1, Dgat 2等[107].
以上这些实验均发现了脂肪酸、甘油三酯及胆固醇的水平可由生物钟组分的突变而发生改变, 也证实了生物钟基因是脂质代谢的关键调节剂[106,108,109]. 不难发现, 一旦机体昼夜节律紊乱, 必然导致脂质代谢失调从而加速肥胖甚至代谢性疾病的发生发展[9,110-112], 这一观点已在动物身上得到验证. 如CLOCK突变和BMAL1敲除的小鼠都表现出葡萄糖耐量下降, 胰岛素分泌减少, 高脂饮食的敏感度增加, 食欲亢进并且超重[55,113]; Per突变的果蝇可检测到脂质代谢中间体的变化(如二酰甘油和酰基肉碱)以及饥饿敏感性的增加[111]. 此外对于人类而言, 还有其他证据暗示上述观点. 如长期夜间光照导致节律紊乱的患者可伴有脂质代谢紊乱[114]; 吃夜宵的频率与肥胖和体重指数(body mass indexes, BMI)的升高呈正相关[115]; 还有因社交时差导致节律紊乱者, 其平均BMI更高, 脂肪含量也更高[116]. 反过来, 肥胖患者的BMAL1, Cry1, Cry2和Per2基因在明(昼)相表达显著增加, 暗(夜)相表达显著下调, 提示生物钟基因及其下游通路的表达也受到脂质代谢紊乱的反馈影响[117]. 目前对于改善生物节律, 减轻脂肪蓄积降低, 患病风险的策略有如下几点: 首先是限制进食时间, 如对生物钟基因突变小鼠限时喂养(在活动阶段10 h可以进食)可以恢复其新陈代谢节律, 保护其免于肥胖[118]. 其次是营养调节, 改变膳食比例. 如投喂蛋白含量较高, 碳水化合物含量较低的食物可以促进小鼠肝肾多个时钟基因的表达节律, 增加了BMAL1和Cry1的平均表达量[119]; 减少饱和脂肪酸(SFA)的摄入或者增加多不饱和脂肪酸(PUFA)的摄入, 比如Omega-3 PUFA, 它甚至被建议作为NAFLD的潜在治疗剂[120]; 还有最近的DHA[121,122], 它可以克服由高脂饮食引起的小鼠脂质代谢昼夜节律紊乱[123]. 此外, 人为补充一些激素可能具有效果, 如褪黑素可以改善高脂喂养小鼠肠道菌群的昼夜节律[124], 具有一定抗肥胖功效. 不过遗憾的是, 上述种种有前景的措施, 其潜在机制尚未明确, 应用人体效果如何仍需进一步研究.
饮食摄入的蛋白质通常在小肠中被降解为氨基酸, 并被吸收运输到肝脏[125]. 氨基酸可用于糖异生, 合成蛋白质, 生成活性分子(如甲硫氨酸腺苷酸化所产生的SAM)以及降解释放出氨参与尿素循环, 所以细胞中的氨基酸很少在保持游离状态. 在进食状态下, 胰岛素受体底物(insulin receptor substrate, IRS)下游激酶AKT激活mTOR-S6激酶途径以促进蛋白质翻译, AKT或S6K1还可以使BMAL1磷酸化, 并将其招募到翻译复合物中, 促进复合物的活性[126,127]. 由于核糖体生物合成[128]和mRNA特定亚组的优先翻译[129]是受昼夜节律调控的, 所以蛋白质合成也具有普遍节律, 尤其在合成体内许多重要分泌蛋白上(如白蛋白, 视黄醇结合蛋白, 甲状腺素运载蛋白等), 它们对肝脏功能而言特别重要.
在夜间禁食期间, 肌肉和肝细胞中的转录因子KLF15可以介导下游酶的节律表达, 这些酶参与了肌肉中氨基酸的动员, 并在肝脏中用于糖异生和尿素循环中氨的再利用[130]. 因此, 血浆中总氨基酸, 支链氨基酸和尿素的水平在人体中表现出昼夜节律, 并在夜间达到峰值[130]. 当给予KLF15-/-小鼠富含蛋白质饮食时, 它们出现了急性代谢紊乱(如低血糖, 高氨血症和尿素生成受损), 从而证明了其对氨基酸代谢的重要性[130]. 此外, 最近研究发现支链氨基酸可通过PI3K-AKT途径负调控KLF15表达[131], 提示了氨基酸在调节代谢方面的潜在机制.
过去二十年, 人们对昼夜节律, 能量代谢和激素稳态之间关系的认识逐步增加, 进而揭示了核心生物钟在协调周围器官生物钟的作用, 并且还发现了既与昼夜节律相关又与代谢调节相关的若干基因. 可以肯定, 昼夜节律是人类生理平衡的重要组分, 而任何串扰都可能打破这种微妙的平衡, 甚至导致严重的病理改变. 即使目前已证实昼夜节律失调与某些疾病发生率攀升有关, 却也很难评估昼夜节律紊乱在这些疾病发展中所占的比重. 因此, 我们需要更多的研究去深入了解生物节律参与代谢、致病过程的分子途径, 这不仅为治疗提供靶点, 还为预防代谢性疾病创造了机遇, 也有益于改善公众健康, 合理指导疾病的预防和健康保健.
学科分类: 胃肠病学和肝病学
手稿来源地: 湖北省
同行评议报告学术质量分类
A级 (优秀): 0
B级 (非常好): B, B, B, B
C级 (良好): C
D级 (一般): D
E级 (差): 0
科学编辑: 刘继红 制作编辑:刘继红
| 1. | Chung S, Son GH, Kim K. Circadian rhythm of adrenal glucocorticoid: its regulation and clinical implications. Biochim Biophys Acta. 2011;1812:581-591. [PubMed] [DOI] |
| 2. | Rana S, Mahmood S. Circadian rhythm and its role in malignancy. J Circadian Rhythms. 2010;8:3. [PubMed] [DOI] |
| 3. | Caufriez A, Leproult R, Copinschi G. Circadian profiles of progesterone, gonadotropins, cortisol and corticotropin in cycling and postmenopausal women. Chronobiol Int. 2018;35:72-79. [PubMed] [DOI] |
| 4. | Daut RA, Hartsock MJ, Tomczik AC, Watkins LR, Spencer RL, Maier SF, Fonken LK. Circadian misalignment has differential effects on affective behavior following exposure to controllable or uncontrollable stress. Behav Brain Res. 2019;359:440-445. [PubMed] [DOI] |
| 5. | Reinke H, Asher G. Circadian Clock Control of Liver Metabolic Functions. Gastroenterology. 2016;150:574-580. [PubMed] [DOI] |
| 6. | Wayland J, Shah F, Samuels K, Seward T, Schroder E, Delisle BP. Disrupting the Circadian Clock Mechanism in Cardiomyocytes Exacerbates the LQT3-related Phenotype in Scn5a(Delta KPQ/+) Mice. Biophys J. 2020;118:103a-a. |
| 7. | Allen NC, Philip NH, Hui L, Zhou X, Franklin RA, Kong Y, Medzhitov R. Desynchronization of the molecular clock contributes to the heterogeneity of the inflammatory response. Sci Signal. 2019;12. [PubMed] [DOI] |
| 8. | Kuehn BM. Resetting the Circadian Clock Might Boost Metabolic Health. JAMA. 2017;317:1303-1305. [PubMed] [DOI] |
| 9. | Hernández-García J, Navas-Carrillo D, Orenes-Piñero E. Alterations of circadian rhythms and their impact on obesity, metabolic syndrome and cardiovascular diseases. Crit Rev Food Sci Nutr. 2020;60:1038-1047. [PubMed] [DOI] |
| 10. | Czeisler CA. SLEEP. Measuring the passage of brain time. Science. 2016;353:648-649. [PubMed] [DOI] |
| 11. | Potter GD, Skene DJ, Arendt J, Cade JE, Grant PJ, Hardie LJ. Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocr Rev. 2016;37:584-608. [PubMed] [DOI] |
| 12. | PITTENDRIGH CS. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol. 1960;25:159-184. [PubMed] [DOI] |
| 13. | Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551-577. [PubMed] [DOI] |
| 14. | Hastings MH, Maywood ES, Brancaccio M. The Mammalian Circadian Timing System and the Suprachiasmatic Nucleus as Its Pacemaker. Biology (Basel). 2019;8. [PubMed] [DOI] |
| 15. | Dibner C, Schibler U. Circadian timing of metabolism in animal models and humans. J Intern Med. 2015;277:513-527. [PubMed] [DOI] |
| 16. | Schibler U. Oxidation of CLOCK boosts circadian rhythms. Nat Cell Biol. 2019;21:1464-1465. [PubMed] [DOI] |
| 17. | Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935-941. [PubMed] [DOI] |
| 18. | Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764-775. [PubMed] [DOI] |
| 19. | Guillaumond F, Dardente H, Giguère V, Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms. 2005;20:391-403. [PubMed] [DOI] |
| 20. | Sato F, Kohsaka A, Bhawal UK, Muragaki Y. Potential Roles of Dec and Bmal1 Genes in Interconnecting Circadian Clock and Energy Metabolism. Int J Mol Sci. 2018;19. [PubMed] [DOI] |
| 21. | Gooley JJ. Circadian regulation of lipid metabolism. Proc Nutr Soc. 2016;75:440-450. [PubMed] [DOI] |
| 22. | Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349-354. [PubMed] [DOI] |
| 23. | Rey G, Cesbron F, Rougemont J, Reinke H, Brunner M, Naef F. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biol. 2011;9:e1000595. [PubMed] [DOI] |
| 24. | Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, Liddle C, Auwerx J, Downes M, Panda S, Evans RM. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485:123-127. [PubMed] [DOI] |
| 25. | Fang B, Everett LJ, Jager J, Briggs E, Armour SM, Feng D, Roy A, Gerhart-Hines Z, Sun Z, Lazar MA. Circadian enhancers coordinate multiple phases of rhythmic gene transcription in vivo. Cell. 2014;159:1140-1152. [PubMed] [DOI] |
| 26. | Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307-320. [DOI] |
| 27. | Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78-83. [PubMed] [DOI] |
| 28. | Robles MS, Cox J, Mann M. In-vivo quantitative proteomics reveals a key contribution of post-transcriptional mechanisms to the circadian regulation of liver metabolism. PLoS Genet. 2014;10:e1004047. [PubMed] [DOI] |
| 29. | Mauvoisin D, Wang J, Jouffe C, Martin E, Atger F, Waridel P, Quadroni M, Gachon F, Naef F. Circadian clock-dependent and -independent rhythmic proteomes implement distinct diurnal functions in mouse liver. Proc Natl Acad Sci USA. 2014;111:167-172. [PubMed] [DOI] |
| 30. | Wang J, Mauvoisin D, Martin E, Atger F, Galindo AN, Dayon L, Sizzano F, Palini A, Kussmann M, Waridel P, Quadroni M, Dulić V, Naef F, Gachon F. Nuclear Proteomics Uncovers Diurnal Regulatory Landscapes in Mouse Liver. Cell Metab. 2017;25:102-117. [PubMed] [DOI] |
| 31. | Aviram R, Manella G, Kopelman N, Neufeld-Cohen A, Zwighaft Z, Elimelech M, Adamovich Y, Golik M, Wang C, Han X, Asher G. Lipidomics Analyses Reveal Temporal and Spatial Lipid Organization and Uncover Daily Oscillations in Intracellular Organelles. Mol Cell. 2016;62:636-648. [PubMed] [DOI] |
| 32. | Adamovich Y, Rousso-Noori L, Zwighaft Z, Neufeld-Cohen A, Golik M, Kraut-Cohen J, Wang M, Han X, Asher G. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 2014;19:319-330. [PubMed] [DOI] |
| 33. | Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011;13:125-137. [PubMed] [DOI] |
| 34. | Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev. 2013;93:107-135. [PubMed] [DOI] |
| 36. | Feng D, Lazar MA. Clocks, metabolism, and the epigenome. Mol Cell. 2012;47:158-167. [PubMed] [DOI] |
| 37. | Asher G, Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell. 2015;161:84-92. [PubMed] [DOI] |
| 38. | Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008;105:15172-15177. [PubMed] [DOI] |
| 39. | Kalsbeek A, la Fleur S, Fliers E. Circadian control of glucose metabolism. Mol Metab. 2014;3:372-383. [PubMed] [DOI] |
| 40. | Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307-320. [PubMed] [DOI] |
| 42. | Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, Downes M, Evans RM. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480:552-556. [PubMed] [DOI] |
| 43. | So AY, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci USA. 2009;106:17582-17587. [PubMed] [DOI] |
| 44. | Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci USA. 2009;106:21453-21458. [PubMed] [DOI] |
| 45. | Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y, Brenner DA, Montminy M, Kay SA. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16:1152-1156. [PubMed] [DOI] |
| 46. | Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786-1789. [PubMed] [DOI] |
| 47. | Yin L, Wu N, Lazar MA. Nuclear receptor Rev-erbalpha: a heme receptor that coordinates circadian rhythm and metabolism. Nucl Recept Signal. 2010;8:e001. [PubMed] [DOI] |
| 48. | Preitner N, Brown S, Ripperger J, Le-Minh N, Damiola F, Schibler U. Orphan nuclear receptors, molecular clockwork, and the entrainment of peripheral oscillators. Novartis Found Symp. 2003;253:89-99; discussion 99-109. [PubMed] |
| 49. | Zhang Y, Fang B, Emmett MJ, Damle M, Sun Z, Feng D, Armour SM, Remsberg JR, Jager J, Soccio RE, Steger DJ, Lazar MA. GENE REGULATION. Discrete functions of nuclear receptor Rev-erbα couple metabolism to the clock. Science. 2015;348:1488-1492. [PubMed] [DOI] |
| 50. | Jarrett RJ, Keen H. Diurnal variation of oral glucose tolerance: a possible pointer to the evolution of diabetes mellitus. Br Med J. 1969;2:341-344. [PubMed] [DOI] |
| 51. | Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043-1045. [PubMed] [DOI] |
| 52. | Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring). 2009;17:2100-2102. [PubMed] [DOI] |
| 53. | Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453-4458. [PubMed] [DOI] |
| 54. | Jacobi D, Liu S, Burkewitz K, Kory N, Knudsen NH, Alexander RK, Unluturk U, Li X, Kong X, Hyde AL, Gangl MR, Mair WB, Lee CH. Hepatic Bmal1 Regulates Rhythmic Mitochondrial Dynamics and Promotes Metabolic Fitness. Cell Metab. 2015;22:709-720. [PubMed] [DOI] |
| 55. | Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627-631. [PubMed] [DOI] |
| 56. | Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, Peek CB, Hong H, Huang W, Omura C, Allred AL, Bradfield CA, Dinner AR, Barish GD, Bass J. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015;350:aac4250. [PubMed] [DOI] |
| 57. | Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54:120-124. [PubMed] [DOI] |
| 58. | Harfmann BD, Schroder EA, Kachman MT, Hodge BA, Zhang X, Esser KA. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet Muscle. 2016;6:12. [PubMed] [DOI] |
| 59. | Dyar KA, Ciciliot S, Wright LE, Biensø RS, Tagliazucchi GM, Patel VR, Forcato M, Paz MI, Gudiksen A, Solagna F, Albiero M, Moretti I, Eckel-Mahan KL, Baldi P, Sassone-Corsi P, Rizzuto R, Bicciato S, Pilegaard H, Blaauw B, Schiaffino S. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab. 2014;3:29-41. [PubMed] [DOI] |
| 60. | Stenvers DJ, van Dorp R, Foppen E, Mendoza J, Opperhuizen AL, Fliers E, Bisschop PH, Meijer JH, Kalsbeek A, Deboer T. Dim light at night disturbs the daily sleep-wake cycle in the rat. Sci Rep. 2016;6:35662. [PubMed] [DOI] |
| 61. | Fonken LK, Nelson RJ. The effects of light at night on circadian clocks and metabolism. Endocr Rev. 2014;35:648-670. [PubMed] [DOI] |
| 62. | Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 2010;107:18664-18669. [PubMed] [DOI] |
| 63. | Opperhuizen AL, Stenvers DJ, Jansen RD, Foppen E, Fliers E, Kalsbeek A. Light at night acutely impairs glucose tolerance in a time-, intensity- and wavelength-dependent manner in rats. Diabetologia. 2017;60:1333-1343. [PubMed] [DOI] |
| 64. | Cheung IN, Zee PC, Shalman D, Malkani RG, Kang J, Reid KJ. Morning and Evening Blue-Enriched Light Exposure Alters Metabolic Function in Normal Weight Adults. PLoS One. 2016;11:e0155601. [PubMed] [DOI] |
| 65. | Albreiki MS, Middleton B, Hampton SM. A single night light exposure acutely alters hormonal and metabolic responses in healthy participants. Endocr Connect. 2017;6:100-110. [PubMed] [DOI] |
| 66. | Toyoura M, Miike T, Tajima S, Matsuzawa S, Konishi Y. Inadequate sleep as a contributor to impaired glucose tolerance: A cross-sectional study in children, adolescents, and young adults with circadian rhythm sleep-wake disorder. Pediatr Diabetes. 2020;21:557-564. [PubMed] [DOI] |
| 67. | Vetter C, Dashti HS, Lane JM, Anderson SG, Schernhammer ES, Rutter MK, Saxena R, Scheer FAJL. Night Shift Work, Genetic Risk, and Type 2 Diabetes in the UK Biobank. Diabetes Care. 2018;41:762-769. [PubMed] [DOI] |
| 68. | Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860-1869. [PubMed] [DOI] |
| 69. | Qian J, Dalla Man C, Morris CJ, Cobelli C, Scheer FAJL. Differential effects of the circadian system and circadian misalignment on insulin sensitivity and insulin secretion in humans. Diabetes Obes Metab. 2018;20:2481-2485. [PubMed] [DOI] |
| 70. | Wefers J, van Moorsel D, Hansen J, Connell NJ, Havekes B, Hoeks J, van Marken Lichtenbelt WD, Duez H, Phielix E, Kalsbeek A, Boekschoten MV, Hooiveld GJ, Hesselink MKC, Kersten S, Staels B, Scheer FAJL, Schrauwen P. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc Natl Acad Sci USA. 2018;115:7789-7794. [PubMed] [DOI] |
| 71. | Chen Z, Yoo SH, Takahashi JS. Development and Therapeutic Potential of Small-Molecule Modulators of Circadian Systems. Annu Rev Pharmacol Toxicol. 2018;58:231-252. [PubMed] [DOI] |
| 72. | He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, Garcia JM, Koike N, Lee CC, Takahashi JS, Yoo SH, Chen Z. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016;23:610-621. [PubMed] [DOI] |
| 73. | Humphries PS, Bersot R, Kincaid J, Mabery E, McCluskie K, Park T, Renner T, Riegler E, Steinfeld T, Turtle ED, Wei ZL, Willis E. Carbazole-containing sulfonamides and sulfamides: Discovery of cryptochrome modulators as antidiabetic agents. Bioorg Med Chem Lett. 2016;26:757-760. [PubMed] [DOI] |
| 74. | Humphries PS, Bersot R, Kincaid J, Mabery E, McCluskie K, Park T, Renner T, Riegler E, Steinfeld T, Turtle ED, Wei ZL, Willis E. Carbazole-containing amides and ureas: Discovery of cryptochrome modulators as antihyperglycemic agents. Bioorg Med Chem Lett. 2018;28:293-297. [PubMed] [DOI] |
| 75. | Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678-693. [PubMed] [DOI] |
| 76. | Chávez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:1679-1694.e3. [PubMed] [DOI] |
| 77. | Reinke H, Asher G. Crosstalk between metabolism and circadian clocks. Nat Rev Mol Cell Biol. 2019;20:227-241. [PubMed] [DOI] |
| 78. | Duane WC, Gilberstadt ML, Wiegand DM. Diurnal rhythms of bile acid production in the rat. Am J Physiol. 1979;236:R175-R179. [PubMed] [DOI] |
| 79. | Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789-799. [PubMed] [DOI] |
| 80. | Lavery DJ, Schibler U. Circadian transcription of the cholesterol 7 alpha hydroxylase gene may involve the liver-enriched bZIP protein DBP. Genes Dev. 1993;7:1871-1884. [PubMed] [DOI] |
| 81. | Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801-810. [PubMed] [DOI] |
| 82. | Haas JT, Francque S, Staels B. Pathophysiology and Mechanisms of Nonalcoholic Fatty Liver Disease. Annu Rev Physiol. 2016;78:181-205. [PubMed] [DOI] |
| 83. | Chiang JYL. Bile acid metabolism and signaling in liver disease and therapy. Liver Res. 2017;1:3-9. [PubMed] [DOI] |
| 84. | Stroeve JH, Brufau G, Stellaard F, Gonzalez FJ, Staels B, Kuipers F. Intestinal FXR-mediated FGF15 production contributes to diurnal control of hepatic bile acid synthesis in mice. Lab Invest. 2010;90:1457-1467. [PubMed] [DOI] |
| 85. | Zhang YK, Guo GL, Klaassen CD. Diurnal variations of mouse plasma and hepatic bile acid concentrations as well as expression of biosynthetic enzymes and transporters. PLoS One. 2011;6:e16683. [PubMed] [DOI] |
| 86. | Duez H, van der Veen JN, Duhem C, Pourcet B, Touvier T, Fontaine C, Derudas B, Baugé E, Havinga R, Bloks VW, Wolters H, van der Sluijs FH, Vennström B, Kuipers F, Staels B. Regulation of bile acid synthesis by the nuclear receptor Rev-erbalpha. Gastroenterology. 2008;135:689-698. [PubMed] [DOI] |
| 87. | Zhang T, Zhao M, Lu D, Wang S, Yu F, Guo L, Wen S, Wu B. REV-ERBα Regulates CYP7A1 Through Repression of Liver Receptor Homolog-1. Drug Metab Dispos. 2018;46:248-258. [PubMed] [DOI] |
| 88. | Govindarajan K, MacSharry J, Casey PG, Shanahan F, Joyce SA, Gahan CG. Unconjugated Bile Acids Influence Expression of Circadian Genes: A Potential Mechanism for Microbe-Host Crosstalk. PLoS One. 2016;11:e0167319. [PubMed] [DOI] |
| 89. | Eggink HM, Oosterman JE, de Goede P, de Vries EM, Foppen E, Koehorst M, Groen AK, Boelen A, Romijn JA, la Fleur SE, Soeters MR, Kalsbeek A. Complex interaction between circadian rhythm and diet on bile acid homeostasis in male rats. Chronobiol Int. 2017;34:1339-1353. [PubMed] [DOI] |
| 90. | Duane WC, Levitt DG, Mueller SM, Behrens JC. Regulation of bile acid synthesis in man. Presence of a diurnal rhythm. J Clin Invest. 1983;72:1930-1936. [PubMed] [DOI] |
| 91. | Li T, Francl JM, Boehme S, Ochoa A, Zhang Y, Klaassen CD, Erickson SK, Chiang JY. Glucose and insulin induction of bile acid synthesis: mechanisms and implication in diabetes and obesity. J Biol Chem. 2012;287:1861-1873. [PubMed] [DOI] |
| 92. | Chaix A, Zarrinpar A. The effects of time-restricted feeding on lipid metabolism and adiposity. Adipocyte. 2015;4:319-324. [PubMed] [DOI] |
| 93. | Husse J, Hintze SC, Eichele G, Lehnert H, Oster H. Circadian clock genes Per1 and Per2 regulate the response of metabolism-associated transcripts to sleep disruption. PLoS One. 2012;7:e52983. [PubMed] [DOI] |
| 94. | Pan X, Zhang Y, Wang L, Hussain MM. Diurnal regulation of MTP and plasma triglyceride by CLOCK is mediated by SHP. Cell Metab. 2010;12:174-186. [PubMed] [DOI] |
| 95. | Pan X, Hussain MM. Clock is important for food and circadian regulation of macronutrient absorption in mice. J Lipid Res. 2009;50:1800-1813. [PubMed] [DOI] |
| 96. | Kudo T, Tamagawa T, Kawashima M, Mito N, Shibata S. Attenuating effect of clock mutation on triglyceride contents in the ICR mouse liver under a high-fat diet. J Biol Rhythms. 2007;22:312-323. [PubMed] [DOI] |
| 97. | Shostak A, Meyer-Kovac J, Oster H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes. 2013;62:2195-2203. [PubMed] [DOI] |
| 98. | Tsai JY, Kienesberger PC, Pulinilkunnil T, Sailors MH, Durgan DJ, Villegas-Montoya C, Jahoor A, Gonzalez R, Garvey ME, Boland B, Blasier Z, McElfresh TA, Nannegari V, Chow CW, Heird WC, Chandler MP, Dyck JR, Bray MS, Young ME. Direct regulation of myocardial triglyceride metabolism by the cardiomyocyte circadian clock. J Biol Chem. 2010;285:2918-2929. [PubMed] [DOI] |
| 99. | Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. [PubMed] [DOI] |
| 100. | Kumar Jha P, Challet E, Kalsbeek A. Circadian rhythms in glucose and lipid metabolism in nocturnal and diurnal mammals. Mol Cell Endocrinol. 2015;418 Pt 1:74-88. [PubMed] [DOI] |
| 101. | Shimba S, Ogawa T, Hitosugi S, Ichihashi Y, Nakadaira Y, Kobayashi M, Tezuka M, Kosuge Y, Ishige K, Ito Y, Komiyama K, Okamatsu-Ogura Y, Kimura K, Saito M. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLoS One. 2011;6:e25231. [PubMed] [DOI] |
| 102. | Fontaine C, Dubois G, Duguay Y, Helledie T, Vu-Dac N, Gervois P, Soncin F, Mandrup S, Fruchart JC, Fruchart-Najib J, Staels B. The orphan nuclear receptor Rev-Erbalpha is a peroxisome proliferator-activated receptor (PPAR) gamma target gene and promotes PPARgamma-induced adipocyte differentiation. J Biol Chem. 2003;278:37672-37680. [PubMed] [DOI] |
| 103. | Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, Lo Sasso G, Moschetta A, Schibler U. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009;7:e1000181. [PubMed] [DOI] |
| 104. | Alenghat T, Meyers K, Mullican SE, Leitner K, Adeniji-Adele A, Avila J, Bućan M, Ahima RS, Kaestner KH, Lazar MA. Nuclear receptor corepressor and histone deacetylase 3 govern circadian metabolic physiology. Nature. 2008;456:997-1000. [PubMed] [DOI] |
| 105. | Feng D, Liu T, Sun Z, Bugge A, Mullican SE, Alenghat T, Liu XS, Lazar MA. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315-1319. [PubMed] [DOI] |
| 106. | Grimaldi B, Bellet MM, Katada S, Astarita G, Hirayama J, Amin RH, Granneman JG, Piomelli D, Leff T, Sassone-Corsi P. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 2010;12:509-520. [PubMed] [DOI] |
| 107. | Barclay JL, Shostak A, Leliavski A, Tsang AH, Jöhren O, Müller-Fielitz H, Landgraf D, Naujokat N, van der Horst GT, Oster H. High-fat diet-induced hyperinsulinemia and tissue-specific insulin resistance in Cry-deficient mice. Am J Physiol Endocrinol Metab. 2013;304:E1053-E1063. [PubMed] [DOI] |
| 108. | Paschos GK, Ibrahim S, Song WL, Kunieda T, Grant G, Reyes TM, Bradfield CA, Vaughan CH, Eiden M, Masoodi M, Griffin JL, Wang F, Lawson JA, Fitzgerald GA. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med. 2012;18:1768-1777. [PubMed] [DOI] |
| 109. | Bugge A, Feng D, Everett LJ, Briggs ER, Mullican SE, Wang F, Jager J, Lazar MA. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26:657-667. [PubMed] [DOI] |
| 110. | Villanueva JE, Livelo C, Trujillo AS, Chandran S, Woodworth B, Andrade L, Le HD, Manor U, Panda S, Melkani GC. Time-restricted feeding restores muscle function in Drosophila models of obesity and circadian-rhythm disruption. Nat Commun. 2019;10:2700. [PubMed] [DOI] |
| 111. | Schäbler S, Amatobi KM, Horn M, Rieger D, Helfrich-Förster C, Mueller MJ, Wegener C, Fekete A. Loss of function in the Drosophila clock gene period results in altered intermediary lipid metabolism and increased susceptibility to starvation. Cell Mol Life Sci. 2020;. [PubMed] [DOI] |
| 112. | Baron KG, Reid KJ, Kim T, Van Horn L, Attarian H, Wolfe L, Siddique J, Santostasi G, Zee PC. Circadian timing and alignment in healthy adults: associations with BMI, body fat, caloric intake and physical activity. Int J Obes (Lond). 2017;41:203-209. [PubMed] [DOI] |
| 113. | Meyer-Kovac J, Kolbe I, Ehrhardt L, Leliavski A, Husse J, Salinas G, Lingner T, Tsang AH, Barclay JL, Oster H. Hepatic gene therapy rescues high-fat diet responses in circadian Clock mutant mice. Mol Metab. 2017;6:512-523. [PubMed] [DOI] |
| 114. | Touitou Y, Reinberg A, Touitou D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017;173:94-106. [PubMed] [DOI] |
| 115. | Nedeltcheva AV, Scheer FA. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2014;21:293-298. [PubMed] [DOI] |
| 116. | Parsons MJ, Moffitt TE, Gregory AM, Goldman-Mellor S, Nolan PM, Poulton R, Caspi A. Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int J Obes (Lond). 2015;39:842-848. [PubMed] [DOI] |
| 117. | Tahira K, Ueno T, Fukuda N, Aoyama T, Tsunemi A, Matsumoto S, Nagura C, Matsumoto T, Soma M, Shimba S, Matsumoto Y. Obesity alters the expression profile of clock genes in peripheral blood mononuclear cells. Arch Med Sci. 2011;7:933-940. [PubMed] [DOI] |
| 118. | Yasumoto Y, Hashimoto C, Nakao R, Yamazaki H, Hiroyama H, Nemoto T, Yamamoto S, Sakurai M, Oike H, Wada N, Yoshida-Noro C, Oishi K. Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice. Metabolism. 2016;65:714-727. [PubMed] [DOI] |
| 119. | Lewis P, Oster H, Korf HW, Foster RG, Erren TC. Food as a circadian time cue - evidence from human studies. Nat Rev Endocrinol. 2020;16:213-223. [PubMed] [DOI] |
| 120. | Parker HM, Cohn JS, O'Connor HT, Garg ML, Caterson ID, George J, Johnson NA. Effect of Fish Oil Supplementation on Hepatic and Visceral Fat in Overweight Men: A Randomized Controlled Trial. Nutrients. 2019;11. [PubMed] [DOI] |
| 121. | Gui L, Chen S, Wang H, Ruan M, Liu Y, Li N, Zhang H, Liu Z. ω-3 PUFAs Alleviate High-Fat Diet-Induced Circadian Intestinal Microbes Dysbiosis. Mol Nutr Food Res. 2019;63:e1900492. [PubMed] [DOI] |
| 122. | Liu Y, Li Q, Wang H, Zhao X, Li N, Zhang H, Chen G, Liu Z. Fish oil alleviates circadian bile composition dysregulation in male mice with NAFLD. J Nutr Biochem. 2019;69:53-62. [PubMed] [DOI] |
| 123. | Chen R, Zuo Z, Li Q, Wang H, Li N, Zhang H, Yu X, Liu Z. DHA substitution overcomes high-fat diet-induced disturbance in the circadian rhythm of lipid metabolism. Food Funct. 2020;11:3621-3631. [PubMed] [DOI] |
| 124. | Yin J, Li Y, Han H, Ma J, Liu G, Wu X, Huang X, Fang R, Baba K, Bin P, Zhu G, Ren W, Tan B, Tosini G, He X, Li T, Yin Y. Administration of Exogenous Melatonin Improves the Diurnal Rhythms of the Gut Microbiota in Mice Fed a High-Fat Diet. mSystems. 2020;5. [PubMed] [DOI] |
| 125. | Zhao J, Zhang X, Liu H, Brown MA, Qiao S. Dietary Protein and Gut Microbiota Composition and Function. Curr Protein Pept Sci. 2019;20:145-154. [PubMed] [DOI] |
| 126. | Dang F, Sun X, Ma X, Wu R, Zhang D, Chen Y, Xu Q, Wu Y, Liu Y. Insulin post-transcriptionally modulates Bmal1 protein to affect the hepatic circadian clock. Nat Commun. 2016;7:12696. [PubMed] [DOI] |
| 127. | Lipton JO, Yuan ED, Boyle LM, Ebrahimi-Fakhari D, Kwiatkowski E, Nathan A, Güttler T, Davis F, Asara JM, Sahin M. The Circadian Protein BMAL1 Regulates Translation in Response to S6K1-Mediated Phosphorylation. Cell. 2015;161:1138-1151. [PubMed] [DOI] |
| 128. | Jouffe C, Cretenet G, Symul L, Martin E, Atger F, Naef F, Gachon F. The circadian clock coordinates ribosome biogenesis. PLoS Biol. 2013;11:e1001455. [PubMed] [DOI] |
| 129. | Atger F, Gobet C, Marquis J, Martin E, Wang J, Weger B, Lefebvre G, Descombes P, Naef F, Gachon F. Circadian and feeding rhythms differentially affect rhythmic mRNA transcription and translation in mouse liver. Proc Natl Acad Sci USA. 2015;112:E6579-E6588. [PubMed] [DOI] |
| 130. | Jeyaraj D, Scheer FA, Ripperger JA, Haldar SM, Lu Y, Prosdocimo DA, Eapen SJ, Eapen BL, Cui Y, Mahabeleshwar GH, Lee HG, Smith MA, Casadesus G, Mintz EM, Sun H, Wang Y, Ramsey KM, Bass J, Shea SA, Albrecht U, Jain MK. Klf15 orchestrates circadian nitrogen homeostasis. Cell Metab. 2012;15:311-323. [PubMed] [DOI] |
| 131. | Liu Y, Dong W, Shao J, Wang Y, Zhou M, Sun H. Branched-Chain Amino Acid Negatively Regulates KLF15 Expression via PI3K-AKT Pathway. Front Physiol. 2017;8:853. [PubMed] [DOI] |