修回日期: 2018-11-05
接受日期: 2018-11-15
在线出版日期: 2019-02-28
家族性腺瘤性息肉病(familial adenomatous polyposis, FAP)是一种癌变倾向较高的常染色体显性遗传病. 预防性外科干预的时机和手术方式的选择在以腺瘤的数量、大小和严重程度为主要决定因素的同时, 应结合多方面情况综合考虑, 在有效预防癌变的同时使患者易于接受. 全结直肠切除、回肠贮袋肛管吻合术(ileal pouch anal anastomosis, IPAA)已经成为FAP患者的首选治疗方案. IPAA能够最大限度减少直肠黏膜残留、降低腺瘤复发癌变风险, 而腹腔镜IPAA具有美观、恢复快、黏连少、妊娠率高等明显优势. FAP患者应由在专业的医疗中心工作的经验丰富的外科医生进行管理, 以便在最佳的时机得到最合理的治疗, 达到长期有效的治疗结果.
核心提要: 本文是一篇关于家族性腺瘤性息肉病患者外科手术治疗时机、方案选择策略相关的文献综述, 总结了目前该疾病外科领域的治疗进展, 帮助读者深入认识该疾病的复杂性和外科治疗的有效性, 以便读者了解该疾病并作以参考.
引文著录: 李凯钰, 刘刚. 家族性腺瘤性息肉病的外科治疗进展. 世界华人消化杂志 2019; 27(4): 252-259
Revised: November 5, 2018
Accepted: November 15, 2018
Published online: February 28, 2019
Familial adenomatous polyposis (FAP) is an autosomal dominant genetic disease with a high tendency to develop colorectal cancer. The timing and choice of preventive surgical interventions should be based on the number, size and severity of adenomas, combined with a variety of considerations, in order to effectively prevent cancer and make patients easy to accept. Total proctocolectomy and ileal pouch-anal anastomosis (IPAA) procedure, which could minimize the residual rectal mucosa and reduce the risk of adenoma recurrence, has become the first choice for patients with FAP. Besides, laparoscopic IPAA has obvious advantages such as cosmetic appearance, quick recovery, little adhesion and high pregnancy rate. Patients with FAP should be managed by experienced surgeons working in specialized medical centers in order to get the most reasonable treatment at the best time and achieve long-term effective outcomes.
- Citation: Li KY, Liu G. Progress in surgical treatment of familial adenomatous polyposis. Shijie Huaren Xiaohua Zazhi 2019; 27(4): 252-259
- URL: https://www.wjgnet.com/1009-3079/full/v27/i4/252.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v27.i4.252
家族性腺瘤性息肉病(familial adenomatous polyposis, FAP)又名腺瘤性肠息肉病(adenomatous polyposis coli, APC), 是由5号染色体上的APC基因突变引起的常染色体显性遗传病[1,2], 其经典表型以结肠和直肠中发生成百上千枚腺瘤性息肉为特征, 具有左半优势[3,4]. FAP癌变倾向较高, 如不进行预防性干预, 在40岁以后几乎100%发生癌变[5]. 临床症状、息肉数量和患者年龄是FAP癌变的主要高危因素, 而在低危个体中腺瘤癌变的可能性依然存在[6]. 衰减型FAP(attenuated form, aFAP)在发病年龄、整体息肉负荷(通常为10-100)和癌症风险[7]等方面表现都更为良性, 并且具有右半优势[8]. 对FAP患者的临床管理的目的主要在于预防癌症死亡和保证生活质量. 迄今为止, 唯一合理的且具有良好预后的预防性手段仍是预防性手术切除[9]. 由于FAP病变主要发生于结肠和直肠, 几乎很少发生于小肠, 这为外科手术根治提供了可能. FAP外科手术的发展近半个世纪发生着巨大变化, 本文就其外科治疗进展作如下综述.
通过外科手术切除FAP患者部分肠段实际上是一种预防性治疗手段, 其手术时机在不同中心甚至不同外科医生间存在差异, 目前尚无明确指南. 在防止癌变发生的基础上, 还应充分考虑患者遗传背景、身体一般情况、教育水平、社会情感、财务状况等多方面综合因素以决定手术时机[10]. 由于突变基因携带者从青春期开始就需要进行结肠镜检查, 故患者的教育、社交需求和财务状况等方面因素在决定手术时机时显得尤为突出, 应予以充分考虑合理规划手术[11].
临床症状作为FAP癌变的高危因素影响着手术时机的决策. FAP的"特异性"症状包括血便、贫血、黏液便、腹泻、反复便秘腹泻交替等[12]. 有症状患者的结直肠癌的发生率约为60%[13], 当患者出现临床症状时往往表明癌变可能已经发生, 故即使是结直肠癌发病率较低的青少年FAP患者, 一旦出现症状也应尽早进行手术治疗[14].
1.2 肠镜表现 特定的肠镜下和组织病理学特征可能给予预防性结直肠切除充分的理由. 研究表明[6], 结肠息肉数量>1000枚的患者较<1000枚的患者癌症风险增加2.3倍. 且由于息肉较多的患者(>1000枚)内镜下检测和预防癌变存在困难, 故即使无症状的严重FAP患者(结肠息肉>1000枚和/或直肠息肉>20枚)一经发现也应尽早手术[14]. 中等量息肉患者(100-1000枚)及少量息肉患者(<100枚)需每年复查结肠镜, 若腺瘤直径<9 mm且无高度不典型增生, 可逐年推迟手术. 若腺瘤数量迅速增多、存在高度不典型增生、或腺瘤直径超过10 mm, 应立即手术治疗.
1.3 年龄 年龄同样决定着手术时机的把握. 年龄低于20岁的患者中, 癌症患病风险约为1%[15], 而在40岁以上的患者中, 65%被诊断为结直肠癌[12]. 此外, 每增加10岁年龄组的癌症风险增加2.4倍, 故FAP患者最好在25岁前行手术治疗[16]. 对于有明确家族史的儿童, 手术可推迟至十几岁[17], 待身体和心智发育成熟后再考虑手术治疗[18].
1.4 硬纤维瘤 硬纤维瘤是影响FAP患者生活质量的最具破坏性的因素, 也是FAP患者死亡的重要原因. 所有FAP患者必须被视为对于硬纤维瘤(肠系膜性纤维瘤病)具有潜在的敏感性. 由于手术创伤会触发硬纤维瘤的生长, 对于已发现硬纤维瘤家族史或个人病史的患者, 建议尽可能安全地推迟预防性结直肠手术[19].
遗传信息是否能够指导手术时机的选择尚未达成共识. APC基因型可能与FAP的严重程度有关. 重度FAP患者基因突变多存在于密码子1250和1464之间, 尤其是密码子1309突变; 而轻度FAP患者的基因突变多发生于基因的末端和外显子9的可变剪接部分[20]. 但由于疾病的发生发展过程相当多变, 即使在同一家系的不同个体间也存在着较大差异, 治疗时机的把握应该根据个体患者的结肠镜检查结果决定, 而非单纯基于基因突变位点[21]. Lynch等[22]人提出了一项以标准化FAP疾病进展为基础的评分系统以期明确阶段特异性干预措施. 该评分系统纳入了临床症状、组织病理学、年龄、职业、社会活动、硬纤维病史或家族史、既往手术、括约肌或骨盆功能障°等诸多因素, 在标准化结直肠疾病进展的评估方面显示出了巨大的潜能, 但其能否在实际应用中实现标准化测量以及干预策略的有效性, 仍需大量研究评估(表1).
| 影响因素 | 手术时机 |
| 出现明显临床症状者(具有高CRC风险) | 尽快手术 |
| 少量息肉的无症状患者 | 可观察随诊 |
| 息肉较大或伴高度不典型增生 | 立即手术 |
| 重度FAP肠镜表现/家族史/基因型 | 尽快手术 |
| 衰减型FAP肠镜表现/家族史/基因型 | 根据患者意愿(建议25岁前) |
| 存在硬纤维瘤家族史或遗传易感性 | 延迟手术(需评估CRC风险) |
FAP外科手术治疗的目的是通过切除已存在病变和可能发生病变的肠段预防结直肠癌的发生发展. 最初, 在结直肠全部切除后直接在腹壁进行永久性末端回肠造口术, 这种方法虽然切除了全部病变肠段, 有效预防了息肉的复发及结直肠癌的发生, 但永久性回肠造口严重影响了患者术后的生活质量, 降低了其社交参与度与社会融入感, 由此引发的情感心理问题也随之而来. 尤其对于年龄较小的患者及其家属, 永久性回肠造口多无法被接受. 而作为一种预防性治疗措施, 永久性回肠造口也并不理想, 目前已很少应用于FAP的预防性治疗.
为了保留肛门功能, 全结直肠切除、回肠-肛管吻合术[23], 部分结直肠切除、盲肠/升结肠-直肠吻合术等术式随后被引入FAP的治疗, 但由于术后便次较多严重降低患者生存质量、存在息肉复发及癌变高风险等原因, 在FAP的临床治疗上并未得到广泛应用. 目前, FAP手术方式的选择主要是结直肠次全切除、回肠-直肠吻合术(ileorectal anastomosis, IRA)和全结直肠切除、回肠贮袋-肛管吻合术(ileal pouch anal anastomosis, IPAA)的选择[24,25].
IRA手术切除了全部结肠, 只保留了部分直肠, 在有效预防了残余结直肠过多带来的FAP复发及癌变高风险的基础上, 保留了直肠的粪便存贮功能防止术后排便次数过多. 它避免了直肠周围神经损伤带来的危险和后遗症, 且手术操作相对简单, 并发症较少, 术后肠功能恢复更接近正常[26]. 但残余直肠仍存在复发风险, 需终身进行结肠镜检查, 及时内镜下切除直肠残留息肉, 且最终可能仍需要切除直肠. 1996年, Vasen等人提出[27]1250位点前基因突变的患者疾病发展更缓慢, 推荐此类患者行IRA手术治疗. IRA被推荐用于直肠腺瘤较少且有轻度表型家族史的年轻患者和轻度FAP患者, 前提是需终身接受结肠镜检查.
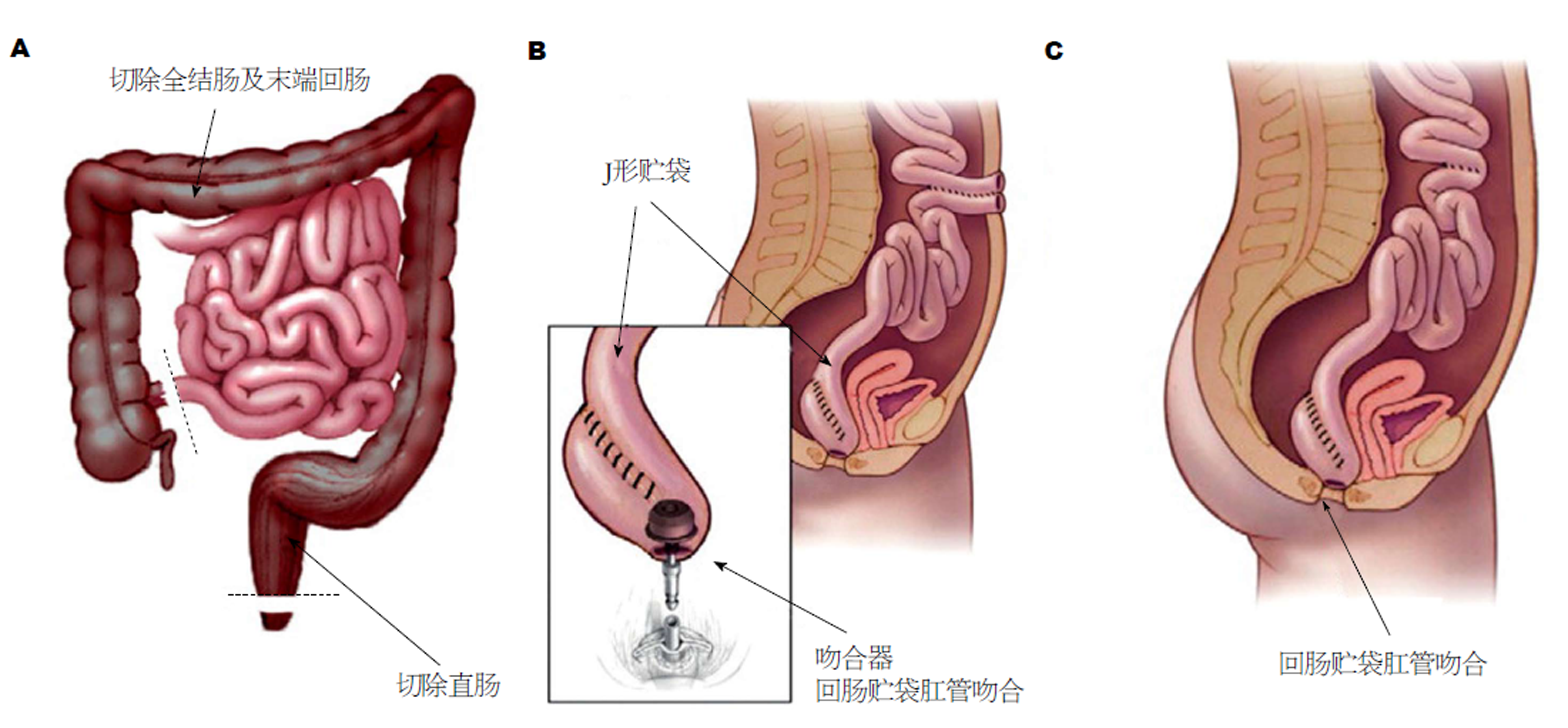
对于重度FAP患者及直肠息肉较多的患者, 切除全部结直肠是十分必要的, 任何直肠黏膜的残留都会带来腺瘤癌变的风险, 而为了减少回肠内容物对肛门的直接刺激, IPAA应运而生(图1). 该术式去除了几乎所有结直肠黏膜, 直肠癌风险最小[28]; 既避免了永久性回肠造口, 又有效控制了便失禁情况, 患者术后生存质量得到明显改善[29]. IPAA在儿童FAP患者中应用的安全性也得到了证实[30]. 但其手术操作相对复杂, 直肠周围神经保护相当重要, 尤其对于年轻患者. 且较低位的吻合往往需要预防性造口, 二次手术打击也应在决定手术方式时予以考虑. 演变而来的还有回肠贮袋-直肠残端吻合术, 在一定程度上降低了手术难度, 但残留的直肠仍存在复发风险, 而手术操作较IRA复杂, 临床已很少使用(表2).
| 术式 | IPAA | IRA |
| 适应证 | 直肠腺瘤数量较多(>20枚)或较大(>2 cm)或伴高度不典型增生者; 结直肠癌变者; 经典表型FAP; 重度表型家族史 | 直肠腺瘤<20枚者; aFAP; 轻度表型家族史; 有生育需求的年轻女性 |
| 优势 | 癌变风险低, 生存质量较高 | 操作相对简单, 功能恢复良好; 并发症较少 |
| 劣势 | 操作复杂, 并发症风险较大; 多需预防性回肠造口, 行二期手术 | 终生直肠癌风险, 再次手术直肠切除可能较大 |
| 并发症 | 盆腔神经损伤, 远期贮袋炎等 | 肠梗阻、吻合口瘘等 |
| 随访 | 肠镜监测贮袋状况 | 肠镜监测直肠有无癌变 |
对于直肠中存在较大息肉的经典表型FAP患者, 推荐首选IPAA手术治疗. IPAA可以更彻底地清除肠黏膜, 最大限度地降低直肠癌的风险, 但由于技术的复杂性, 这一术式对外科医生手术技术要求较高[31,32], 且存在较高的并发症发生率[3,33], 如盆神经损伤, 可能导致泌尿功能和性功能障°, 女性的生育能力下降[34], 术后贮袋失败废弃风险[35,36]等. 外科医生的经验和技术可能会影响最终的术式选择及预后[37].
IRA作为保留直肠的预防性结肠切除术, 术后具有良好的功能结果, 但术后残余直肠腺瘤复发癌变风险较高, 需要终生进行肠镜检查, 监测直肠疾病进展. 在IPAA出现以后, 术后直肠癌的发生率从15%-40%降低至不到10%[38,39]. IRA通常推荐用于直肠息肉少的患者、aFAP、轻度表型家族史以及有怀孕需求的年轻女性, 其余患者均应接受IPAA[39,40].
IPAA和IRA的选择实际上是是否切除直肠的抉择. 芬兰一项研究表明[41], 结肠切除30年后, 二次行直肠切除术的累积风险为53%. 尽管长期监测, 仍有44%的人发生癌变. 这种风险与息肉的严重程度密切相关[42], 20个直肠腺瘤的阈值是决定是否切除直肠的合理方法[43]. 在经验丰富的FAP治疗中心推荐直肠息肉大于20枚的患者行IPAA术. 而手术治疗多年后腺瘤仍可能在回肠贮袋内发生[44], 同时存在一定的恶变风险[40,45].
2016年, Church等[11]人提出存在硬纤维瘤高风险的患者应行腹腔镜IRA手术, 其术后硬纤维瘤发生风险可能较低, 应尽量避免使用IPAA术, 尤其是避免使用腹腔镜IPAA. 因为在13%的案例中硬纤维瘤很可能在全结直肠切除后影响贮袋下拉至盆腔行IPAA手术, 但还没有数据表明术后发生硬纤维瘤会影响IRA以后的直肠切除[28]. 如果已患有硬纤维瘤的患者需要行全结直肠切除术, 肠切除后多采用末端回肠造口术.
微创技术的出现减轻了腹部结直肠切除术后患者的虚弱和痛苦程度, 腹腔镜手术的优势显而易见. 腹腔镜技术在直肠癌治疗中的应用价值已得到证实[46], 一些研究也验证了其在溃疡性结肠炎和FAP患者治疗中的有效性、可行性及安全性[47]. 而这些疾病通常累及年轻、有活力、自身形象意识较强的患者, 是微创技术应用的理想领域. 因其较开腹手术术后瘢痕小、更加美观而易被年轻人接受[48]. 除了能减小体表瘢痕外, 腹腔镜技术还可能具有其他潜在优势.
回顾腹腔镜手术相关文献, 尽管手术时间可能相对较长, 但在生存率[49]、并发症发生率、再次手术概率等方面与开腹手术组相比无明显差异[50]. 腹腔镜对术野的放大作用使得解剖层面能够显现得更加清晰, 无血管平面的游离使手术失血更少[51,52]. 此外, 由于腹腔镜手术创伤小, 感染风险更小[53], 患者术后恢复较快, 能够更快地恢复肠道的连续性[54]. 有大量证据表明[55], 腹腔镜手术对组织骚扰小, 术后腹腔和盆腔黏连较少, 可降低术后肠梗阻风险[56]、提高生育能力[57]. 术后妊娠率较高[57,58]使腹腔镜IPAA手术成为年轻女性FAP患者的最佳选择. 一项前瞻性随机对照研究表明[59], 通过腹腔镜进行结直肠切除、回肠贮袋肛管吻合术似乎没有任何缺点, 尽管这项研究因受试者多要求入腹腔镜手术组而被迫提前终止.
1978年, Parks等[60]首次描述了在黏膜切除术后, 于齿状线高度进行手工缝合的方法, 完成回肠贮袋-肛管吻合术. 这种方法理论上可以去除所有具有肿瘤潜能的黏膜, 但仍可能残存部分黏膜岛的报道一出, 证明在溃疡性结肠炎和FAP患者中除去所有风险黏膜的假设优势证明并不充分. 尽管黏膜切除可以降低腺瘤和癌症形成的风险, 但却会影响术后肛门功能[61]. 1986年, Heald[62]提出了双吻合器吻合法, 除了技术上更容易、更快捷以外, 与手工缝合相比, 由于括约肌操作较少、吻合口上方保留了部分直肠袖带, 这一方法能达到较好的吻合效果且术后肛门功能恢复更好. 但同时, 术后腺瘤复发风险可能更高, 也可能提高直肠癌的发病率[63,64]. 长久以来, 吻合器吻合和手工缝合利弊的比较一直受到学者们的关注. 一项Meta分析显示[61], 吻合器组的整体功能效果和可控性更好, 但吻合口愈合不良的发生率较高, 这在一项仅包含FAP患者的研究中得到证实[59]. 有研究表明[65,66], 手缝组的脓毒性并发症发生率较高, 可能产生肛门刺激症状, 且存在一定的吻合口瘘和吻合口狭窄发生率.
总体来说, 吻合器IPAA的优点在于它比手工缝合更简单, 吻合更确切, 并发症少, 具有较高的安全性[61]. 而其缺点是残余直肠或肛管移行区(anal transition zone, ATZ)易导致腺瘤复发, 这种风险约为手缝IPAA的两倍[67], 而不予干预复发腺瘤的ATZ最终可能导致癌变[68]. 但无论手缝还是吻合器吻合, 残余的直肠黏膜都可能导致腺瘤复发, 故每年进行结肠镜检查对所有回肠贮袋患者都是十分必要的[68]. 此外, 而由于目前研究随访时间较短, 且缺乏大规模的前瞻性研究, 残余直肠相关腺瘤形成的远期风险尚不清楚. 但随着近期FAP患者腺瘤复发率较高的报道的出现, 一些学者可能改变其对于吻合器技术的偏好.
学科分类: 胃肠病学和肝病学
手稿来源地: 天津市
同行评议报告分类
A级 (优秀): A
B级 (非常好): 0
C级 (良好): C
D级 (一般): 0
E级 (差): E
编辑: 崔丽君 电编:张砚梁
| 1. | Bodmer WF, Bailey CJ, Bodmer J, Bussey HJ, Ellis A, Gorman P, Lucibello FC, Murday VA, Rider SH, Scambler P. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987;328:614-616. [PubMed] [DOI] |
| 2. | Bisgaard ML, Fenger K, Bülow S, Niebuhr E, Mohr J. Familial adenomatous polyposis (FAP): frequency, penetrance, and mutation rate. Hum Mutat. 1994;3:121-125. [PubMed] [DOI] |
| 3. | Ganschow P, Pfeiffer U, Hinz U, Leowardi C, Herfarth C, Kadmon M. Quality of life ten and more years after restorative proctocolectomy for patients with familial adenomatous polyposis coli. Dis Colon Rectum. 2010;53:1381-1387. [PubMed] [DOI] |
| 4. | Guillem JG, Smith AJ, Calle JP, Ruo L. Gastrointestinal polyposis syndromes. Curr Probl Surg. 1999;36:217-323. [PubMed] |
| 5. | Rhodes M, Bradburn DM. Overview of screening and management of familial adenomatous polyposis. Gut. 1992;33:125-131. [PubMed] |
| 6. | Debinski HS, Love S, Spigelman AD, Phillips RK. Colorectal polyp counts and cancer risk in familial adenomatous polyposis. Gastroenterology. 1996;110:1028-1030. [PubMed] |
| 7. | Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis. 2009;4:22. [PubMed] [DOI] |
| 8. | Aretz S, Vasen HF, Olschwang S. Clinical Utility Gene Card for: Familial adenomatous polyposis (FAP) and attenuated FAP (AFAP)--update 2014. Eur J Hum Genet. 2015;23. [PubMed] [DOI] |
| 9. | Yamadera M, Ueno H, Kobayashi H, Konishi T, Ishida F, Yamaguchi T, Hinoi T, Inoue Y, Kanemitsu Y, Tomita N, Ishida H, Sugihara K. Current status of prophylactic surgical treatment for familial adenomatous polyposis in Japan. Surg Today. 2017;47:690-696. [PubMed] [DOI] |
| 10. | Chittleborough TJ, Warrier SK, Heriot AG, Kalady M, Church J. Dispelling misconceptions in the management of familial adenomatous polyposis. ANZ J Surg. 2017;87:441-445. [PubMed] [DOI] |
| 11. | Church JM. Controversies in the surgery of patients with familial adenomatous polyposis and Lynch syndrome. Fam Cancer. 2016;15:447-451. [PubMed] [DOI] |
| 12. | Croner RS, Brueckl WM, Reingruber B, Hohenberger W, Guenther K. Age and manifestation related symptoms in familial adenomatous polyposis. BMC Cancer. 2005;5:24. [PubMed] [DOI] |
| 13. | Bülow S. Results of national registration of familial adenomatous polyposis. Gut. 2003;52:742-746. [PubMed] |
| 14. | Church JM, McGannon E, Burke C, Clark B. Teenagers with familial adenomatous polyposis: what is their risk for colorectal cancer? Dis Colon Rectum. 2002;45:887-889. [PubMed] |
| 15. | Vasen HF, Möslein G, Alonso A, Aretz S, Bernstein I, Bertario L, Blanco I, Bülow S, Burn J, Capella G, Colas C, Engel C, Frayling I, Friedl W, Hes FJ, Hodgson S, Järvinen H, Mecklin JP, Møller P, Myrhøi T, Nagengast FM, Parc Y, Phillips R, Clark SK, de Leon MP, Renkonen-Sinisalo L, Sampson JR, Stormorken A, Tejpar S, Thomas HJ, Wijnen J. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut. 2008;57:704-713. [PubMed] [DOI] |
| 16. | Kobayashi H, Ishida H, Ueno H, Hinoi T, Inoue Y, Ishida F, Kanemitsu Y, Konishi T, Yamaguchi T, Tomita N, Matsubara N, Watanabe T, Sugihara K. Association between the age and the development of colorectal cancer in patients with familial adenomatous polyposis: a multi-institutional study. Surg Today. 2017;47:470-475. [PubMed] [DOI] |
| 17. | McGrath DR, Spigelman AD. In the beginning there was colectomy: current surgical options in familial adenomatous polyposis. Hered Cancer Clin Pract. 2004;2:153-160. [PubMed] [DOI] |
| 18. | Warrier SK, Kalady MF. Familial adenomatous polyposis: challenges and pitfalls of surgical treatment. Clin Colon Rectal Surg. 2012;25:83-89. [PubMed] [DOI] |
| 19. | Sturt NJ, Clark SK. Current ideas in desmoid tumours. Fam Cancer. 2006;5:275-285; discussion 287-288. [PubMed] [DOI] |
| 20. | Nieuwenhuis MH, Mathus-Vliegen LM, Slors FJ, Griffioen G, Nagengast FM, Schouten WR, Kleibeuker JH, Vasen HF. Genotype-phenotype correlations as a guide in the management of familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2007;5:374-378. [PubMed] [DOI] |
| 21. | Friedl W, Caspari R, Sengteller M, Uhlhaas S, Lamberti C, Jungck M, Kadmon M, Wolf M, Fahnenstich J, Gebert J, Möslein G, Mangold E, Propping P. Can APC mutation analysis contribute to therapeutic decisions in familial adenomatous polyposis? Experience from 680 FAP families. Gut. 2001;48:515-521. [PubMed] |
| 22. | Lynch PM, Morris JS, Wen S, Advani SM, Ross W, Chang GJ, Rodriguez-Bigas M, Raju GS, Ricciardiello L, Iwama T, Rossi BM, Pellise M, Stoffel E, Wise PE, Bertario L, Saunders B, Burt R, Belluzzi A, Ahnen D, Matsubara N, Bülow S, Jespersen N, Clark SK, Erdman SH, Markowitz AJ, Bernstein I, De Haas N, Syngal S, Moeslein G. A proposed staging system and stage-specific interventions for familial adenomatous polyposis. Gastrointest Endosc. 2016;84:115-125.e4. [PubMed] [DOI] |
| 23. | Kolster CE, Moore D, Carpenter S, Filler R, Shandling B, Wesson D, Sherman P. [Ileoanal anastomosis with and without reservoir in children and adolescents with ulcerative rectocolitis and familial polyposis]. GEN. 1989;43:261-265. [PubMed] |
| 24. | Vasen HF, van Duijvendijk P, Buskens E, Bülow C, Björk J, Järvinen HJ, Bülow S. Decision analysis in the surgical treatment of patients with familial adenomatous polyposis: a Dutch-Scandinavian collaborative study including 659 patients. Gut. 2001;49:231-235. [PubMed] |
| 25. | Wolthuis AM, Leonard D, Kartheuser A, Bruyninx L, Van De Stadt J, Van Cutsem E, D'Hoore A. Different surgical strategies in the treatment of familial adenomatous polyposis: what's the role of the ileorectal anastomosis? Acta Gastroenterol Belg. 2011;74:435-437. [PubMed] |
| 26. | Church JM, Fazio VW, Lavery IC, Oakley JR, Milsom J, McGannon E. Quality of life after prophylactic colectomy and ileorectal anastomosis in patients with familial adenomatous polyposis. Dis Colon Rectum. 1996;39:1404-1408. [PubMed] |
| 27. | Vasen HF, van der Luijt RB, Slors JF, Buskens E, de Ruiter P, Baeten CG, Schouten WR, Oostvogel HJ, Kuijpers JH, Tops CM, Meera Khan P. Molecular genetic tests as a guide to surgical management of familial adenomatous polyposis. Lancet. 1996;348:433-435. [PubMed] |
| 28. | Church JM, Xhaja X, Warrier SK, Laguardia L, O'Malley M, Burke C, Kalady MF. Desmoid tumors do not prevent proctectomy following abdominal colectomy and ileorectal anastomosis in patients with familial adenomatous polyposis. Dis Colon Rectum. 2014;57:343-347. [PubMed] [DOI] |
| 29. | Köhler LW, Pemberton JH, Zinsmeister AR, Kelly KA. Quality of life after proctocolectomy. A comparison of Brooke ileostomy, Kock pouch, and ileal pouch-anal anastomosis. Gastroenterology. 1991;101:679-684. [PubMed] |
| 30. | Diederen K, Sahami SS, Tabbers MM, Benninga MA, Kindermann A, Tanis PJ, Oomen MW, de Jong JR, Bemelman WA. Outcome after restorative proctocolectomy and ileal pouch-anal anastomosis in children and adults. Br J Surg. 2017;104:1640-1647. [PubMed] [DOI] |
| 31. | Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606-1619. [PubMed] [DOI] |
| 32. | Rencuzogullari A, Stocchi L, Costedio M, Gorgun E, Kessler H, Remzi FH. Characteristics of learning curve in minimally invasive ileal pouch-anal anastomosis in a single institution. Surg Endosc. 2017;31:1083-1092. [PubMed] [DOI] |
| 33. | Fazio VW, Kiran RP, Remzi FH, Coffey JC, Heneghan HM, Kirat HT, Manilich E, Shen B, Martin ST. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg. 2013;257:679-685. [PubMed] [DOI] |
| 34. | Olsen KØ, Juul S, Bülow S, Järvinen HJ, Bakka A, Björk J, Oresland T, Laurberg S. Female fecundity before and after operation for familial adenomatous polyposis. Br J Surg. 2003;90:227-231. [PubMed] [DOI] |
| 35. | Sahami S, Bartels SA, D'Hoore A, Young Fadok T, Tanis PJ, de Buck van Overstraeten A, Wolthuis AM, Buskens CJ, Bemelman WA. External validation of a prognostic model of preoperative risk factors for failure of restorative proctocolectomy. Colorectal Dis. 2017;19:181-187. [PubMed] [DOI] |
| 36. | Quinn KP, Lightner AL, Pendegraft RS, Enders FT, Boardman LA, Raffals LE. Pouchitis Is a Common Complication in Patients With Familial Adenomatous Polyposis Following Ileal Pouch-Anal Anastomosis. Clin Gastroenterol Hepatol. 2016;14:1296-1301. [PubMed] [DOI] |
| 37. | Tanaka M, Kanemitsu Y, Ueno H, Kobayashi H, Konishi T, Ishida F, Yamaguchi T, Hinoi T, Inoue Y, Tomita N, Ishida H, Sugihara K. Prognostic impact of hospital volume on familial adenomatous polyposis: a nationwide multicenter study. Int J Colorectal Dis. 2017;32:1489-1498. [PubMed] [DOI] |
| 38. | Smith KD, Rodriguez-Bigas MA. Role of surgery in familial adenomatous polyposis and hereditary nonpolyposis colorectal cancer (Lynch syndrome). Surg Oncol Clin N Am. 2009;18:705-715. [PubMed] [DOI] |
| 39. | Bülow S, Bülow C, Vasen H, Järvinen H, Björk J, Christensen IJ. Colectomy and ileorectal anastomosis is still an option for selected patients with familial adenomatous polyposis. Dis Colon Rectum. 2008;51:1318-1323. [PubMed] [DOI] |
| 40. | Campos FG, Perez RO, Imperiale AR, Seid VE, Nahas SC, Cecconello I. Surgical treatment of familial adenomatous polyposis: ileorectal anastomosis or restorative proctolectomy? Arq Gastroenterol. 2009;46:294-299. [PubMed] |
| 41. | Koskenvuo L, Renkonen-Sinisalo L, Järvinen HJ, Lepistö A. Risk of cancer and secondary proctectomy after colectomy and ileorectal anastomosis in familial adenomatous polyposis. Int J Colorectal Dis. 2014;29:225-230. [PubMed] [DOI] |
| 42. | Church J, Burke C, McGannon E, Pastean O, Clark B. Risk of rectal cancer in patients after colectomy and ileorectal anastomosis for familial adenomatous polyposis: a function of available surgical options. Dis Colon Rectum. 2003;46:1175-1181. [PubMed] [DOI] |
| 43. | Church J, Burke C, McGannon E, Pastean O, Clark B. Predicting polyposis severity by proctoscopy: how reliable is it? Dis Colon Rectum. 2001;44:1249-1254. [PubMed] |
| 44. | Wasmuth HH, Tranø G, Myrvold HE, Aabakken L, Bakka A. Adenoma formation and malignancy after restorative proctocolectomy with or without mucosectomy in patients with familial adenomatous polyposis. Dis Colon Rectum. 2013;56:288-294. [PubMed] [DOI] |
| 45. | Tonelli F, Ficari F, Bargellini T, Valanzano R. Ileal pouch adenomas and carcinomas after restorative proctocolectomy for familial adenomatous polyposis. Dis Colon Rectum. 2012;55:322-329. [PubMed] [DOI] |
| 46. | Bonjer HJ, Deijen CL, Haglind E; COLOR II Study Group. A Randomized Trial of Laparoscopic versus Open Surgery for Rectal Cancer. N Engl J Med. 2015;373:194. [PubMed] [DOI] |
| 47. | da Luz Moreira A, Church JM, Burke CA. The evolution of prophylactic colorectal surgery for familial adenomatous polyposis. Dis Colon Rectum. 2009;52:1481-1486. [PubMed] [DOI] |
| 48. | Ueno H, Kobayashi H, Konishi T, Ishida F, Yamaguchi T, Hinoi T, Kanemitsu Y, Inoue Y, Tomita N, Matsubara N, Komori K, Ozawa H, Nagasaka T, Hasegawa H, Koyama M, Akagi Y, Yatsuoka T, Kumamoto K, Kurachi K, Tanakaya K, Yoshimatsu K, Watanabe T, Sugihara K, Ishida H. Prevalence of laparoscopic surgical treatment and its clinical outcomes in patients with familial adenomatous polyposis in Japan. Int J Clin Oncol. 2016;21:713-722. [PubMed] [DOI] |
| 49. | Seshadri RA, Swaminathan R, Srinivasan A. Laparoscopic versus open surgery for rectal cancer after neoadjuvant chemoradiation: Long-term outcomes of a propensity score matched study. J Surg Oncol. 2018;117:506-513. [PubMed] [DOI] |
| 50. | Campos FG, Real Martinez CA, Monteiro de Camargo MG, Cesconetto DM, Nahas SC, Cecconello I. Laparoscopic Versus Open Restorative Proctocolectomy for Familial Adenomatous Polyposis. J Laparoendosc Adv Surg Tech A. 2018;28:47-52. [PubMed] [DOI] |
| 51. | Zhang X, Wu Q, Hu T, Gu C, Bi L, Wang Z. Laparoscopic Versus Conventional Open Surgery in Intersphincteric Resection for Low Rectal Cancer: A Systematic Review and Meta-Analysis. J Laparoendosc Adv Surg Tech A. 2018;28:189-200. [PubMed] [DOI] |
| 52. | Hida K, Okamura R, Sakai Y, Konishi T, Akagi T, Yamaguchi T, Akiyoshi T, Fukuda M, Yamamoto S, Yamamoto M, Nishigori T, Kawada K, Hasegawa S, Morita S, Watanabe M; Japan Society of Laparoscopic Colorectal Surgery. Open versus Laparoscopic Surgery for Advanced Low Rectal Cancer: A Large, Multicenter, Propensity Score Matched Cohort Study in Japan. Ann Surg. 2018;268:318-324. [PubMed] [DOI] |
| 53. | Quintana JM, Anton-Ladislao A, Lázaro S, Gonzalez N, Bare M, de Larrea NF, Redondo M, Briones E, Escobar A, Sarasqueta C, Garcia-Gutierrez S; REDISSEC-CARESS/CCR group. Outcomes of open versus laparoscopic surgery in patients with rectal cancer. Int J Colorectal Dis. 2018;33:99-103. [PubMed] [DOI] |
| 54. | Fajardo AD, Dharmarajan S, George V, Hunt SR, Birnbaum EH, Fleshman JW, Mutch MG. Laparoscopic versus open 2-stage ileal pouch: laparoscopic approach allows for faster restoration of intestinal continuity. J Am Coll Surg. 2010;211:377-383. [PubMed] [DOI] |
| 55. | Indar AA, Efron JE, Young-Fadok TM. Laparoscopic ileal pouch-anal anastomosis reduces abdominal and pelvic adhesions. Surg Endosc. 2009;23:174-177. [PubMed] [DOI] |
| 56. | Dolejs S, Kennedy G, Heise CP. Small bowel obstruction following restorative proctocolectomy: affected by a laparoscopic approach? J Surg Res. 2011;170:202-208. [PubMed] [DOI] |
| 57. | Beyer-Berjot L, Maggiori L, Birnbaum D, Lefevre JH, Berdah S, Panis Y. A total laparoscopic approach reduces the infertility rate after ileal pouch-anal anastomosis: a 2-center study. Ann Surg. 2013;258:275-282. [PubMed] [DOI] |
| 58. | Bartels SA, DʼHoore A, Cuesta MA, Bensdorp AJ, Lucas C, Bemelman WA. Significantly increased pregnancy rates after laparoscopic restorative proctocolectomy: a cross-sectional study. Ann Surg. 2012;256:1045-1048. [PubMed] [DOI] |
| 59. | Ganschow P, Treiber I, Hinz U, Leowardi C, Büchler MW, Kadmon M. Residual rectal mucosa after stapled vs. handsewn ileal J-pouch-anal anastomosis in patients with familial adenomatous polyposis coli (FAP)--a critical issue. Langenbecks Arch Surg. 2015;400:213-219. [PubMed] [DOI] |
| 60. | Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J. 1978;2:85-88. [PubMed] |
| 61. | Lovegrove RE, Constantinides VA, Heriot AG, Athanasiou T, Darzi A, Remzi FH, Nicholls RJ, Fazio VW, Tekkis PP. A comparison of hand-sewn versus stapled ileal pouch anal anastomosis (IPAA) following proctocolectomy: a meta-analysis of 4183 patients. Ann Surg. 2006;244:18-26. [PubMed] [DOI] |
| 62. | Heald RJ, Allen DR. Stapled ileo-anal anastomosis: a technique to avoid mucosal proctectomy in the ileal pouch operation. Br J Surg. 1986;73:571-572. [PubMed] |
| 63. | von Roon AC, Will OC, Man RF, Neale KF, Phillips RK, Nicholls RJ, Clark SK, Tekkis PP. Mucosectomy with handsewn anastomosis reduces the risk of adenoma formation in the anorectal segment after restorative proctocolectomy for familial adenomatous polyposis. Ann Surg. 2011;253:314-317. [PubMed] [DOI] |
| 64. | Friederich P, de Jong AE, Mathus-Vliegen LM, Dekker E, Krieken HH, Dees J, Nagengast FM, Vasen HF. Risk of developing adenomas and carcinomas in the ileal pouch in patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2008;6:1237-1242. [PubMed] [DOI] |
| 65. | Kirat HT, Remzi FH, Kiran RP, Fazio VW. Comparison of outcomes after hand-sewn versus stapled ileal pouch-anal anastomosis in 3,109 patients. Surgery. 2009;146:723-729; discussion 729-730. [PubMed] [DOI] |
| 66. | Lewis WG, Kuzu A, Sagar PM, Holdsworth PJ, Johnston D. Stricture at the pouch-anal anastomosis after restorative proctocolectomy. Dis Colon Rectum. 1994;37:120-125. [PubMed] |
| 67. | Ozdemir Y, Kalady MF, Aytac E, Kiran RP, Erem HH, Church JM, Remzi FH. Anal transitional zone neoplasia in patients with familial adenomatous polyposis after restorative proctocolectomy and IPAA: incidence, management, and oncologic and functional outcomes. Dis Colon Rectum. 2013;56:808-814. [PubMed] [DOI] |
| 68. | Church J. Ileoanal pouch neoplasia in familial adenomatous polyposis: an underestimated threat. Dis Colon Rectum. 2005;48:1708-1713. [PubMed] [DOI] |
| 69. | Dafnis G. Early and late surgical outcomes of ileal pouch-anal anastomosis within a defined population in Sweden. Eur J Gastroenterol Hepatol. 2016;28:842-849. [PubMed] [DOI] |
| 70. | Lightner AL, Kelley SR, Larson DW. Robotic Platform for an IPAA. Dis Colon Rectum. 2018;61:869-874. [PubMed] [DOI] |