修回日期: 2020-05-07
接受日期: 2020-06-09
在线出版日期: 2020-07-08
近年来, 长链非编码RNA (long non-coding RNA, lncRNA)与肿瘤的关系受到越来越多的关注, lncRNA的异常表达通过参与胃癌(gastric cancer, GC)细胞增殖、侵袭、迁移等生物学行为, 在GC发生中发挥着致癌/抑癌基因的作用. 本文通过总结相关文献, 对lncRNA的研究现状、检测技术、在GC发生、进展、预后及耐药相关潜在标志物的作用机制进行分析, 以期lncRNA能作为GC靶基因在早期发现、早期治疗、有效改善化疗耐药中发挥重要作用, 提高患者生存质量, 达到GC临床个体化精准治疗.
核心提要: 长链非编码RNA (long non-coding RNA, lncRNA)在胃癌(gastric cancer, GC)发生中发挥着致癌/抑癌基因的作用, 因此探讨lncRNA在GC发生、进展、预后及耐药相关潜在标志物的作用机制, 以期lncRNA能作为GC靶基因在诊断及有效改善化疗耐药中发挥重要作用.
引文著录: 李芳, 陈子豪, 檀碧波, 李勇. 长非编码RNA作为胃癌发生、进展及预后相关潜在标志物的研究进展. 世界华人消化杂志 2020; 28(13): 544-552
Revised: May 7, 2020
Accepted: June 9, 2020
Published online: July 8, 2020
In recent years, more and more attention has been paid to the relationship between long non-coding RNAs (lncRNAs) and tumor. Abnormal expression of lncRNAs plays an oncogenic or tumor-suppressing role in gastric cancer (GC) by participating in the biological behaviors of GC cells, such as proliferation, invasion, and migration. By summarizing the relevant literature, this paper discusses the research status, detection technology, and mechanism of action of lncRNAs in GC, as well as their potential as markers for occurrence, progression, prognosis, and drug resistance of GC. It is expected that lncRNAs can play an important role in early detection, early treatment, and effective improvement of chemotherapy resistance of GC to achieve personalized precise treatment of this malignancy.
- Citation: Li F, Chen ZH, Tan BB, Li Y. Long non-coding RNAs as potential markers for occurrence, progression, and prognosis of gastric cancer. Shijie Huaren Xiaohua Zazhi 2020; 28(13): 544-552
- URL: https://www.wjgnet.com/1009-3079/full/v28/i13/544.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v28.i13.544
胃癌(gastric cancer, GC)在全球最常见的癌症类型中位列第四, 死亡率位列第三[1]. 全世界每年约有700000例新确诊GC患者[2], 严重影响着人类健康. 许多GC患者诊断时即为晚期, 尽管在该病的诊断和治疗方面取得了许多进展, 如手术方式的改进、辅助放疗及术前新辅助化疗的进行, 增加了可切除性GC的机会[3], 但GC的预后仍然很差, 5年生存率偏低[4]. 因此, 寻找能够准确预测或反映胃癌个体癌症风险、判断不良预后的新型生物标志物对患者实施个体化精准诊治策略具有重要意义. 以往认为肿瘤是由蛋白质编码基因发生突变引起的, 近年来发现, 长链非编码RNA (long non-coding RNA, lncRNA)能够在表观遗传学水平如染色体重塑、转录及转录后调控水平调控基因的表达, 转录后可导致基因沉默或激活, 参与多种肿瘤的发生发展, 可通过调控血管生成相关因子的表达、内皮细胞的增殖、迁移, 最终影响血管生成[5-8](表1). LncRNA因其在人类疾病中的作用而受到越来越多的关注, lncRNA的异常表达也与GC密切相关, 通过参与GC细胞增殖、侵袭、迁移等生物学行为, 影响GC进展及预后. 传统的化疗由于肿瘤细胞的多药耐药而疗效有限, 肿瘤细胞的耐药机制尚不清楚. 因此胃癌相关lncRNA可作为GC早期诊断、预测预后、探讨耐药机制的潜在生物标志物.
| LncRNA | 功能 | 引用文献 |
| Airn | 父体特异性沉默 | Aris等[61] |
| CCAT1-L | 影响myc基因的表达 | Sun等[62] |
| CCAT1-S | 影响myc基因的表达 | Xiang等[63] |
| CDKN2B-HS1 | 抑制癌基因CDKN2A/CDKN2B的表达 | Yap等[64] |
| GAS5 | 抑制miR-21的表达 | Duan等[65] |
| GClnc1 | 招募WDR5和KAT2A进行特定的组蛋白修饰 | Gu等[66] |
| H19 | 母体表达, 出生后的大部分组织中表达迅速下调 | Yoshimura等[67]; Shen等[68] |
| HOTAIR | 形成多个组蛋白修饰复合物, 参与组蛋白修饰反应 | Tsai等[69]; Somarowthu等[70] |
| Linc-P21 | 在P53通路中发挥重要作用, P53激活细胞凋亡 | Huarte等[71] |
| MALAT1 | 高表达于乳腺、结肠和前列腺中高表达; 与许多疾病相关 | Li等[72]; Anko等[73] |
| MIAT | 调节微血管功能障碍 | Yan等[74] |
| MEG3 | 抑制癌症发生 | Zhang等[75] |
| Rsx | 调节X染色体沉默 | Plath等[76] |
| SPA1 | 激素受体共同-激活剂 | Novikova等[77] |
| XIST | 调节X染色体沉默 | Duszczyk等[78]; Wutz等[79]; Hasegawa等[80] |
真核生物基因组可能转录几种类型的RNA, 包括蛋白编码mRNA、短和长非编码RNA. 从这些RNA中, 发现人类细胞中的非编码RNA比编码RNA多. 根据DNA基因组中, 76%的人类基因组DNA转录成RNA, 仅有2%的基因组DNA被翻译成蛋白, 证明存在大量的非编码RNA. 近年来, 对小分子RNA (micro RNA, miRNA)、小干扰RNA (small interference RNA, siRNA)、核仁小RNAs等短链非编码RNA的研究较多. 与此同时, lncRNA正受到越来越多的关注, lncRNA不仅被认为是基因组的副产品, 而且具有丰富的防御细胞功能, 许多功能与人类疾病有关[9].
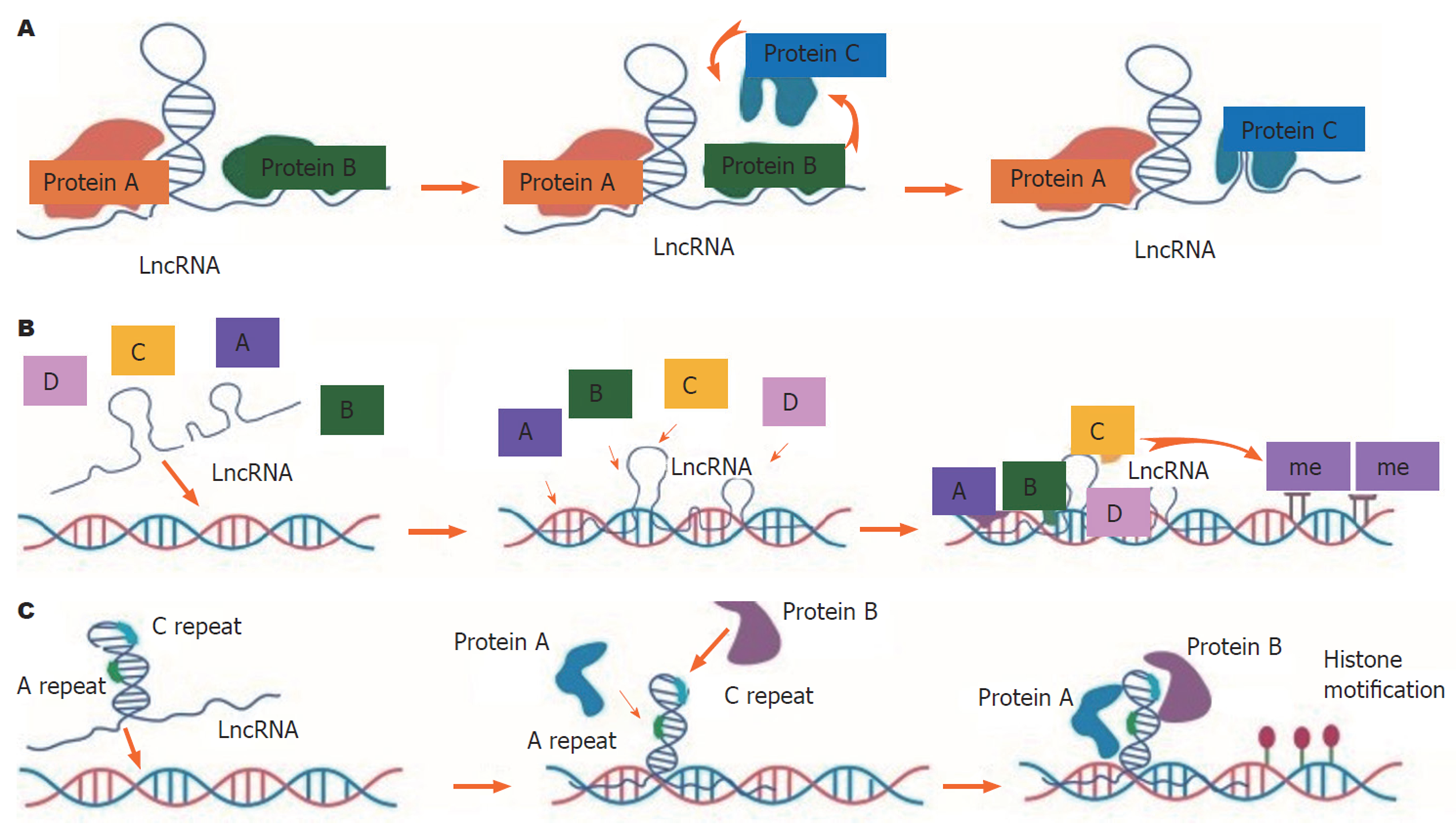
LncRNA是一类长度超过200个核苷酸的的非编码RNA. 大多数lncRNA是由RNA聚合酶II合成的. 根据lncRNA在基因组上为主将其分为5类: (1)反义型; (2)增强子型; (3)基因内型; (4)基因间型; (5)双向型. LncRNA在染色质、DNA、转录和转录后水平调控基因表达, 影响mRNA的转录、剪切、翻译、输出、输入和稳定性, 从而影响各种疾病的发生和预后. 调控机制可能包括干扰编码基因的翻译, 抑制聚合酶II的活性, 促进转录后修饰, 与功能蛋白结合, 或作为小分子RNA的前体物质与染色体结合, 调控信号通路[10]. 与短链非编码RNA相比, lncRNA的机制功能多样化, 增加了该基因家族的复杂性, 而短链非编码RNA多被认为是基因调控的结果[8](图1). 目前可能是由于lncRNA的低表达水平和组织特异性, 对其功能缺乏深入了解. 通过像DNA元素百科全书、哺乳动物基因组的功能注释、基因型-组织表达和GENCODE这样的基因组计划, 已经预测出了超过60000个lncRNA, 一些已经被证实与某些疾病密切相关. LncRNA通过与转录起始位点的相互作用、作为转录协同激活因子或作为蛋白质支架, 已被证明具有转录因子招募者的功能. LncRNA可能作为分子诱饵捕获转录因子, 从而限制转录因子与DNA结合位点的结合. 除了转录调控外, lncRNA还通过调控mRNA剪接、抑制转译、充当miRNA海绵或竞争miRNA在mRNA上的结合位点, 从而在mRNA的加工、成熟和稳定性方面发挥作用[11]. 一些lncRNA也可以编码小肽, 表明这些lncRNA可以作为一个双功能转录本, 即作为lncRNA或蛋白模板[12]. 近年来, 越来越多的lncRNA被发现在致癌过程中发挥重要作用[13,14].
LncRNA与DNA、RNA和蛋白质相互作用, 发挥功能, 使其复杂性提高到一个新的水平. 传统的mRNA功能研究方法在lncRNA研究中效率低下, 由于lncRNA在表达上有特异性高峰的特征, 拷贝数也很低, 这些特征使检测变得极为困难. 虽然某些lncRNA组织特异性的出现有助于生物标志物的发展, 但一些情况下若出现在细胞核内, 再利用RNA干扰进行功能研究时可能会造成困难. 因此, 研究lncRNA的方法应该是高效的, 具有更高的靶向性和分辨率, 在分子水平上具有高度的可操作性.
对lncRNA的研究主要手段是对细胞或组织提取的RNA进行定量和定性分析, 常用方法有PCR、芯片技术、测序技术、Northern blot等[15]. 传统RNA原位杂交选择放射性标记、荧光标记、生物素标记的RNA探针, 检测特异性及灵敏性较差. 针对RNA长度超过300碱基的lncRNA可采用RNAscope技术, 它是基于独特的探针设计和信号放大的原位杂交方法, 显著提高了检测的特异性和灵敏度, 已经被广泛用于lncRNA的研究.
(1)采用lncRNA芯片、RNA-seq测序等方法进行lncRNA表达谱筛选分析. RNA-seq测序对于低丰度lncRNA无法进行准确定量, 芯片比RNA-seq更适合低丰度lncRNA表达谱的检测; (2)通过生物信息学的方法筛选出具有表达差异的lncRNA, 预测lncRNA靶基因; (3)通过构建lncRNA过表达载体进行过表达功能试验的验证; (4)通过siRNA、shRNA等方法沉默lncRNA, 敲低lncRNA表达后检测其对相关疾病信号通路相关基因表达的影响和对细胞增值、侵袭、凋亡、转移的影响; (5)通过RNA pull-down、RNA免疫沉淀、ChIRP-seq等技术检测与lncRNA结合的DNA、RNA及蛋白质; (6)通过构建动物移植瘤模型实验, 导入lncRNA表达质粒或siRNA后, 通过PCR、免疫组织化学、Western blot等方法检测相关指标的变化, 从而揭示lncRNA在疾病发生及进展中的作用机制.
大量研究表明, lncRNA在肿瘤发生和肿瘤进展中起着关键作用. 特别是lncRNA表达异常可能伴随DNA损伤、免疫逃逸以及癌细胞的细胞代谢紊乱. LncRNA还与上皮间充质转化(epithelial-mesenchymal transition, EMT)以及对细胞干性的调控密切相关. LncRNA目前在GC中研究尚少, 研究显示其异常表达与GC的发生、发展、侵袭、转移及预后等相关, 有可能作为GC诊断和预后的潜在标志物, 成为新的治疗靶点. Cao等[16]利用生物信息学方法检测显示, 有88种lncRNA在GC组织中存在异常表达. LncRNA的异常表达与GC的发生发展密切相关, 通过多种分子机制实现. 某些lncRNA可以与DNA、RNA 和蛋白质相互作用, 参与GC的发生和发展, 如MALAT1、GHET1和TINCR等可通过碱基互补配对与mRNA结合或与RNA结合蛋白形成复合物, 介导mRNA的稳定性和拼接. 还有些lncRNA, 如ANRIL、GACAT3、H19、MEG3和TUSC7通过与miR-NAs 结合发挥生物学作用. ANRIL、H19、HOTAIR、MALAT1和PVT1, 可以通过招募组蛋白修饰物, 抑制靶基因的顺式或反式转录. CCAT1、GAPLINC、GAS5、H19、MEG3和PWRN1分别通过调节肿瘤抑制因子p53、癌基因c-myc发挥致癌或抑癌作用[17-19]. 细胞凋亡被认为是抑制细胞生长的另一种途径, caspase-3和-9是细胞凋亡的两个关键成员. TP73-AS1在GC组织标本和细胞中转录水平升高, 其高转录与肿瘤大小偏大和TNM分期偏晚密切相关, 提示TP73-AS1可能是GC的致瘤因子; TP73-AS1基因的敲除显著降低了GC细胞的生长和集落形成能力; TP73-AS1沉默后Bax、caspase-3和-9升高, 而Bcl-2降低, TP73-AS1可以通过Bcl-2/Caspase-3途径保护细胞凋亡[20]. HOTAIR在GC中表达水平越高, 存活率越低. GC细胞株转染HOTAIR后, HOTAIR水平显著升高, miR-618水平明显下降, 而转染si-HOTAIR-2则导致HOTAIR下调, miR-618上调, 由此预测miR-618是HOTAIR的直接靶点, 在GC组织和细胞中被下调[21]. PVT1在胃发育不良及GC组织中的表达显著升高, 与GC发生关系密切[22].
TP73-AS1、HOTAIR、PVT1等lncRNA在GC发生过程中发挥着重要的作用, 其发挥作用的分子学机制有待进一步研究.
LncRNA与miRNA及其靶基因之间内源性竞争的调控模式与GC的发展进程密切相关, 如bc032469、GAPLINC和HOTAIR等. HOXA11-AS通过与PRC2/LSD1/DNMT1相互作用和海绵吸附miR-1297促进细胞生长和GC的侵袭潜能; HOXA11-AS可能与WDR5促进β-catenin转录, 结合EZH2 p21抑制转录, 并通过与STAU1交互诱导KLF2 mRNA降解, 促进GC生长和转移[23,24]. lnc00153在GC中表达增加, 通过激活EGFR/PI3K/Akt通路促进肿瘤生长和迁移[25]. lnc01939低表达与GC进展呈正相关, 高表达的患者总生存和无进展生存期预后更好, lnc01939是GC患者的独立保护因素, 可能在GC的进展和转移中起到抑制作用[26]. lnc00473是一个基因间lncRNA, 位于人类染色体6q27, 是幽门螺杆菌感染的人胃上皮细胞和组织中严重失调的lncRNA之一[27], 高表达与组织学类型分化差、临床分期晚、淋巴结转移和远处转移相关[28], 过表达与临床进展相关. RP11-789C1.1被认为是一种抑癌因子, 在GC中下调, 作为miRNA-5003-3p的内源竞争RNA (competing endogenous RNA, ceRNA)海绵, 并通过EMT进一步在GC的进展和转移中发挥作用, 提示可能作为GC转移的潜在标志物[29]. M26317在61.17%的GC组织中表达上调, 与患者年龄、肿瘤大小、Lauren分型、浸润深度、淋巴结及远处转移、TNM分期、预后均有显著相关性, 提示M26317在GC发生发展过程中影响肿瘤的进展[30]. 在GC细胞中发现LOC285194的显著衰减和Wnt信号通路的强烈激活, 沉默LOC285194可促进GC细胞集落形成、增殖、侵袭和迁移, 抑制细胞凋亡, 且LOC285194的下调通过激活Wnt信号转导促进了GC的进展, 而过表达LOC285194则可抑制GC的发展[31]. LINP1可下调RBM5, 在体内外均能显著促进GC的生长, 抑制其凋亡[32]. Ftx通过上调HK2水平促进GC进展[33], IGFL2-AS1的下调抑制了GC的发展, IGFL2-AS1/miR-802/ARPP19轴在GC的进展和转移中起关键作用[34]. TEAD4调控的MNX1-AS1部分通过抑制EZH2/BTG2和激活miR-6785-5p/BCL2导致GC进展[35]. CR749391通过海绵miR-181a作为调节KLF6表达的ceRNA, 可能是GC中的关键调控因子, CR749391的下调可能是GC进展相关的新标志物[36]. GCRL1在体内外均可调节胃细胞的增殖和转移, 通过海绵化miR-885-3p促进细胞增殖和转移, 从而在GC细胞中积极调节CDK4, 预示了包括GCRL1、miR-885-3p和CDK4GC进展的新调控轴, 也可能成为GC潜在的治疗靶点[37].
LncRNA通过结合转录因子, 干扰其与基因启动子区的结合, 调控转录; 作为分子海绵, 吸附miRNA, 抑制其与mRNA结合; 通过调控蛋白活性, 影响基因转录和表达; 与mRNA结合, 抑制翻译, 影响剪切, 影响mRNA的稳定性, lncRNA的失调可能与GC恶性进展密切相关[38].
LncRNA的多样性和异质性使得肿瘤发生过程更加复杂. lnc00473高表达的GC患者总生存期明显缩短, 高表达是GC患者总生存期的独立不良预后因素. 沉默lnc00473对GC细胞活力没有影响, 但通过调节MMP2、MMP9、ecin和Vimentin的表达, 抑制了GC细胞的迁移和侵袭. lnc00473水平与TCGA数据库中GC患者无病生存时间和总生存时间呈负相关[28]. lnc00483通过上调SPAG9和激活MAPKs在体内外GC模型中促进增殖和抑制凋亡, 对GC的诊断和预后具有相当大的预测价值[39]. UFC1过表达可促进GC细胞的增殖、迁移和侵袭, 沉默后则抑制; 通过海绵吸附miR-498和作为lin28b的ceRNA发挥其致癌活性, UFC1表达水平高与GC患者病情进展及预后不良有关[40]. AFAP1-AS1过表达与GC患者生存时间缩短有关, 并促进GC细胞恶性生物学行为, 部分显著提高GC细胞E-cadherin蛋白、降低N-cadherin蛋白表达水平, 调节EMT过程, 进而影响GC预后[41,42]. MEG3在GC中表达水平明显降低, 过表达可抑制GC的肿瘤进展, 下调MEG3与GC预后不良有关[43,44]. SNHG8通过靶向miR-491/PDGFRA轴促进了GC细胞的增殖和侵袭, 从而影响GC预后[45]. GAPLINC作为miR-211-3p的分子海绵调控CD44, 增强肿瘤的迁移和侵袭, 过表达增加了G2/M细胞周期阶段的细胞数量, 增强了体内肿瘤的生长, 对细胞增殖和细胞周期进程的增强则依赖于MAPK1, 对GC预后预测具有重要价值[46].
LncRNA通过靶向调控miRNA, 从而进一步调控靶基因影响胃癌预后, 因此, 靶向lncRNA可能是一种较好的肿瘤治疗方法.
目前术前和术后联合化疗治疗方案的实施, 提高了GC患者的整体生存率, 但耐药仍然是有效进行癌症化疗的障碍之一[47]. 顺铂是进展期胃癌患者的主要化疗药物. LncRNA 已被证实参与胃癌自噬相关耐药(顺铂或长春新碱)过程[48,49], 抑制自噬可以增加GC细胞对顺铂的敏感性, 降低GC细胞的多药耐药[50]. HULC在耐药GC细胞中高表达, 促进了耐药GC细胞的自噬, 增强GC耐药细胞对顺铂的耐药性[51]. MALAT1通过调节顺铂耐药GC细胞中miR-30b/ATG5轴, 诱导自噬, 增强顺铂耐药[52]. UCA1通过影响凋亡通路调节GC中阿霉素的化学敏感性, 敲低UCA1通过上调RARP和下调Bcl-2来加速阿霉素诱导的GC细胞凋亡[53]. ANRIL在顺铂耐药和5-氟尿嘧啶耐药的GC组织和细胞中上调, ANRIL沉默可通过下调MDR1和MRP1逆转多药耐药[54]. SNHG5在顺铂耐药GC细胞中高表达, 通过介导凋亡和耐药相关基因调控GC顺铂耐药[53]. miR-145-5p可直接靶向多药耐药蛋白1, 增加化疗药物的毒性. MACC1-AS1通过拮抗miR-145-5p促进干细胞形成和耐药[55]. AK022798参与了顺铂抗性SGC7901/DDP和BGC823/DDP的形成过程, 其表达受Notch 1调控, Notch 1增强了细胞的耐药能力, 加重了肿瘤细胞的恶化, 通过干扰AK022798的表达, MRP1和P-gp的表达及细胞增殖下降, SGC7901/DDP和BGC823/DDP细胞的凋亡增加, 靶向AK022798表达可能为晚期GC的治疗提供一种新的方法[56]. PVT1可以激活抗凋亡因子Bcl-2的表达, 在PVT1高表达的GC患者中, PVT1增强了GC对5-Fu的耐药性, 基于非5-Fu的化疗方案可能是更好的治疗选择[57]. miR-27b通过miR-27b/CCNG1/P53/miR-508-5p轴参与GC细胞多耐药的调控. UCA1与miR-27b在GC组织中的表达呈负相关, UCA1的下调恢复了miR-27b在GC细胞中的表达, UCA1-miR-27b轴参与调控GC细胞的化学敏感性[58], 通过灭活THOR/SOX9调节轴可以减弱SCG7901-ddp细胞对顺铂的耐药, THOR在顺铂耐药中的作用, 为靶向THOR治疗GC新发或获得性耐药患者提供了新的选择[59]. HOTAIR作为内源性海绵, 通过竞争性结合和抑制miR-126的表达, 激活PI3K/AKT/MRP1通路的表达和活性, 诱导miR-126靶向VEGFA和PIK3R2在GC细胞中的表达. HOTAIR过表达可通过诱导肿瘤细胞增殖和减少凋亡, 促进GC顺铂耐药[60].
目前化疗药物耐药是限制GC临床治疗效果的一项重要因素, 从lncRNA着手攻克肿瘤耐药问题成为治疗热点. 随着越来越多GC耐药相关lncRNA陆续被研究发现, 但仍处于基础研究阶段, 如何将lncRNA耐药相关的基础研究转化为临床领域的应用还需要多中心、大数据的进一步研究.
由于高通量测序技术、基因组图谱以及生物信息学工具的快速发展, 已经发现了lncRNAs的大量信息. LncRNA在GC发生中发挥着致癌/抑癌基因的作用, 通过多种相关分子机制及信号通路影响着GC的进展及预后. 化疗耐药是临床治疗GC面临的困境, 直接影响着患者的生存. 某些lncRNA可增加或减少对抗肿瘤药物的敏感性, 由于多数研究目前集中在体外细胞学和动物学实验, 对人体肿瘤的具体应用和临床实施还存在着诸多挑战. 早发现、早治疗、有效改善化疗耐药仍然是提高GC患者生存率的关键, 有望通过检测GC发生、进展及预后相关的lncRNA潜在标志物, 预测个体化GC诊治策略, 实现临床实践中的精准治疗.
学科分类: 胃肠病学和肝病学
手稿来源地: 河北省
同行评议报告学术质量分类
A级 (优秀): 0
B级 (非常好): 0
C级 (良好): 0
D级 (一般): D
E级 (差): 0
科学编辑: 张晗 制作编辑:刘继红
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [PubMed] [DOI] |
| 2. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [PubMed] [DOI] |
| 3. | Ba MC, Ba Z, Long H, Cui SZ, Gong YF, Yan ZF, Lin KP, Wu YB, Tu YN. LncRNA AC093818.1 accelerates gastric cancer metastasis by epigenetically promoting PDK1 expression. Cell Death Dis. 2020;11:64. [PubMed] [DOI] |
| 4. | Tang CT, Liang Q, Yang L, Lin XL, Wu S, Chen Y, Zhang XT, Gao YJ, Ge ZZ. RAB31 Targeted by MiR-30c-2-3p Regulates the GLI1 Signaling Pathway, Affecting Gastric Cancer Cell Proliferation and Apoptosis. Front Oncol. 2018;8:554. [PubMed] [DOI] |
| 5. | Ghafouri-Fard S, Esmaeili M, Taheri M. H19 lncRNA: Roles in tumorigenesis. Biomed Pharmacother. 2020;123:109774. [PubMed] [DOI] |
| 7. | Sun LL, Li WD, Lei FR, Li XQ. The regulatory role of microRNAs in angiogenesis-related diseases. J Cell Mol Med. 2018;22:4568-4587. [PubMed] [DOI] |
| 8. | Wang C, Wang L, Ding Y, Lu X, Zhang G, Yang J, Zheng H, Wang H, Jiang Y, Xu L. LncRNA Structural Characteristics in Epigenetic Regulation. Int J Mol Sci. 2017;18:2659. [PubMed] [DOI] |
| 9. | Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17:47-62. [PubMed] [DOI] |
| 10. | Geisler S, Coller J. RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol. 2013;14:699-712. [PubMed] [DOI] |
| 11. | Lau E. Non-coding RNA: Zooming in on lncRNA functions. Nat Rev Genet. 2014;15:574-575. [PubMed] [DOI] |
| 12. | Richard JLC, Eichhorn PJA. Deciphering the roles of lncRNAs in breast development and disease. Oncotarget. 2018;9:20179-20212. [PubMed] [DOI] |
| 13. | Mao Z, Li H, Du B, Cui K, Xing Y, Zhao X, Zai S. LncRNA DANCR promotes migration and invasion through suppression of lncRNA-LET in gastric cancer cells. Biosci Rep. 2017;37:BSR20171070. [PubMed] [DOI] |
| 14. | Zhou Y, Chen Y, Ding W, Hua Z, Wang L, Zhu Y, Qian H, Dai T. LncRNA UCA1 impacts cell proliferation, invasion, and migration of pancreatic cancer through regulating miR-96/FOXO3. IUBMB Life. 2018;70:276-290. [PubMed] [DOI] |
| 15. | Goodwin S, McPherson JD, McCombie WR. Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet. 2016;17:333-351. [PubMed] [DOI] |
| 16. | Cao WJ, Wu HL, He BS, Zhang YS, Zhang ZY. Analysis of long non-coding RNA expression profiles in gastric cancer. World J Gastroenterol. 2013;19:3658-3664. [PubMed] [DOI] |
| 17. | Li T, Mo X, Fu L, Xiao B, Guo J. Molecular mechanisms of long noncoding RNAs on gastric cancer. Oncotarget. 2016;7:8601-8612. [PubMed] [DOI] |
| 18. | Fang XY, Pan HF, Leng RX, Ye DQ. Long noncoding RNAs: novel insights into gastric cancer. Cancer Lett. 2015;356:357-366. [PubMed] [DOI] |
| 19. | Chen Z, Ju H, Yu S, Zhao T, Jing X, Li P, Jia J, Li N, Tan B, Li Y. Prader-Willi region non-protein coding RNA 1 suppressed gastric cancer growth as a competing endogenous RNA of miR-425-5p. Clin Sci (Lond). 2018;132:1003-1019. [PubMed] [DOI] |
| 20. | Zhang W, Zhai Y, Wang W, Cao M, Ma C. Enhanced expression of lncRNA TP73-AS1 predicts unfavorable prognosis for gastric cancer and promotes cell migration and invasion by induction of EMT. Gene. 2018;678:377-383. [PubMed] [DOI] |
| 21. | Xun J, Wang C, Yao J, Gao B, Zhang L. Long Non-Coding RNA HOTAIR Modulates KLF12 to Regulate Gastric Cancer Progression via PI3K/ATK Signaling Pathway by Sponging miR-618. Onco Targets Ther. 2019;12:10323-10334. [PubMed] [DOI] |
| 22. | Niu J, Song X, Zhang X. Regulation of lncRNA PVT1 on miR-125 in metastasis of gastric cancer cells. Oncol Lett. 2020;19:1261-1266. [PubMed] [DOI] |
| 23. | Sun M, Nie F, Wang Y, Zhang Z, Hou J, He D, Xie M, Xu L, De W, Wang Z, Wang J. LncRNA HOXA11-AS Promotes Proliferation and Invasion of Gastric Cancer by Scaffolding the Chromatin Modification Factors PRC2, LSD1, and DNMT1. Cancer Res. 2016;76:6299-6310. [PubMed] [DOI] |
| 24. | Jiang J, Wang X, Gao G, Liu X, Chang H, Xiong R, Lu J, Sun Z. Silencing of lncRNA HOXA11-AS inhibits cell migration, invasion, proliferation, and promotes apoptosis in human glioma cells via upregulating microRNA-125a: in vitro and in vivo studies. Am J Transl Res. 2019;11:6382-6392. [PubMed] |
| 25. | Zhou J, Zhi X, Wang L, Wang W, Li Z, Tang J, Wang J, Zhang Q, Xu Z. Linc00152 promotes proliferation in gastric cancer through the EGFR-dependent pathway. J Exp Clin Cancer Res. 2015;34:135. [PubMed] [DOI] |
| 26. | Chen CL, Ke Q, Luo M, Gao ZY, Li ZJ, Luo ZG, Liu DB. Loss of LINC01939 expression predicts progression and poor survival in gastric cancer. Pathol Res Pract. 2018;214:1539-1543. [PubMed] [DOI] |
| 27. | Zhang L, Wang Y, Li X, Xia X, Li N, He R, He H, Han C, Zhao W. ZBTB7A Enhances Osteosarcoma Chemoresistance by Transcriptionally Repressing lncRNALINC00473-IL24 Activity. Neoplasia. 2017;19:908-918. [PubMed] [DOI] |
| 28. | Zhang W, Song Y. LINC00473 predicts poor prognosis and regulates cell migration and invasion in gastric cancer. Biomed Pharmacother. 2018;107:1-6. [PubMed] [DOI] |
| 29. | Chen Z, Wu J, Huang W, Peng J, Ye J, Yang L, Yuan Y, Chen C, Zhang C, Cai S, He Y, Wu S, Song W. Long Non-Coding RNA RP11-789C1.1 Suppresses Epithelial to Mesenchymal Transition in Gastric Cancer Through the RP11-789C1.1/MiR-5003/E-Cadherin Axis. Cell Physiol Biochem. 2018;47:2432-2444. [PubMed] [DOI] |
| 30. | Li L, Wang YY, Mou XZ, Ye ZY, Zhao ZS. Up-regulation of long noncoding RNA M26317 correlates with tumor progression and poor prognosis in gastric cancer. Hum Pathol. 2018;78:44-53. [PubMed] [DOI] |
| 31. | Zhong B, Wang Q, He J, Xiong Y, Cao J. LncRNA LOC285194 modulates gastric carcinoma progression through activating Wnt/β-catenin signaling pathway. Cancer Med. 2020;9:2181-2189. [PubMed] [DOI] |
| 32. | Lu XC, Zhou HY, Wu J, Jin Y, Yao XM, Wu XY. LncRNA LINP1 promotes proliferation and inhibits apoptosis of gastric cancer cells by repressing RBM5. Eur Rev Med Pharmacol Sci. 2020;24:137-144. [PubMed] [DOI] |
| 33. | Zhu L, Jia R, Zhang J, Li X, Qin C, Zhao Q. Quantitative Proteomics Analysis Revealed the Potential Role of lncRNA Ftx in Promoting Gastric Cancer Progression. Proteomics Clin Appl. 2020;14:e1900053. [PubMed] [DOI] |
| 34. | Ma Y, Liu Y, Pu YS, Cui ML, Mao ZJ, Li ZZ, He L, Wu M, Wang JH. LncRNA IGFL2-AS1 functions as a ceRNA in regulating ARPP19 through competitive binding to miR-802 in gastric cancer. Mol Carcinog. 2020;59:311-322. [PubMed] [DOI] |
| 35. | Shuai Y, Ma Z, Liu W, Yu T, Yan C, Jiang H, Tian S, Xu T, Shu Y. TEAD4 modulated LncRNA MNX1-AS1 contributes to gastric cancer progression partly through suppressing BTG2 and activating BCL2. Mol Cancer. 2020;19:6. [PubMed] [DOI] |
| 36. | Shi S, Li D, Li Y, Feng Z, Du Y, Nie Y. LncRNA CR749391 acts as a tumor suppressor to upregulate KLF6 expression via interacting with miR-181a in gastric cancer. Exp Ther Med. 2020;19:569-578. [PubMed] [DOI] |
| 37. | Lin Z, Zhou Z, Guo H, He Y, Pang X, Zhang X, Liu Y, Ao X, Li P, Wang J. Long noncoding RNA gastric cancer-related lncRNA1 mediates gastric malignancy through miRNA-885-3p and cyclin-dependent kinase 4. Cell Death Dis. 2018;9:607. [PubMed] [DOI] |
| 38. | Yu Z, Zeng J, Liu H, Wang T, Yu Z, Chen J. Role of HDAC1 in the progression of gastric cancer and the correlation with lncRNAs. Oncol Lett. 2019;17:3296-3304. [PubMed] [DOI] |
| 39. | Li D, Yang M, Liao A, Zeng B, Liu D, Yao Y, Hu G, Chen X, Feng Z, Du Y, Zhou Y, He J, Nie Y. Linc00483 as ceRNA regulates proliferation and apoptosis through activating MAPKs in gastric cancer. J Cell Mol Med. 2018;22:3875-3886. [PubMed] [DOI] |
| 40. | Zhang X, Liang W, Liu J, Zang X, Gu J, Pan L, Shi H, Fu M, Huang Z, Zhang Y, Qian H, Jiang P, Xu W. Long non-coding RNA UFC1 promotes gastric cancer progression by regulating miR-498/Lin28b. J Exp Clin Cancer Res. 2018;37:134. [PubMed] [DOI] |
| 41. | Han X, Wang L, Ning Y, Li S, Wang Z. Long non-coding RNA AFAP1-AS1 facilitates tumor growth and promotes metastasis in colorectal cancer. Biol Res. 2016;49:36. [PubMed] [DOI] |
| 42. | Luo HL, Huang MD, Guo JN, Fan RH, Xia XT, He JD, Chen XF. AFAP1-AS1 is upregulated and promotes esophageal squamous cell carcinoma cell proliferation and inhibits cell apoptosis. Cancer Med. 2016;5:2879-2885. [PubMed] [DOI] |
| 43. | Sun M, Xia R, Jin F, Xu T, Liu Z, De W, Liu X. Downregulated long noncoding RNA MEG3 is associated with poor prognosis and promotes cell proliferation in gastric cancer. Tumour Biol. 2014;35:1065-1073. [PubMed] [DOI] |
| 44. | Dan J, Wang J, Wang Y, Zhu M, Yang X, Peng Z, Jiang H, Chen L. LncRNA-MEG3 inhibits proliferation and metastasis by regulating miRNA-21 in gastric cancer. Biomed Pharmacother. 2018;99:931-938. [PubMed] [DOI] |
| 45. | Zhang P, Li S, Chen Z, Lu Y, Zhang H. LncRNA SNHG8 promotes proliferation and invasion of gastric cancer cells by targeting the miR-491/PDGFRA axis. Hum Cell. 2020;33:123-130. [PubMed] [DOI] |
| 46. | He Y, Wu Z, Qiu C, Wang X, Xiang Y, Lu T, He Y, Shang T, Zhu Q, Wang X, Zeng Q, Zhang H, Li D. Long non-coding RNA GAPLINC promotes angiogenesis by regulating miR-211 under hypoxia in human umbilical vein endothelial cells. J Cell Mol Med. 2019;23:8090-8100. [PubMed] [DOI] |
| 47. | Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J, Savastano B, Mabilia A, Lieto E, Ciardiello F, De Vita F. Treatment of gastric cancer. World J Gastroenterol. 2014;20:1635-1649. [PubMed] [DOI] |
| 48. | Xi Z, Si J, Nan J. LncRNA MALAT1 potentiates autophagyassociated cisplatin resistance by regulating the microRNA30b/autophagyrelated gene 5 axis in gastric cancer. Int J Oncol. 2019;54:239-248. [PubMed] [DOI] |
| 49. | YiRen H, YingCong Y, Sunwu Y, Keqin L, Xiaochun T, Senrui C, Ende C, XiZhou L, Yanfan C. Long noncoding RNA MALAT1 regulates autophagy associated chemoresistance via miR-23b-3p sequestration in gastric cancer. Mol Cancer. 2017;16:174. [PubMed] [DOI] |
| 50. | Kumar A, Singh UK, Chaudhary A. Targeting autophagy to overcome drug resistance in cancer therapy. Future Med Chem. 2015;7:1535-1542. [PubMed] [DOI] |
| 51. | Liu T, Liu Y, Wei C, Yang Z, Chang W, Zhang X. LncRNA HULC promotes the progression of gastric cancer by regulating miR-9-5p/MYH9 axis. Biomed Pharmacother. 2020;121:109607. [PubMed] [DOI] |
| 52. | Zhang YF, Li CS, Zhou Y, Lu XH. Propofol facilitates cisplatin sensitivity via lncRNA MALAT1/miR-30e/ATG5 axis through suppressing autophagy in gastric cancer. Life Sci. 2020;244:117280. [PubMed] [DOI] |
| 53. | Li M, Zhang YY, Shang J, Xu YD. LncRNA SNHG5 promotes cisplatin resistance in gastric cancer via inhibiting cell apoptosis. Eur Rev Med Pharmacol Sci. 2019;23:4185-4191. [PubMed] [DOI] |
| 54. | Verstraelen J, Reichl S. Multidrug resistance-associated protein (MRP1, 2, 4 and 5) expression in human corneal cell culture models and animal corneal tissue. Mol Pharm. 2014;11:2160-2171. [PubMed] [DOI] |
| 55. | He W, Liang B, Wang C, Li S, Zhao Y, Huang Q, Liu Z, Yao Z, Wu Q, Liao W, Zhang S, Liu Y, Xiang Y, Liu J, Shi M. MSC-regulated lncRNA MACC1-AS1 promotes stemness and chemoresistance through fatty acid oxidation in gastric cancer. Oncogene. 2019;38:4637-4654. [PubMed] [DOI] |
| 56. | Hang Q, Sun R, Jiang C, Li Y. Notch 1 promotes cisplatin-resistant gastric cancer formation by upregulating lncRNA AK022798 expression. Anticancer Drugs. 2015;26:632-640. [PubMed] [DOI] |
| 57. | Du P, Hu C, Qin Y, Zhao J, Patel R, Fu Y, Zhu M, Zhang W, Huang G. LncRNA PVT1 Mediates Antiapoptosis and 5-Fluorouracil Resistance via Increasing Bcl2 Expression in Gastric Cancer. J Oncol. 2019;2019:9325407. [PubMed] [DOI] |
| 58. | Fang Q, Chen X, Zhi X. Long Non-Coding RNA (LncRNA) Urothelial Carcinoma Associated 1 (UCA1) Increases Multi-Drug Resistance of Gastric Cancer via Downregulating miR-27b. Med Sci Monit. 2016;22:3506-3513. [PubMed] [DOI] |
| 59. | Song H, Xu Y, Shi L, Xu T, Fan R, Cao M, Xu W, Song J. LncRNA THOR increases the stemness of gastric cancer cells via enhancing SOX9 mRNA stability. Biomed Pharmacother. 2018;108:338-346. [PubMed] [DOI] |
| 60. | Yan J, Dang Y, Liu S, Zhang Y, Zhang G. LncRNA HOTAIR promotes cisplatin resistance in gastric cancer by targeting miR-126 to activate the PI3K/AKT/MRP1 genes. Tumour Biol. 2016; Epub ahead of print. [PubMed] [DOI] |
| 61. | Aris RM, Lester GE, Renner JB, Winders A, Denene Blackwood A, Lark RK, Ontjes DA. Efficacy of pamidronate for osteoporosis in patients with cystic fibrosis following lung transplantation. Am J Respir Crit Care Med. 2000;162:941-946. [PubMed] [DOI] |
| 62. | Sun TT, He J, Liang Q, Ren LL, Yan TT, Yu TC, Tang JY, Bao YJ, Hu Y, Lin Y, Sun D, Chen YX, Hong J, Chen H, Zou W, Fang JY. LncRNA GClnc1 Promotes Gastric Carcinogenesis and May Act as a Modular Scaffold of WDR5 and KAT2A Complexes to Specify the Histone Modification Pattern. Cancer Discov. 2016;6:784-801. [PubMed] [DOI] |
| 63. | Xiang JF, Yang L, Chen LL. The long noncoding RNA regulation at the MYC locus. Curr Opin Genet Dev. 2015;33:41-48. [PubMed] [DOI] |
| 64. | Yap KL, Li S, Muñoz-Cabello AM, Raguz S, Zeng L, Mujtaba S, Gil J, Walsh MJ, Zhou MM. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell. 2010;38:662-674. [PubMed] [DOI] |
| 65. | Duan Z, Choy E, Nielsen GP, Rosenberg A, Iafrate J, Yang C, Schwab J, Mankin H, Xavier R, Hornicek FJ. Differential expression of microRNA (miRNA) in chordoma reveals a role for miRNA-1 in Met expression. J Orthop Res. 2010;28:746-752. [PubMed] [DOI] |
| 66. | Gu Y, Chen T, Li G, Yu X, Lu Y, Wang H, Teng L. LncRNAs: emerging biomarkers in gastric cancer. Future Oncol. 2015;11:2427-2441. [PubMed] [DOI] |
| 67. | Yoshimura H, Matsuda Y, Yamamoto M, Kamiya S, Ishiwata T. Expression and role of long non-coding RNA H19 in carcinogenesis. Front Biosci (Landmark Ed). 2018;23:614-625. [PubMed] [DOI] |
| 68. | Shen Y, Zhang S, Li H, Ren Y, Liu H. Efficient synthesis of lactic acid by aerobic oxidation of glycerol on Au-Pt/TiO2 catalysts. Chemistry. 2010;16:7368-7371. [PubMed] [DOI] |
| 69. | Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689-693. [PubMed] [DOI] |
| 70. | Somarowthu S, Legiewicz M, Chillón I, Marcia M, Liu F, Pyle AM. HOTAIR forms an intricate and modular secondary structure. Mol Cell. 2015;58:353-361. [PubMed] [DOI] |
| 71. | Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, Attardi LD, Regev A, Lander ES, Jacks T, Rinn JL. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409-419. [PubMed] [DOI] |
| 72. | Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92-D97. [PubMed] [DOI] |
| 73. | Ankö ML, Neugebauer KM. RNA-protein interactions in vivo: global gets specific. Trends Biochem Sci. 2012;37:255-262. [PubMed] [DOI] |
| 74. | Yan B, Yao J, Liu JY, Li XM, Wang XQ, Li YJ, Tao ZF, Song YC, Chen Q, Jiang Q. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res. 2015;116:1143-1156. [PubMed] [DOI] |
| 75. | Zhang X, Rice K, Wang Y, Chen W, Zhong Y, Nakayama Y, Zhou Y, Klibanski A. Maternally expressed gene 3 (MEG3) noncoding ribonucleic acid: isoform structure, expression, and functions. Endocrinology. 2010;151:939-947. [PubMed] [DOI] |
| 76. | Plath K, Fang J, Mlynarczyk-Evans SK, Cao R, Worringer KA, Wang H, de la Cruz CC, Otte AP, Panning B, Zhang Y. Role of histone H3 lysine 27 methylation in X inactivation. Science. 2003;300:131-135. [PubMed] [DOI] |
| 77. | Novikova IV, Hennelly SP, Sanbonmatsu KY. Structural architecture of the human long non-coding RNA, steroid receptor RNA activator. Nucleic Acids Res. 2012;40:5034-5051. [PubMed] [DOI] |
| 78. | Duszczyk MM, Wutz A, Rybin V, Sattler M. The Xist RNA A-repeat comprises a novel AUCG tetraloop fold and a platform for multimerization. RNA. 2011;17:1973-1982. [PubMed] [DOI] |