修回日期: 2019-06-21
接受日期: 2019-08-19
在线出版日期: 2019-12-28
手足口病(hand-foot-mouth disease, HFMD)是全球性传染病, 感染人群以婴幼儿为主, 而肠道病毒71型(enterovirus 71, EV71)是主要病原体. 感染EV71除表现HFMD外, 还能感染神经系统和其他脏器, 引起无菌性脑膜炎、脑干脑炎和脊髓灰质炎样的麻痹性疾病, 对儿童健康造成严重危害, 已引起高度重视. 目前EV71感染HFMD的发病机制仍不清楚, 且无有效治疗方法. 本文从病毒基因重组和自发突变、宿主基因及受体位点等方面讨论了EV71感染发病的影响因素, 并通过动物感染模型的研究对EV71感染HFMD的发病机制进行简要综述; 探讨发病机制研究中面临的主要问题, 为疾病的防控、临床治疗和有效疫苗的研究提供启发.
核心提要: 本文从肠道病毒71型(enterovirus 71, EV71)基因重组和突变、宿主基因及受体位点等方面探讨了EV71感染手足口病(hand-foot-mouth disease, HFMD)的影响因素, 并通过EV71感染模型探究HFMD的发病机制, 阐明EV71疫苗研究中存在的问题, 为疾病的防控、临床治疗和疫苗研究提供参考.
引文著录: 王春荣. 肠道病毒71型感染手足口病发病机制的研究. 世界华人消化杂志 2019; 27(24): 1465-1472
Revised: June 21, 2019
Accepted: August 19, 2019
Published online: December 28, 2019
Hand-foot-mouth disease (HFMD) is a global infectious disease. The infected population is mainly infants and young children. Enterovirus 71 (EV71) is the main pathogen. In addition to HFMD, EV71 infection can also affect the nervous system and other organs, resulting in aseptic meningitis, brainstem encephalitis, and poliomyelitis-like paralysis, causing serious harm to children's health. At present, the pathogenesis of HFMD caused by EV71 is still unclear, and there is no effective treatment. In this paper, we discuss the factors influencing EV71 infection from the aspects of virus gene recombination and spontaneous mutation, host genes, and receptor sites, review the pathogenesis of HFMD caused by EV71 based on the study findings from animal infection models, and explore the main problems in the study of pathogenesis of this condition, in order to provide reference for the prevention and treatment of HFMD and for the development of new drugs or effective vaccines for EV71 infection.
- Citation: Wang CR. Pathogenesis of hand-foot-mouth disease caused by enterovirus 71. Shijie Huaren Xiaohua Zazhi 2019; 27(24): 1465-1472
- URL: https://www.wjgnet.com/1009-3079/full/v27/i24/1465.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v27.i24.1465
肠道病毒71型(enterovirus 71, EV71)是引起手足口病(hand-foot-mouth disease, HFMD)的主要病原体之一. EV71感染以5岁以下的婴幼儿为主, 引起的HFMD多为自限性, 但有些感染可引起严重的神经系统疾病, 如无菌性脑膜炎、脑干脑炎和脊髓灰质炎样的麻痹性疾病, 甚至死亡[1-3], 已引起高度关注. 目前尚无有效的EV71治疗方法.
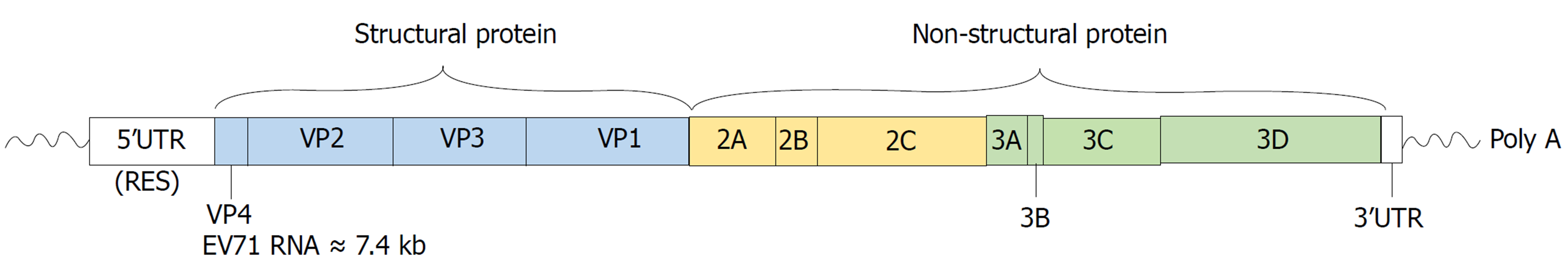
EV71属小RNA病毒科肠道病毒属, 基因组为单股正链RNA, 长度约为7.4 kb. 基因组两端为5'和3'非编码区(untranslated region, UTR), 中间为连续的开放阅读框, 编码含有约2194个氨基酸的多聚蛋白(图1)[4], 依据VP1核苷酸序列的差异, 可将EV71分为A、B、C 3个基因型[5]. 目前EV71感染HFMD的发病机制仍未明确, 病毒基因结构与保证其生存、循环和逃避免疫等因素之间的关系尚不清楚. 本文就EV71病毒和宿主相互作用机制的影响因素进行概述, 通过EV71感染动物模型的研究探讨HFMD的发病机制, 为HFMD的防控、开发治疗EV71的新型药物或有效疫苗提供参考.
1962年, 有学者在脊髓灰质炎病毒研究中报告了第一例RNA重组, 此后许多研究[6,7]表明重组在肠道病毒进化中具有重要意义并经常发生. 通过种内和种间50个非翻译区域的重组, 肠道病毒的进化和演变也已被证实[8]. 以往研究显示, 当从严重HFMD患者分离的EV71亚基因型C4毒株与从轻度患者分离的毒株进行比较时, 大多数突变位于50-NTR和VP1区域, 有的仅在VP1中观察到10个突变[9-12]. 然而, 当将2株EV71亚基因型C4a与C4进行比较时, 突变几乎跨越了整个基因组, 从50-NTR到30-NTR[13,14]. 通过量化每一个突变基因所赋予的毒力程度来研究这些毒株的毒力, 可以更好地了解它们究竟如何发挥作用, 这将是非常有说服力的.
有趣的是, 当对2000年新加坡严重HFMD暴发中分离出的致命EV71株进行分析时, 发现它与非致命株仅在核苷酸5262处有所不同: 致命株在基因组的5262位携带一个苏氨酸, 而非致命株中该 位置为丙氨酸[15]. 为了鉴定这个位置的苏氨酸是否导致了该毒株的致死性, Yee等[16]通过核苷酸的定点诱变对致命株(C41)和非致命株(C10)的毒力进行了量化; 与致命株相比, 5262位(A5262G)的突变导致非致命株RNA拷贝数减少75%, 斑块形成能力降低90%.
为了进一步评估EV71亚基因型B4株基因组的特异性突变对毒力的影响, Yee等[16]在其基因组多位点(158、475、486和487)诱导突变并缺失50-NTR区域475-486核苷酸部位的11个碱基对, 结果显示突变体475(C475T)和缺失体PD(50-NTR中475到486)表现出明显的低细胞病变效应和极低的RNA拷贝数; 然而, 对RNA拷贝数的分析表明, 突变体A486G在RD细胞中仍能产生高水平的RNA, 而突变体G487A在RD细胞中的拷贝数降低.这些数据为EV71亚基因型B4株毒力的影响因素提供了佐证.
以往研究表明, 国内分离的致命和非致命毒株之间存在氨基酸差异[10,14,17,18]. 这是否意味着某些毒力较高的毒株能够引起大规模的疫情爆发? 然而, EV71病毒每次复制时出现错误的可能性很大, 这也提出了另一个问题, 即单个基因突变是否真是致死的根本原因, 或者更确切地说, 携带多个氨基酸替换的突变群体是否增加了病毒种群的适应性, 从而赋予其更高的致病性?在正常情况下, 病毒基因组复制并产生数百种子代病毒, 这些子代病毒可能因一个位置的氨基酸替代而有所不同, 在接下来的几轮复制中, 可能产生更复杂的突变体而与原始序列有显著差异, 这些突变体的集合还可能形成一个准物种[18]. 关于重组和/或自发突变导致的EV71致命毒株是否是HFMD暴发的唯一理由, 或是否可能存在具有不同毒力的不同准种之间的合作, 这些问题都有待证实.
在以小鼠为感染模型的研究中, 发现EV71 VP1的145位氨基酸或VP2的149位氨基酸的点突变是新生小鼠能否成功感染EV71适应株的决定性因素[20]. 除编码序列外, 非编码区域序列如内部核糖体进入位点(internal ribosome entry site, IRES)的突变也影响EV71对小鼠的感染毒性[9]. 而感染组织倾向性也可能与病毒序列变异有关, 从呼吸道、胃肠道、中枢神经系统和血液标本中分离的EV71序列的比较显示, 不同来源的EV71核苷酸存在差异. 进一步的研究表明, EV71 VP1的97位氨基酸与病毒的嗜神经组织特性有关[21]. 近期研究显示, EV71 VP1突变体L97R被认为更具毒性, 可能是因为它能更好地与硫酸肝素结合[22], 而硫酸肝素是许多病毒(包括EV71)的常见附着受体[23]. 与此相反的是, VP1突变体145G与硫酸肝素的结合比突变体145E更好, 其神经毒力却被认为比后者小[24], 可能是因为硫酸肝素优先吸附在大量非靶细胞上, 导致突变体VP1-145G的捕获衰减. 但EV71 VP1的硫酸肝素结合特性与病毒毒力是否呈正相关或负相关, 目前并没有确切结论.
同种同亚型的EV71病毒在不同的宿主会产生不同的临床症状, 地区分布不同临床表现也不同[25], 这除了与病毒的毒力差别有关外, 还可能决定于不同的宿主对病毒的反应不同. 人类白细胞抗原(human leukocyte antigen, HLA)基因是一种免疫相关基因, 在亚洲人中多见, 表型频率分布可达17%-35%, 而在白人中少见(表型频率分布仅0%-1%). 据此, 中国台湾学者通过病例对照研究, 发现HLA-A33基因与病毒的易感性相关, 并发现HLA-A2基因与肺水肿的发生相关[26]. 根据该基因在不同人群分布的差异或许可以解释在过去为什么EV71多在中国台湾、马来西亚以及中国内地、日本等亚太地区爆发. 细胞毒性T淋巴细胞抗原4(cytotoxic T lymphocyte antigen 4, CTLA-4)是T细胞毒性和耐受性的重要调节因子, 其多态性与EV71感染儿童脑炎有关[27]. 其他遗传多态性, 如肿瘤坏死因子和干扰素受体1[28,29], 在EV71患者中也被发现与疾病严重程度相关.
EV71所致HFMD, 不同个体的临床表型复杂多样, 这除了与病毒因素有关外, 更重要的是由病毒和宿主的相互作用决定的, 尤其是不同个体对病毒发生的免疫反应不同. EV71感染首先通过与宿主细胞表面的特异性受体结合而吸附于宿主细胞表面, 然后进入宿主细胞并将病毒核酸释放入细胞进行繁殖扩增, 属于细胞内感染. 与体液免疫功能比较, 细胞免疫与非体液免疫功能对EV71感染患儿的影响更大[30,31]. 在免疫应答过程中, 众多细胞因子在机体内通过旁分泌、自分泌或内分泌等方式发挥作用, 具有多效性、重叠性、拮抗性、协同性等多种生理特性, 形成了十分复杂的细胞因子调节网络[3], 而宿主的遗传因素通过控制某些细胞因子的产量或反应性调控免疫状态.
宿主细胞表面的受体在病毒感染和侵入机体的过程中发挥关键性作用, 决定了病毒的宿主和组织嗜性. 目前报道的较为明确的EV71受体包括溶酶体整合膜蛋白2(lysosomal integral membrane protein 2, SCARB2)和P选择素糖蛋白配体1(P-selectin glycoprotein ligand 1, PSGLl). SCARB2是一种双链跨膜蛋白, 主要分布于溶酶体及基质, 参与细胞膜运输、内含体和溶酶体的重组等过程. EV71可通过VPl区结合游离的SCARB2受体而进入细胞内, 这个过程能被SCARB2的抗体所抑制[32]. 此外, SCARB2受体蛋白的表达能使那些对EV71不易感的细胞变得有利于病毒的繁殖[33]. Chen等[34]研究证实, EV71通过其五聚体周围的"口袋"区与SCARB2结合使病毒颗粒构象改变, 导致病毒释放基因组RNA完成感染过程. PSGLl主要存在于白细胞表面, 在炎症早期与EV71结合引起炎性因子的释放[35]. 也有研究发现, Toll样受体作为重要的病原模式识别受体能够识别EV71, 与宿主抵抗病毒感染和炎性反应等有直接关系, 可能参与介导EV71感染所致混合性拮抗反应综合征[36], 另有研究报告了几种EV71的替代受体[23,37,38].
最常见的EV71感染细胞系是横纹肌肉瘤起源的人恶性胚胎横纹肌瘤细胞(rhabdomyosarcoma, RD)细胞系[39]. 这种RD细胞株能产生大量的病毒颗粒, 因此常被用于动物实验[40]和病毒结构研究[41]中EV71的扩增. 除RD细胞外, 用于脊髓灰质炎病毒感染的各种细胞株, 如HELA和HEK293, 也易受EV71感染和转染[42,43]. 这些细胞系常被用作病毒蛋白功能研究的平台. 一些神经细胞系, 包括sk-n-sh、nsc-34和sf268, 已被用于研究宿主对EV71的反应[44]、系统发育分析[45]和抗病毒药物鉴定[46].
除RD细胞外, 许多细胞系被用于EV71分子病毒学、病毒-宿主相互作用或药物筛选的研究, 如THP-1和人PBMC被用于研究EV71感染的先天性免疫[43]. 到目前为止, RD细胞系似乎是文献中最常用的金标准, 部分原因可能是它具有不同EV71基因型的高产率[47]. 随着越来越多的实验室使用相同的RD细胞系, 未来更容易交叉比较来自不同实验室的结果. 由于这些细胞系模型在某种程度上与人类自然感染相似, 因此还需要另一种方法来验证通过细胞培养系统获得的发现, 在这方面, 动物模型可以为研究者对EV71体内感染和发病机制的理解提供更加完整和有说服力的证据.
从细胞培养系统中获得的实验结果需要在动物模型和人类临床试验中进行验证. 目前有四种小鼠感染模型[48], 这些模型正被积极地用于抗病毒研究. 在这些模型中, 有一个共同点就是经常使用肢体瘫痪和死亡率来评估疾病的严重程度.
4.2.1 依赖于EV71适应性毒株的小鼠模型: 成人通常对EV71感染拥有抵抗力, 同样成年小鼠也不容易感染EV71临床分离株. 为了解决这个问题, 研究人员在ICR小鼠模型中开发出适合小鼠感染的EV71毒株MP4[49-51]. 将亲本EV71注射到一天龄ICR小鼠体内, 从脑组织中分离的适应性EV71毒株被再次用于连续的注射适应循环, 第四轮循环得到的EV71被指定为MP4[50]. 与其亲本EV71株相比, MP4的细胞毒性更强, 产生的斑块更大. 由于EV71是一种具有准物种性质的RNA病毒, 小鼠适应性毒株往往会积累一些突变, 而这些适应性突变在人类自然感染过程中不容易被发现. 除了MP4, 免疫缺陷的nod/scid小鼠模型中也产生了适应性EV71毒株[52]. 对临床分离株与小鼠适应株的氨基酸序列进行比较, 发现两者在VP1、VP3和蛋白酶2A等结构蛋白存在显著差异[52]. 因此, 目前还不能确定用EV71适应株感染小鼠得到的研究结果是否可以扩展到人类对自然毒株感染情况.
4.2.2 免疫缺陷小鼠模型: 宿主的免疫系统对病毒感染起着至关重要的作用[53]. 免疫缺陷小鼠, 即使没有携带任何人类病毒感染受体, 也可以支持EV71临床分离株的感染, 而不是依赖于小鼠适应株.
有研究显示宿主体内的干扰素是防止EV71感染和发病的必要条件[54]. AG129小鼠, 由于缺乏干扰素或干扰素受体, 当通过静脉注射或口服途径感染非小鼠适应性EV71时, 会出现肢体瘫痪和死亡, 在肠道和口腔感染的中枢神经系统中都能检测到病毒蛋白[55].与小鼠AG129相似, 小鼠A129(缺乏干扰素受体)也可感染小鼠非适应株EV71[56]. 此外, stat-1是干扰素信号传导过程中的关键转录因子, stat-1基因敲除小鼠G129(缺乏干扰素受体)通过粪-口途径可成功感染B型和C型EV71临床分离株, 感染的小鼠后肢瘫痪, 中枢神经系统中含有丰富的病毒蛋白[40], 支持干扰素信号在保护中枢神经系统免受EV71感染机制中的重要作用.
除先天免疫外, 体液免疫在EV71感染和发病中也起着重要作用. 缺乏T和B淋巴细胞的nod/scid小鼠可以感染小鼠适应性EV71[52]. 然而, 既然小鼠适应EV71也可以感染具有免疫活性的小鼠[50], nod/scid小鼠模型中缺失的T淋巴细胞和B淋巴细胞是否真正对免受小鼠适应EV71感染的保护中起作用? 进一步研究显示, nod/scid小鼠接种EV71临床分离株发生了肢体麻痹, 上述问题得到解决. 尽管肢体瘫痪已被用作小鼠模型中评估EV71发病机制的常见标志, 但在人类自然感染中, 急性弛缓性麻痹的发生频率不如HFMD. 有趣的是, 在nod/scid模型中, 首次在感染了临床分离株的小鼠模型中观察到一种类似HFMD的皮疹表型. 此外, 炎症性因子IL-23/IL-17轴似乎在感染的nod/scid小鼠模型中被激活.这些有趣的现象是否在nod/scid模型具有特异性, 或者可以推广到其他小鼠模型或人类患者, 还有待观察.
此外, 干扰素-γ诱导蛋白-10(interferon-gamma-inducible protein-10, IP-10)是EV71患者高表达的趋化因子[57]. 与野生型对照小鼠相比, IP-10敲除小鼠感染小鼠适应性EV71后的死亡率更高, 表明IP-10在EV71感染和发病机制中的保护作用[58].
4.2.3 转基因小鼠模型: 近年来, 许多EV71感染过程中的细胞受体[33,35,37,59]被提出, 这些受体促进了非敏感细胞系如小鼠成纤维细胞L929对EV71的感染. 然而, 表达人PSGL-1的转基因小鼠不能支持EV71临床分离株的感染[60]. 在这些报道的细胞受体中, 迄今为止只有人scarb2(hscarb2)被证明支持体内EV71感染.
以往报道有两种不同的hscarb2-tg小鼠模型. 在ef-1a-hscarb2模型[61]中, ef-1a启动子被用来驱动C57b/6小鼠背景中hscarb2的表达; 在sc2-hscarb2模型[62]中, 一个天然的hscarb2启动子在人scarb2-bac克隆中驱动c57b/6小鼠背景中的转基因表达. 这两种模型都能感染EV71, 发展成肢体瘫痪, 并在肌肉和神经组织中检测到病毒蛋白. 然而, 上述hscarb2Tg小鼠模型还不能有效支持口腔感染[61,62], 而这对于儿童是一个重要的传播途径.
4.2.4 杂交小鼠模型: 如上所述, hscarb2受体和免疫缺陷都有助于EV71感染和发病. 因此, 将转基因hscarb2和宿主免疫缺陷结合可能会进一步提高感染效率. 事实上, 通过杂交培育hscarb2-tg和stat-1ko小鼠产生了一个新的杂交小鼠模型. 与其亲本小鼠相比, 该杂交小鼠模型更易于应用. 例如, 杂交小鼠在2 wk大的时候, 仍然可以感染不同基因型的EV71, 需要的滴度(pfu)比亲本小鼠低1000倍[63]. EV71感染的杂交小鼠在中枢神经系统(如中脑和脊髓)中表现出高密度的病毒蛋白. 与stat-1ko模型一样, 尽管EV71感染的杂交小鼠出现肢体瘫痪, 但在肌肉组织中没有检测到病毒RNA和蛋白. 这表明, 瘫痪完全源于中枢神经系统损伤, 而不是肌肉破坏. 这种新的混合(hscarb2-tg/stat-1-ko)模型可以作为一个评估药物或疫苗疗效的平台[63].
尽管这种杂交小鼠模型对研究EV71相关的神经病变和抗病毒治疗是一个更为敏感的系统, 但由于缺乏干扰素信号, 不能成为研究免疫调节剂治疗的良好模型.
EV71一般通过密切的人与人接触传播, 因此, 疫苗接种可能是控制EV71感染的最佳方法. 目前研究的EV71疫苗有多种类型, 包括灭活病毒疫苗[64-66]、病毒样颗粒疫苗[67]、DNA疫苗[68]、亚单位疫苗[69]和减毒活疫苗[70].
灭活病毒EV71疫苗的开发进展迅速, 是目前EV71疫苗中最先进的候选疫苗[65,66,71-73], 健康儿童接种灭活EV71疫苗5年后的免疫持续性仍然较高[74]. 2015年12月, 中国食品药品监督管理局批准了两种预防HFMD的灭活EV71疫苗[75]. 由于灭活的EV71疫苗不能复制, 因此出于安全考虑, 它们比减毒活疫苗更受欢迎. 然而, 灭活疫苗的生产成本和潜在的供应问题使其在实际应用中受到了限制[76].
病毒样颗粒(VLPS)疫苗与灭活EV71疫苗不同, VLPS疫苗的优点是能够同时以其天然构象呈现EV71衣壳蛋白的所有表面表位[75,77]. 尽管VLPS疫苗的效力低于灭活疫苗, 但实验动物模型研究表明, VLP能产生保护性中和抗体, 并对疫苗中不存在的多种亚型产生交叉反应[77]. 与VLP相关的问题是其稳定性、净化和生产成本. 其他类型的EV71疫苗(DNA疫苗、亚单位疫苗和减毒活疫苗)尚处于早期发展阶段, 目前正在对小鼠和非人类灵长类动物进行临床前试验[75].
EV71感染在亚太地区引起了大规模的HFMD暴发, 已引起社会各界的高度关注. 但是, 目前尚无有效的EV71治疗药物, EV71感染发病机制的研究也没有确切结论, 还有很多问题需要解决. EV71疫苗是保护儿童免受感染的有效方法, 然而, 目前没有一种EV71疫苗能提供有效的保护作用来防止其他肠道病毒的感染, 开发能覆盖多种肠道病毒的疫苗仍是一种前瞻性的选择.
在今后的研究中, (1)应建立一个全球性EV71感染监测网络, 持续的监测有助于识别和发现新的EV71变种; (2)迫切需要有效的抗病毒药物来对抗亚洲和世界各地频繁发生的EV71感染; (3)应深入研究EV71感染模型中病毒种群的行为及其与发病机制的关系, 因为目前还不清楚致命感染是由单一毒株导致, 还是由人群中携带不同突变的病原体的合作引起, 对这个问题的解答可以为设计一种有效的EV71疫苗铺平道路; 最后, 应进一步寻找新的特异性EV71受体, 特别是加强嗜神经性受体的研究, 可为特效的靶向药物的研究提供科学依据.
学科分类: 胃肠病学和肝病学
手稿来源地: 山东省
同行评议报告分类
A级 (优秀): 0
B级 (非常好): B, B, B
C级 (良好): C, C
D级 (一般): 0
E级 (差): 0
编辑: 崔丽君 电编:刘继红
| 1. | Teoh HL, Mohammad SS, Britton PN, Kandula T, Lorentzos MS, Booy R, Jones CA, Rawlinson W, Ramachandran V, Rodriguez ML, Andrews PI, Dale RC, Farrar MA, Sampaio H. Clinical Characteristics and Functional Motor Outcomes of Enterovirus 71 Neurological Disease in Children. JAMA Neurol. 2016;73:300-307. [PubMed] [DOI] |
| 2. | 郑 亚明, 常 昭瑞, 姜 黎黎, 嵇 红, 陈 国平, 罗 平, 潘 静静, 田 晓灵, 魏 雷雷, 霍 达, 缪 梓萍, 邹 晓妮, 陈 建华, 廖 巧红. 手足口病重症病例分析:基于全国手足口病监测试点数据. 中华流行病学杂志. 2017;38:759-762. [DOI] |
| 3. | Duan G, Yang H, Shi L, Sun W, Sui M, Zhang R, Wang X, Wang F, Zhang W, Xi Y, Fan Q. Serum inflammatory cytokine levels correlate with hand-foot-mouth disease severity: a nested serial case-control study. PLoS One. 2014;9:e112676. [PubMed] [DOI] |
| 4. | Shih C, Liao CC, Chang YS, Wu SY, Chang CS, Liou AT. Immunocompetent and Immunodeficient Mouse Models for Enterovirus 71 Pathogenesis and Therapy. Viruses. 2018;10:674. [PubMed] [DOI] |
| 5. | Chan YF, Sam IC, AbuBakar S. Phylogenetic designation of enterovirus 71 genotypes and subgenotypes using complete genome sequences. Infect Genet Evol. 2010;10:404-412. [PubMed] [DOI] |
| 6. | Hirst GK. Genetic recombination with Newcastle disease virus, polioviruses, and influenza. Cold Spring Harb Symp Quant Biol. 1962;27:303-309. [PubMed] |
| 7. | Ledinko N. Genetic recombination with poliovirus type 1. Studies of crosses between a normal horse serum-resistant mutant and several guanidine-resistant mutants of the same strain. Virology. 1963;20:107-119. [PubMed] |
| 8. | Muslin C, Joffret ML, Pelletier I, Blondel B, Delpeyroux F. Evolution and Emergence of Enteroviruses through Intra- and Inter-species Recombination: Plasticity and Phenotypic Impact of Modular Genetic Exchanges in the 5' Untranslated Region. PLoS Pathog. 2015;11:e1005266. [PubMed] [DOI] |
| 9. | Yeh MT, Wang SW, Yu CK, Lin KH, Lei HY, Su IJ, Wang JR. A single nucleotide in stem loop II of 5'-untranslated region contributes to virulence of enterovirus 71 in mice. PLoS One. 2011;6:e27082. [PubMed] [DOI] |
| 10. | Liu Y, Fu C, Wu S, Chen X, Shi Y, Zhou B, Zhang L, Zhang F, Wang Z, Zhang Y, Fan C, Han S, Yin J, Peng B, Liu W, He X. A novel finding for enterovirus virulence from the capsid protein VP1 of EV71 circulating in mainland China. Virus Genes. 2014;48:260-272. [PubMed] [DOI] |
| 11. | Nishimura Y, Lee H, Hafenstein S, Kataoka C, Wakita T, Bergelson JM, Shimizu H. Enterovirus 71 binding to PSGL-1 on leukocytes: VP1-145 acts as a molecular switch to control receptor interaction. PLoS Pathog. 2013;9:e1003511. [PubMed] [DOI] |
| 12. | Yuan S, Li G, Wang Y, Gao Q, Wang Y, Cui R, Altmeyer R, Zou G. Identification of Positively Charged Residues in Enterovirus 71 Capsid Protein VP1 Essential for Production of Infectious Particles. J Virol. 2015;90:741-752. [PubMed] [DOI] |
| 13. | Wen HL, Si LY, Yuan XJ, Hao SB, Gao F, Chu FL, Sun CX, Wang ZY. Complete genome sequencing and analysis of six enterovirus 71 strains with different clinical phenotypes. Virol J. 2013;10:115. [PubMed] [DOI] |
| 14. | Li R, Zou Q, Chen L, Zhang H, Wang Y. Molecular analysis of virulent determinants of enterovirus 71. PLoS One. 2011;6:e26237. [PubMed] [DOI] |
| 15. | Singh S, Poh CL, Chow VT. Complete sequence analyses of enterovirus 71 strains from fatal and non-fatal cases of the hand, foot and mouth disease outbreak in Singapore (2000). Microbiol Immunol. 2002;46:801-808. [PubMed] |
| 16. | Yee PT, Tan KO, Othman I, Poh CL. Identification of molecular determinants of cell culture growth characteristics of Enterovirus 71. Virol J. 2016;13:194. [PubMed] [DOI] |
| 17. | Chang SC, Li WC, Chen GW, Tsao KC, Huang CG, Huang YC, Chiu CH, Kuo CY, Tsai KN, Shih SR, Lin TY. Genetic characterization of enterovirus 71 isolated from patients with severe disease by comparative analysis of complete genomes. J Med Virol. 2012;84:931-939. [PubMed] [DOI] |
| 18. | Li P, Yue Y, Song N, Li B, Meng H, Yang G, Li Z, An L, Qin L. Genome analysis of enterovirus 71 strains differing in mouse pathogenicity. Virus Genes. 2016;52:161-171. [PubMed] [DOI] |
| 19. | Lauring AS, Andino R. Quasispecies theory and the behavior of RNA viruses. PLoS Pathog. 2010;6:e1001005. [PubMed] [DOI] |
| 20. | Huang SW, Wang YF, Yu CK, Su IJ, Wang JR. Mutations in VP2 and VP1 capsid proteins increase infectivity and mouse lethality of enterovirus 71 by virus binding and RNA accumulation enhancement. Virology. 2012;422:132-143. [PubMed] [DOI] |
| 21. | Cordey S, Petty TJ, Schibler M, Martinez Y, Gerlach D, van Belle S, Turin L, Zdobnov E, Kaiser L, Tapparel C. Identification of site-specific adaptations conferring increased neural cell tropism during human enterovirus 71 infection. PLoS Pathog. 2012;8:e1002826. [PubMed] [DOI] |
| 22. | Tseligka ED, Sobo K, Stoppini L, Cagno V, Abdul F, Piuz I, Meylan P, Huang S, Constant S, Tapparel C. A VP1 mutation acquired during an enterovirus 71 disseminated infection confers heparan sulfate binding ability and modulates ex vivo tropism. PLoS Pathog. 2018;14:e1007190. [PubMed] [DOI] |
| 23. | Tan CW, Poh CL, Sam IC, Chan YF. Enterovirus 71 uses cell surface heparan sulfate glycosaminoglycan as an attachment receptor. J Virol. 2013;87:611-620. [PubMed] [DOI] |
| 24. | Kobayashi K, Sudaka Y, Takashino A, Imura A, Fujii K, Koike S. Amino Acid Variation at VP1-145 of Enterovirus 71 Determines Attachment Receptor Usage and Neurovirulence in Human Scavenger Receptor B2 Transgenic. Mice. J Virol. 2018;92. [PubMed] [DOI] |
| 25. | McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev. 2002;26:91-107. [PubMed] [DOI] |
| 26. | Chang LY, Chang IS, Chen WJ, Huang YC, Chen GW, Shih SR, Juang JL, Shih HM, Hsiung CA, Lin TY, Huang LM. HLA-A33 is associated with susceptibility to enterovirus 71 infection. Pediatrics. 2008;122:1271-1276. [PubMed] [DOI] |
| 27. | Yang KD, Yang MY, Li CC, Lin SF, Chong MC, Wang CL, Chen RF, Lin TY. Altered cellular but not humoral reactions in children with complicated enterovirus 71 infections in Taiwan. J Infect Dis. 2001;183:850-856. [PubMed] [DOI] |
| 28. | Li JA, Chen ZB, Lv TG, Han ZL, Liu PP. Impact of endothelial nitric oxide synthase gene polymorphism on severity of enterovirus 71-infection in Chinese children. Clin Biochem. 2013;46:1842-1847. [PubMed] [DOI] |
| 29. | Zou R, Zhang G, Li S, Wang W, Yuan J, Li J, Wang Y, Lin Y, Deng Y, Zhou B, Gao GF, Liu Y. A functional polymorphism in IFNAR1 gene is associated with susceptibility and severity of HFMD with EV71 infection. Sci Rep. 2015;5:18541. [PubMed] [DOI] |
| 30. | Chang LY, Hsiung CA, Lu CY, Lin TY, Huang FY, Lai YH, Chiang YP, Chiang BL, Lee CY, Huang LM. Status of cellular rather than humoral immunity is correlated with clinical outcome of enterovirus 71. Pediatr Res. 2006;60:466-471. [PubMed] [DOI] |
| 31. | Yee PTI, Poh CL. T Cell Immunity To Enterovirus 71 Infection In Humans And Implications For Vaccine Development. Int J Med Sci. 2018;15:1143-1152. [PubMed] [DOI] |
| 32. | Patel KP, Bergelson JM. Receptors identified for hand, foot and mouth virus. Nat Med. 2009;15:728-729. [PubMed] [DOI] |
| 33. | Yamayoshi S, Yamashita Y, Li J, Hanagata N, Minowa T, Takemura T, Koike S. Scavenger receptor B2 is a cellular receptor for enterovirus 71. Nat Med. 2009;15:798-801. [PubMed] [DOI] |
| 34. | Chen P, Song Z, Qi Y, Feng X, Xu N, Sun Y, Wu X, Yao X, Mao Q, Li X, Dong W, Wan X, Huang N, Shen X, Liang Z, Li W. Molecular determinants of enterovirus 71 viral entry: cleft around GLN-172 on VP1 protein interacts with variable region on scavenge receptor B 2. J Biol Chem. 2012;287:6406-6420. [PubMed] |
| 35. | Nishimura Y, Shimizu H. Identification of P-selectin glycoprotein ligand-1 as one of the cellular receptors for enterovirus 71. Uirusu. 2009;59:195-203. [PubMed] |
| 36. | Denizot M, Neal JW, Gasque P. Encephalitis due to emerging viruses: CNS innate immunity and potential therapeutic targets. J Infect. 2012;65:1-16. [PubMed] [DOI] |
| 37. | Su PY, Wang YF, Huang SW, Lo YC, Wang YH, Wu SR, Shieh DB, Chen SH, Wang JR, Lai MD, Chang CF. Cell surface nucleolin facilitates enterovirus 71 binding and infection. J Virol. 2015;89:4527-4538. [PubMed] [DOI] |
| 38. | Ren XX, Ma L, Liu QW, Li C, Huang Z, Wu L, Xiong SD, Wang JH, Wang HB. The molecule of DC-SIGN captures enterovirus 71 and confers dendritic cell-mediated viral trans-infection. Virol J. 2014;11:47. [PubMed] [DOI] |
| 39. | Han JF, Cao RY, Tian X, Yu M, Qin ED, Qin CF. Producing infectious enterovirus type 71 in a rapid strategy. Virol J. 2010;7:116. [PubMed] [DOI] |
| 40. | Liao CC, Liou AT, Chang YS, Wu SY, Chang CS, Lee CK, Kung JT, Tu PH, Yu YY, Lin CY, Lin JS, Shih C. Immunodeficient mouse models with different disease profiles by in vivo infection with the same clinical isolate of enterovirus 71. J Virol. 2014;88:12485-12499. [PubMed] [DOI] |
| 41. | Lyu K, Wang GC, He YL, Han JF, Ye Q, Qin CF, Chen R. Crystal structures of enterovirus 71 (EV71) recombinant virus particles provide insights into vaccine design. J Biol Chem. 2015;290:3198-3208. [PubMed] [DOI] |
| 42. | Wang B, Xi X, Lei X, Zhang X, Cui S, Wang J, Jin Q, Zhao Z. Enterovirus 71 protease 2Apro targets MAVS to inhibit anti-viral type I interferon responses. PLoS Pathog. 2013;9:e1003231. [PubMed] [DOI] |
| 43. | Wang W, Xiao F, Wan P, Pan P, Zhang Y, Liu F, Wu K, Liu Y, Wu J. EV71 3D Protein Binds with NLRP3 and Enhances the Assembly of Inflammasome Complex. PLoS Pathog. 2017;13:e1006123. [PubMed] [DOI] |
| 44. | Too IH, Yeo H, Sessions OM, Yan B, Libau EA, Howe JL, Lim ZQ, Suku-Maran S, Ong WY, Chua KB, Wong BS, Chow VT, Alonso S. Enterovirus 71 infection of motor neuron-like NSC-34 cells undergoes a non-lytic exit pathway. Sci Rep. 2016;6:36983. [PubMed] [DOI] |
| 45. | Yu P, Bao L, Xu L, Li F, Lv Q, Deng W, Xu Y, Qin C. Neurotropism In Vitro and Mouse Models of Severe and Mild Infection with Clinical Strains of Enterovirus 71. Viruses. 2017;9. [PubMed] [DOI] |
| 46. | Shih SR, Weng KF, Stollar V, Li ML. Viral protein synthesis is required for Enterovirus 71 to induce apoptosis in human glioblastoma cells. J Neurovirol. 2008;14:53-61. [PubMed] [DOI] |
| 47. | Fukuhara M, Iwami S, Sato K, Nishimura Y, Shimizu H, Aihara K, Koyanagi Y. Quantification of the dynamics of enterovirus 71 infection by experimental-mathematical investigation. J Virol. 2013;87:701-705. [PubMed] [DOI] |
| 48. | Shih C, Liao CC, Chang YS, Wu SY, Chang CS, Liou AT. Immunocompetent and Immunodeficient Mouse Models for Enterovirus 71 Pathogenesis and Therapy. Viruses. 2018;10. [PubMed] [DOI] |
| 49. | Chen IC, Wang SM, Yu CK, Liu CC. Subneutralizing antibodies to enterovirus 71 induce antibody-dependent enhancement of infection in newborn mice. Med Microbiol Immunol. 2013;202:259-265. [PubMed] [DOI] |
| 50. | Wang YF, Chou CT, Lei HY, Liu CC, Wang SM, Yan JJ, Su IJ, Wang JR, Yeh TM, Chen SH, Yu CK. A mouse-adapted enterovirus 71 strain causes neurological disease in mice after oral infection. J Virol. 2004;78:7916-7924. [PubMed] [DOI] |
| 51. | Lin YW, Chang KC, Kao CM, Chang SP, Tung YY, Chen SH. Lymphocyte and antibody responses reduce enterovirus 71 lethality in mice by decreasing tissue viral loads. J Virol. 2009;83:6477-6483. [PubMed] [DOI] |
| 52. | Arita M, Ami Y, Wakita T, Shimizu H. Cooperative effect of the attenuation determinants derived from poliovirus sabin 1 strain is essential for attenuation of enterovirus 71 in the NOD/SCID mouse infection model. J Virol. 2008;82:1787-1797. [PubMed] [DOI] |
| 53. | Zhang Y, Li J, Li Q. Immune Evasion of Enteroviruses Under Innate Immune Monitoring. Front Microbiol. 2018;9:1866. [PubMed] [DOI] |
| 54. | Sun J, Ennis J, Turner JD, Chu JJ. Single dose of an adenovirus vectored mouse interferon-α protects mice from lethal EV71 challenge. Antiviral Res. 2016;134:207-215. [PubMed] [DOI] |
| 55. | Khong WX, Yan B, Yeo H, Tan EL, Lee JJ, Ng JK, Chow VT, Alonso S. A non-mouse-adapted enterovirus 71 (EV71) strain exhibits neurotropism, causing neurological manifestations in a novel mouse model of EV71 infection. J Virol. 2012;86:2121-2131. [PubMed] [DOI] |
| 56. | Caine EA, Partidos CD, Santangelo JD, Osorio JE. Adaptation of enterovirus 71 to adult interferon deficient mice. PLoS One. 2013;8:e59501. [PubMed] [DOI] |
| 57. | Wang SM, Lei HY, Yu CK, Wang JR, Su IJ, Liu CC. Acute chemokine response in the blood and cerebrospinal fluid of children with enterovirus 71-associated brainstem encephalitis. J Infect Dis. 2008;198:1002-1006. [PubMed] [DOI] |
| 58. | Shen FH, Tsai CC, Wang LC, Chang KC, Tung YY, Su IJ, Chen SH. Enterovirus 71 infection increases expression of interferon-gamma-inducible protein 10 which protects mice by reducing viral burden in multiple tissues. J Gen Virol. 2013;94:1019-1027. [PubMed] [DOI] |
| 59. | Yang SL, Chou YT, Wu CN, Ho MS. Annexin II binds to capsid protein VP1 of enterovirus 71 and enhances viral infectivity. J Virol. 2011;85:11809-11820. [PubMed] [DOI] |
| 60. | Liu J, Dong W, Quan X, Ma C, Qin C, Zhang L. Transgenic expression of human P-selectin glycoprotein ligand-1 is not sufficient for enterovirus 71 infection in mice. Arch Virol. 2012;157:539-543. [PubMed] [DOI] |
| 61. | Lin YW, Yu SL, Shao HY, Lin HY, Liu CC, Hsiao KN, Chitra E, Tsou YL, Chang HW, Sia C, Chong P, Chow YH. Human SCARB2 transgenic mice as an infectious animal model for enterovirus 71. PLoS One. 2013;8:e57591. [PubMed] [DOI] |
| 62. | Fujii K, Nagata N, Sato Y, Ong KC, Wong KT, Yamayoshi S, Shimanuki M, Shitara H, Taya C, Koike S. Transgenic mouse model for the study of enterovirus 71 neuropathogenesis. Proc Natl Acad Sci USA. 2013;110:14753-14758. [PubMed] [DOI] |
| 63. | Liou AT, Wu SY, Liao CC, Chang YS, Chang CS, Shih C. A new animal model containing human SCARB2 and lacking stat-1 is highly susceptible to EV71. Sci Rep. 2016;6:31151. [PubMed] [DOI] |
| 64. | Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, Zhang YT, Yao X, Chu K, Chen QH, Hu YM, Wu X, Liu P, Zhu LY, Gao F, Jin H, Chen YJ, Dong YY, Liang YC, Shi NM, Ge HM, Liu L, Chen SG, Ai X, Zhang ZY, Ji YG, Luo FJ, Chen XQ, Zhang Y, Zhu LW, Liang ZL, Shen XL. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2013;381:2024-2032. [PubMed] [DOI] |
| 65. | Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X, Tan X, Wang L, Mao Q, Wu J, Hu Y, Ji T, Song L, Liang Q, Zhang B, Gao Q, Li J, Wang S, Hu Y, Gu S, Zhang J, Yao G, Gu J, Wang X, Zhou Y, Chen C, Zhang M, Cao M, Wang J, Wang H, Wang N. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med. 2014;370:818-828. [PubMed] [DOI] |
| 66. | Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z, Zhang Y, Li Y, Mao Q, Wang J, Jiang L, Dong C, Che Y, Huang T, Jiang Z, Xie Z, Wang L, Liao Y, Liang Y, Nong Y, Liu J, Zhao H, Na R, Guo L, Pu J, Yang E, Sun L, Cui P, Shi H, Wang J, Li Q. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med. 2014;370:829-837. [PubMed] [DOI] |
| 67. | Chung YC, Ho MS, Wu JC, Chen WJ, Huang JH, Chou ST, Hu YC. Immunization with virus-like particles of enterovirus 71 elicits potent immune responses and protects mice against lethal challenge. Vaccine. 2008;26:1855-1862. [PubMed] [DOI] |
| 68. | Tung WS, Bakar SA, Sekawi Z, Rosli R. DNA vaccine constructs against enterovirus 71 elicit immune response in mice. Genet Vaccines Ther. 2007;5:6. [PubMed] [DOI] |
| 69. | Foo DG, Alonso S, Phoon MC, Ramachandran NP, Chow VT, Poh CL. Identification of neutralizing linear epitopes from the VP1 capsid protein of Enterovirus 71 using synthetic peptides. Virus Res. 2007;125:61-68. [PubMed] [DOI] |
| 70. | Xu J, Qian Y, Wang S, Serrano JM, Li W, Huang Z, Lu S. EV71: an emerging infectious disease vaccine target in the Far East? Vaccine. 2010;28:3516-3521. [PubMed] [DOI] |
| 71. | Wei M, Meng F, Wang S, Li J, Zhang Y, Mao Q, Hu Y, Liu P, Shi N, Tao H, Chu K, Wang Y, Liang Z, Li X, Zhu F. 2-Year Efficacy, Immunogenicity, and Safety of Vigoo Enterovirus 72 Vaccine in Healthy Chinese Children: A Randomized Open-Label Study. J Infect Dis. 2017;215:56-63. [PubMed] [DOI] |
| 72. | Yi EJ, Shin YJ, Kim JH, Kim TG, Chang SY. Enterovirus 71 infection and vaccines. Clin Exp Vaccine Res. 2017;6:4-14. [PubMed] [DOI] |
| 73. | Liu L, Mo Z, Liang Z, Zhang Y, Li R, Ong KC, Wong KT, Yang E, Che Y, Wang J, Dong C, Feng M, Pu J, Wang L, Liao Y, Jiang L, Tan SH, David P, Huang T, Zhou Z, Wang X, Xia J, Guo L, Wang L, Xie Z, Cui W, Mao Q, Liang Y, Zhao H, Na R, Cui P, Shi H, Wang J, Li Q. Immunity and clinical efficacy of an inactivated enterovirus 71 vaccine in healthy Chinese children: a report of further observations. BMC Med. 2015;13:226. [PubMed] [DOI] |
| 74. | Hu Y, Zeng G, Chu K, Zhang J, Han W, Zhang Y, Li J, Zhu F. Five-year immunity persistence following immunization with inactivated enterovirus 71 type (EV71) vaccine in healthy children: A further observation. Hum Vaccin Immunother. 2018;14:1517-1523. [PubMed] [DOI] |
| 75. | Reed Z, Cardosa MJ. Status of research and development of vaccines for enterovirus 71. Vaccine. 2016;34:2967-2970. [PubMed] [DOI] |
| 76. | Chou AH, Liu CC, Chang JY, Jiang R, Hsieh YC, Tsao A, Wu CL, Huang JL, Fung CP, Hsieh SM, Wang YF, Wang JR, Hu MH, Chiang JR, Su IJ, Chong PC. Formalin-inactivated EV71 vaccine candidate induced cross-neutralizing antibody against subgenotypes B1, B4, B5 and C4A in adult volunteers. PLoS One. 2013;8:e79783. [PubMed] [DOI] |