修回日期: 2019-05-11
接受日期: 2019-06-04
在线出版日期: 2019-09-08
随着天然药物, 代谢调控药物等传统药物在肝细胞癌(hepatocellular carcinoma, HCC)治疗的应用报道, 老药新用途成为HCC药物治疗研究的新亮点. 具有降脂, 抗炎作用的植物源性药物姜黄素近来被发现可抑制HCC生长, 转移而呈现抗癌活性, 成为潜在的抗HCC治疗药物. 本文针对姜黄素抗HCC作用机制的新进展进行总结, 综述.
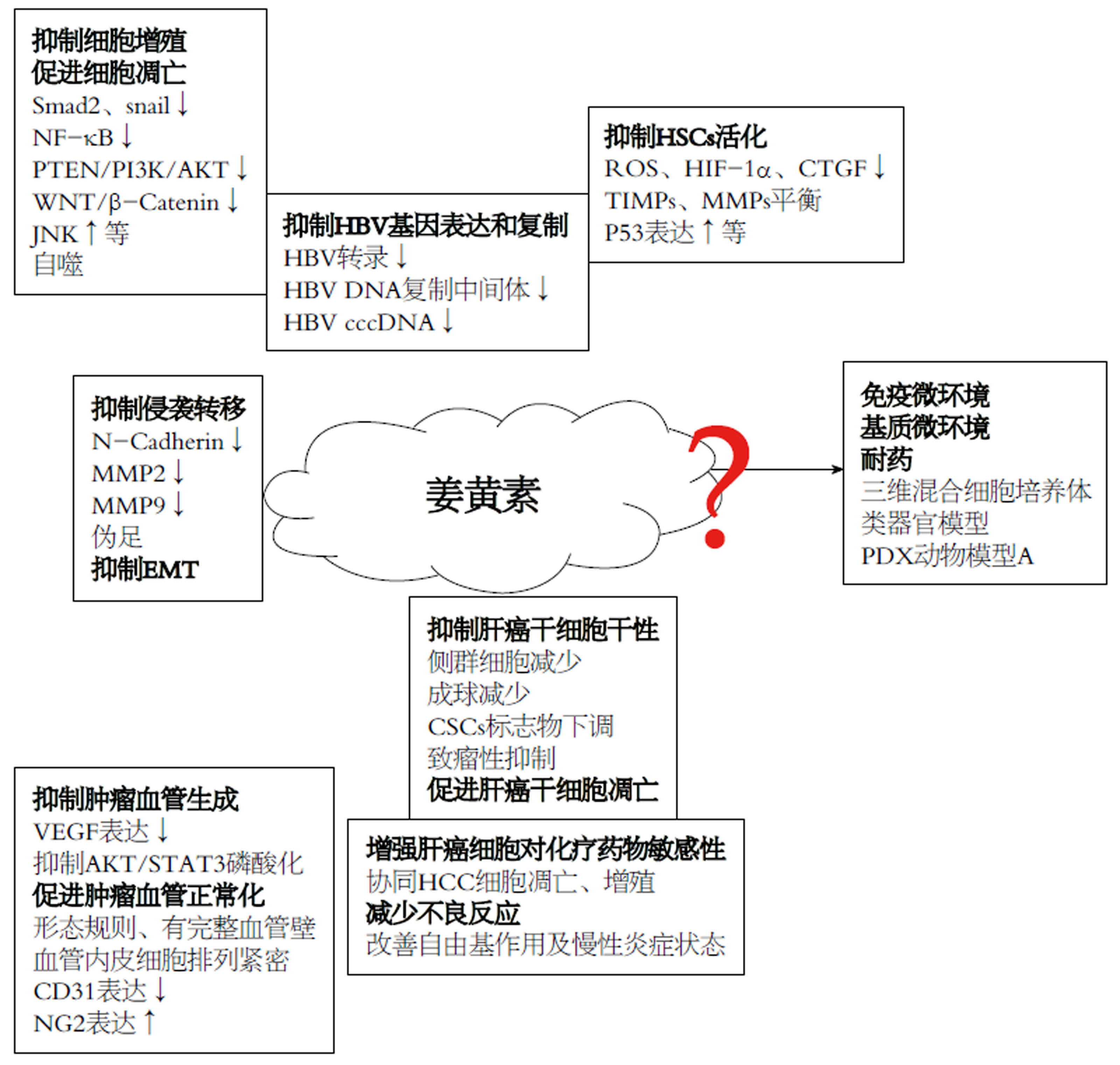
核心提要: 本文对姜黄素抗肝细胞癌(hepatocellular carcinoma, HCC)作用机制的研究进展进行了总结综述, 重点介绍其抑制HCC生长, 侵袭转移, 干性, 血管生成, 肝星状细胞活性, 乙肝病毒表达复制的作用机制, 提出姜黄素抗HCC研究面临问题及未来发展方向.
引文著录: 李苗, 任正刚, 崔杰峰. 姜黄素抗肝细胞癌作用机制新进展. 世界华人消化杂志 2019; 27(17): 1043-1049
Revised: May 11, 2019
Accepted: June 4, 2019
Published online: September 8, 2019
With the application of traditional drugs in the treatment of hepatocellular carcinoma (HCC), such as natural medicinal herbs and metabolic regulators, the new functions of traditional drugs have been revealed in the study of anti-HCC drug therapy. Curcumin, a plant-derived drug with hypolipidemic and anti-inflammation effects, has recently been found to exhibit anti-cancer activity due to its inhibitory effects on HCC growth and metastasis. Therefore, it may act as a potential anti-cancer drug for HCC treatment. This article summarizes the advances in the understanding of the action mechanism of curcumin on HCC.
- Citation: Li M, Ren ZG, Cui JF. Advances in understanding of mechanism of anti-hepatocellular carcinoma effects of curcumin. Shijie Huaren Xiaohua Zazhi 2019; 27(17): 1043-1049
- URL: https://www.wjgnet.com/1009-3079/full/v27/i17/1043.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v27.i17.1043
肝细胞癌(hepatocellular carcinoma, HCC)是消化系统最常见的恶性肿瘤之一, 在世界范围内其发病率位列恶性肿瘤第六位, 癌症相关死亡原因位列第二位, 且近年呈增长趋势[1,2]. HCC起病隐匿, 早期无明显临床症状, 诊断时往往已处中晚期. 中晚期HCC治疗手段目前主要以肝动脉化疗栓塞, 索拉非尼, 化疗(奥沙利铂, 氟尿嘧啶, 顺铂, 多柔比星等)为主, 但预后不佳[3-8]. 靶向药物(如仑伐替尼, 瑞戈非尼, 卡博替尼等)及免疫治疗药物(如PD-1抗体, PD-L1抗体)在部分中晚期HCC患者中虽然显示出一定疗效, 但与索拉非尼相比, 靶向药物总生存期(overall survival, OS)并无明显优势, 中位生存期也仅11 mo左右[9]. 而免疫治疗药物无论单独应用, 亦或与靶向药物或血管内皮生长因子(vascular endothelial growth factor, VEGF)抑制剂等联合应用, 应答率也仅处10%-40%之间[10].药物毒副作用, 耐药, 以及应答患者比例偏低依然是HCC药物治疗亟待解决的主要问题[11].
随着天然药物, 代谢调控药物等传统药物(姜黄素, 二甲双胍等)在HCC治疗的应用报道, 老药新用途逐渐成为近年HCC药物治疗研究的新亮点. 植物源性的天然药物具有低毒副作用, 较高抗癌效果等特征, 长期以来一直是抗肿瘤药物筛选宝库. 姜黄素是从姜科姜黄属植物姜黄根茎中提取的一种酚类色素[12]. 传统医学认为姜黄有通经止痛, 活血行气, 驱寒消炎等功效, 广泛用于降血脂, 消炎利胆, 保肝等[13]. 近年研究显示姜黄素可调控细胞周期, 抑制肿瘤发生, 转移而呈现抗癌活性[14-18], 且其对正常细胞毒性低, 副作用小[15], 成为肿瘤干预潜在的药物选择. 本文针对姜黄素抗HCC作用机制的研究新进展进行总结, 综述.
细胞生命活动基于完整细胞周期, 当某些因素导致G1/S和G2/M检测点控制功能阻断, 会使细胞持续增殖而失去控制, 细胞增殖与凋亡失去平衡, 导致肿瘤发生[19]. 研究显示, 姜黄素可阻滞HCC细胞周期, 使其不能进行有效有丝分裂, 也可调控一些蛋白和基因产物的表达(如Cyclin-D1, Bcl-2, Bax, caspase-3等), 干扰或阻断肿瘤细胞增殖及凋亡信号转导, 导致HCC细胞增殖抑制, 凋亡增加, 且作用呈时间, 剂量依赖性[20,21]. 报道发现, 姜黄素可活化HCC细胞SMMC-7721中JNK信号通路, 抑制ERK和p38 MAPK信号通路, 下调Bcl-2和Survivin表达, 同时上调Bax和半胱天冬酶-3(caspase-3)表达, 而抑制HCC细胞增殖, 增加其凋亡[22,23]. 其他研究也显示, 姜黄素抑制HCC细胞SMMC-7721增殖, 促进其凋亡, 并发现姜黄素处理组HCC细胞Notch表达降低, 认为Notch信号通路可能参与姜黄素对HCC细胞的抑制作用[24]. 姜黄素也可刺激HCC细胞MHCC97H活性氧(reactive oxygen species, ROS)生成, 以浓度依赖性方式抑制细胞增殖, 同时胞内ROS增加激活TLR-4/MyD-88信号通路, 使caspase-8和caspase-3活化, 导致HCC细胞凋亡增加[25]. HCC细胞HepG2的相关研究发现, 姜黄素显著降低HepG2细胞内热休克蛋白70(heat shock protein 70, HSP70)和TLR4水平, 使HepG2保持在DNA合成S期, 抑制HepG2细胞增殖, 并促进细胞凋亡[26]. 姜黄素还可下调GPC3/wnt/β-catenin信号通路抑制HCC细胞HepG2增殖, 诱导凋亡, 并降低自噬途径标记蛋白Sequestosome-1(SQSTM1)浓度, 提示自噬途径激活可能参与姜黄素诱导HCC细胞凋亡过程[27,28]. 姜黄素与甘草酸联合应用使HCC细胞HepG2阻滞于G1期, 细胞增殖受阻, 且细胞凋亡增加, 裸鼠皮下移植瘤模型证实姜黄素和甘草酸通过上调PTEN表达, 抑制PI3K/Akt通路活化, 发挥抗癌作用[29]. 此外, 姜黄素诱导细胞色素C(cytochrome C), caspase-3, caspase-9释放和聚ADP核糖聚合酶(poly ADP-ribose polymerase, PARP)裂解, 通过线粒体凋亡途径诱导HCC细胞H22凋亡[30]. 姜黄素也可抑制头颈部鳞状细胞癌转录因子NF-κB的转录活性, 使及其下游基因产物(COX-2, Cyclin-D1)表达降低, 从而显著降低癌细胞增殖[31].
侵袭转移是肿瘤细胞重要恶性特征之一, 也是绝大多数肿瘤致死原因, 能否降低, 抑制肿瘤细胞侵袭转移是抗癌药物评估的关键. Lin等[32]发现, 姜黄素抑制基质金属蛋白酶-9(matrix metalloproteinases 9, MMP-9)分泌, 从而抑制HCC细胞SK-Hep-1侵袭转移能力. 应用姜黄素类似物处理HCC细胞Huh7和HepG2, HCC细胞MMP-2, MMP-9和N-钙粘蛋白表达明显减少, 而E-钙粘蛋白表达上调, HCC细胞迁徙转移能力显著降低, 上述改变与ROS依赖性JNK通路活化关联, 而姜黄素抑制剂可逆转上述改变, 恢复HCC细胞运动, 转移能力[33]. 另一种姜黄素类似物二苯基二氟酮(diphenyl difluoroketone, EF24), 可减少HCC细胞HCCLM-3和HepG2膜表面丝状伪足形成, 还可抑制HCC细胞中整合素-细胞外基质相互作用信号通路的关键调节因子Src磷酸化, 而抑制HCC迁移, 侵袭[34].
上皮间质转化(epithelial-mesenchymal transitions, EMT)是肿瘤侵袭转移的早期分子事件. TGF-β1通过Smad依赖性和Smad非依赖性途径诱导HCCEMT发生, 姜黄素被发现通过抑制Smad2磷酸化及核转位, 下调Snail转录表达, 逆转TGF-β1诱导的HCC细胞EMT[35]. 此外, 姜黄素通过分别抑制MAPK/AP-1信号通路激活, 阻断PI3K/Akt/NF-κB信号通路活化, 抑制c-Met 依赖性PI3K/Akt/mTOR信号通路活化, 导致E-钙粘蛋白表达增加, N-钙粘蛋白和波形蛋白表达降低, 而相应地逆转乳腺癌[36], 胰腺癌[37]和肺癌[38]细胞EMT发生.
肿瘤干细胞(cancer stem cells, CSCs)能够维持肿瘤形成和分化, 具有自我更新特征, 是肿瘤复发和治疗抵抗的重要原因[39]. Wang等[40]评估姜黄素对HCC干细胞(liver cancer stem cells, LCSCs)增殖和凋亡的影响, 发现姜黄素通过抑制PI3K/AKT/mTOR信号通路活化而抑制LCSCs增殖, 同时上调凋亡相关蛋白(caspase-3, caspase-9, Bax)表达, 下调Bcl-2表达, 释放细胞色素C, 诱导LCSCs凋亡. 也有报道显示, 在对姜黄素敏感HCC细胞(Huh7, PLC)中, 姜黄素可导致CSCs选择性耗竭, 包括侧群细胞减少, 成球减少, CSCs标志物下调及致瘤性抑制等, 并认为该作用是通过抑制NF-κB活化, 降低组蛋白去乙酰化酶活性实现的[41]. 还有研究发现, 姜黄素通过抑制芳香烃受体表达及其下游ERK, SK1磷酸化, 进而抑制邻苯二甲酸酯诱导的HCC细胞(Huh7, PLC/PRF/5)中CSC样细胞比例增加及CSC样细胞干性特征增强[42].
肿瘤的发生, 进展与其诱发新生血管的生成密切关联. VEGF/VEGFR通路是血管内皮细胞生长, 增殖, 迁移最重要分子调控信号[43,44]. 研究显示, 姜黄素能显著下调HCC细胞HepG2和H22中VEGF表达, 抑制血管生成进而抑制HCC进展[21,45]. 在HCC细胞SK-Hep-1中的研究发现, 姜黄素通过降低AKT和STAT3磷酸化而抑制AKT/STAT3活化, 抑制肿瘤血管形成[46]. Tang等[47]联合应用姜黄素和黄芪多糖干预HepG2来源裸鼠HCC原位移植瘤, 发现肿瘤血管稀疏, 生长均匀, 形态规则且有完整血管壁, 血管分支较少, 血管内皮细胞排列紧密, CD31表达降低, 硫酸软骨素蛋白多糖2(neuron-glial antigen 2, NG2)表达增加, 提示干预后的肿瘤组织血管趋于正常化. 故姜黄素除对血管生成有抑制作用外, 还可诱导肿瘤血管正常化, 改善肿瘤血管形态结构, 促进肿瘤血管成熟.
肝星状细胞(hepatic stellate cells, HSCs)是HCC微环境重要细胞组分, 其活化及其表型改变在HCC侵袭转移中发挥重要作用, 静息HSCs在肝纤维化期间被激活, 分泌生长因子和细胞因子, 且过度生成胞外基质蛋白, 促进癌细胞的侵袭和增殖[48,49]. 姜黄素可通过多途径, 靶向多位点抑制HSCs活化, 如血小板衍生生长因子-β受体(platelet-derived growth factor-βreceptor, PDGF-βR), toll样受体(toll-like receptors, TLRs), MMPs, 过氧化物酶体增殖物激活受体(peroxisome proliferator-activated receptors, PPARc), 炎性细胞因子等; 姜黄素还可阻断HSCs瘦素信号通路; 调节HSCs葡萄糖代谢, 脂质代谢; 调节组织MMP抑制剂(tissue inhibitor of matrix metalloproteinases, TIMPs)和MMPs平衡[49], 进而影响HCC侵袭转移. 研究发现, 活化HSCs分泌可溶性因子如白细胞介素-6(interleukin-6, IL-6), VEGF和基质衍生因子-1(stromal-derived factor-1, SDF-1), 促进HCC细胞 HepG2进展; 活化的HSCs还可促进HepG2细胞ROS产生, 上调缺氧诱导因子-1α(hypoxia-inducible factor-1α, HIF-1α)表达, 促进血管生成, EMT及侵袭能力; 姜黄素可显著抑制HSCs的上述作用, 减少HepG2细胞 ROS和HIF-1α表达, 在HepG2细胞中敲除HIF-1α或其下游基因结缔组织生长因子(connective tissue growth factor, CTGF)可逆转姜黄素的抑制作用; 此外, 姜黄素可上调核因子E2相关因子2(nuclear factor E2-related factor 2, Nrf2)和谷胱甘肽诱导ROS清除, 降低HIF-1α稳定性以抑制CTGF表达, 从而起到干预HCC作用[50]. Jin等[48]研究分析姜黄素对活化HSCs衰老的影响, 发现姜黄素上调衰老标志物P16, P21和Hmga1表达诱导HSCs衰老, 同时下调HSCs活化标志物α-平滑肌肌动蛋白和α1(1)前胶原含量, 认为姜黄素诱导HSCs衰老与PPARγ激活依赖性机制促进P53表达有关[50].
乙肝病毒(hepatitis B virus, HBV)感染是我国HCC患者主要病因, HBV基因复制与HCC不良预后相关[51,52], 针对HBV治疗可减少HCC肺转移[52], 改善HCC预后[53]. 姜黄素可通过抑制HBV基因表达和复制发挥抑制HCC进展作用. 研究发现, 姜黄素治疗降低HBsAg和HBeAg表达, 以及细胞内HBV DNA复制中间体和HBV cccDNA, 且该作用呈时间, 剂量依赖性; 此外, 姜黄素处理后, 组蛋白H3乙酰化水平降低, 并伴随H3-和H4-结合的cccDNA减少, 提示姜黄素通过下调cccDNA结合的组蛋白乙酰化抑制HBV基因复制[54]. 过氧化物酶体增殖物激活受体γ共激活因子1α(peroxisome proliferator-activated receptor γ coactivator-1α, PGC-1 α)是一种饥饿诱导蛋白质, 可启动糖原异生级联, 有效地共激活HBV转录, 有研究认为姜黄素通过下调PGC-1α抑制HBV基因表达和复制[55]. 故姜黄素可作为HBV补充治疗, 通过控制HBV感染间接对HCC发挥抑制作用.
姜黄素可增强HCC细胞对化疗药物敏感性. 姜黄素与化疗药物顺铂[56], 多柔比星[57,58]等制成共载脂质体联合应用, 比化疗药物单独使用具有更强抗肿瘤活性, 更高的细胞毒性及更低的IC50. 其中原因可能与姜黄素协同HCC细胞凋亡, 抑制HCC增殖和血管生成有关, 研究显示, 与多柔比星单药比较, 姜黄素与多柔比星联合用药, HCC细胞SMMC 7721 Caspase-3和Bax/Bcl-2比例明显增加, C-myc, PCNA和VEGF表达明显降低[57]. 其他学者相关研究还发现, 与多柔比星单药比较, 姜黄素与多柔比星联合用药组HCC细胞BEL7402的MDR1, Bcl-2和HIF-1α基因mRNA水平以及P-gp, Bcl-2和HIF-1α蛋白水平均明显降低, 提示姜黄素可能参与逆转多柔比星耐药[58]. 此外, 姜黄素有抗炎作用, 靶向各种炎症介质, 如环氧合酶-2, 诱导型一氧化氮合酶和NF-κB, 削弱促炎和促纤维化细胞因子释放, 抑制自由基产生, 改善自由基作用及慢性炎症状态, 增强HCC细胞对化疗药物敏感性, 减少不良反应[59].
尽管植物源性的天然药物姜黄素抑制HCC机制研究取得一些进展, 但由于HCC发生与进展多伴随炎性(HBV/丙型肝炎病毒)和硬度背景(纤维化/硬化), 加之HCC细胞周边物化微环境调控认识的相对滞后与不足, 姜黄素对HCC细胞抑制研究目前依然多集中于其对HCC细胞增殖, 凋亡的体外细胞机制研究(图1). 而姜黄素对HCC基质微环境, 免疫微环境的影响, 炎性, 硬度背景对姜黄素抑制作用的抵抗, 以及姜黄素体内药物敏感情况及耐受机制等研究尚处于探索起步阶段. 随着三维混合细胞培养体系, 组织硬度模拟培养平台, 类器官模型及人源肿瘤异种移植模型(PDX动物模型)等新型研究手段的成熟与应用, 必将极大地推动姜黄素对HCC抑制作用机制再认识, 加速其抗HCC治疗的临床应用.
学科分类: 胃肠病学和肝病学
手稿来源地: 上海市
同行评议报告分类
A级 (优秀): A, A
B级 (非常好): B
C级 (良好): 0
D级 (一般): 0
E级 (差): 0
编辑: 崔丽君 电编:刘继红
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [PubMed] [DOI] |
| 2. | Mak LY, Cruz-Ramón V, Chinchilla-López P, Torres HA, LoConte NK, Rice JP, Foxhall LE, Sturgis EM, Merrill JK, Bailey HH, Méndez-Sánchez N, Yuen MF, Hwang JP. Global Epidemiology, Prevention, and Management of Hepatocellular Carcinoma. Am Soc Clin Oncol Educ Book. 2018;38:262-279. [PubMed] [DOI] |
| 3. | Colombo M, Sangiovanni A. Treatment of hepatocellular carcinoma: beyond international guidelines. Liver Int. 2015;35 Suppl 1:129-138. [PubMed] [DOI] |
| 4. | European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [PubMed] [DOI] |
| 5. | Goossens N, Koessler T, Spahr L, Negro F. [Hepatocellular carcinoma: updated management guidelines]. Rev Med Suisse. 2018;14:1508-1511. [PubMed] |
| 6. | Lu X, Zhao H, Yang H, Mao Y, Sang X, Miao R, Xu Y, Du S, Xu H, Chi T, Yang Z, Zhong S, Huang J. A prospective clinical study on early recurrence of hepatocellular carcinoma after hepatectomy. J Surg Oncol. 2009;100:488-493. [PubMed] [DOI] |
| 7. | Drews RE, Shulman LN. Update in hematology and oncology. Ann Intern Med. 2010;152:655-662. [PubMed] [DOI] |
| 8. | Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-855. [PubMed] [DOI] |
| 9. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [PubMed] [DOI] |
| 10. | Okusaka T, Ikeda M. Immunotherapy for hepatocellular carcinoma: current status and future perspectives. ESMO Open. 2018;3:e000455. [PubMed] [DOI] |
| 11. | Eso Y, Marusawa H. Novel approaches for molecular targeted therapy against hepatocellular carcinoma. Hepatol Res. 2018;48:597-607. [PubMed] [DOI] |
| 12. | Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric: from farm to pharmacy. Biofactors. 2013;39:2-13. [PubMed] [DOI] |
| 13. | Khan H, Ullah H, Nabavi SM. Mechanistic insights of hepatoprotective effects of curcumin: Therapeutic updates and future prospects. Food Chem Toxicol. 2019;124:182-191. [PubMed] [DOI] |
| 14. | Bose S, Panda AK, Mukherjee S, Sa G. Curcumin and tumor immune-editing: resurrecting the immune system. Cell Div. 2015;10:6. [PubMed] [DOI] |
| 15. | Sak K. Chemotherapy and dietary phytochemical agents. Chemother Res Pract. 2012;2012:282570. [PubMed] [DOI] |
| 16. | Park W, Amin AR, Chen ZG, Shin DM. New perspectives of curcumin in cancer prevention. Cancer Prev Res (Phila). 2013;6:387-400. [PubMed] [DOI] |
| 17. | Chikara S, Nagaprashantha LD, Singhal J, Horne D, Awasthi S, Singhal SS. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018;413:122-134. [PubMed] [DOI] |
| 18. | Li W, Guo Y, Zhang C, Wu R, Yang AY, Gaspar J, Kong AN. Dietary Phytochemicals and Cancer Chemoprevention: A Perspective on Oxidative Stress, Inflammation, and Epigenetics. Chem Res Toxicol. 2016;29:2071-2095. [PubMed] [DOI] |
| 19. | Rawat D, Shrivastava S, Naik RA, Chhonker SK, Mehrotra A, Koiri RK. An Overview of Natural Plant Products in the Treatment of Hepatocellular Carcinoma. Anticancer Agents Med Chem. 2018;18:1838-1859. [PubMed] [DOI] |
| 20. | You Z, Li B, Xu J, Chen L, Ye H. Curcumin suppress the growth of hepatocellular carcinoma via down-regulating SREBF1. Oncol Res. 2018;. [PubMed] [DOI] |
| 21. | Pan Z, Zhuang J, Ji C, Cai Z, Liao W, Huang Z. Curcumin inhibits hepatocellular carcinoma growth by targeting VEGF expression. Oncol Lett. 2018;15:4821-4826. [PubMed] [DOI] |
| 22. | Zhang YJ, Xiang H, Liu JS, Li D, Fang ZY, Zhang H. Study on the mechanism of AMPK signaling pathway and its effect on apoptosis of human hepatocellular carcinoma SMMC-7721 cells by curcumin. Eur Rev Med Pharmacol Sci. 2017;21:1144-1150. [PubMed] |
| 23. | Yu J, Zhou X, He X, Dai M, Zhang Q. Curcumin induces apoptosis involving bax/bcl-2 in human hepatoma SMMC-7721 cells. Asian Pac J Cancer Prev. 2011;12:1925-1929. [PubMed] |
| 24. | Liu ZC, Yang ZX, Zhou JS, Zhang HT, Huang QK, Dang LL, Liu GX, Tao KS. Curcumin regulates hepatoma cell proliferation and apoptosis through the Notch signaling pathway. Int J Clin Exp Med. 2014;7:714-718. [PubMed] |
| 25. | Li PM, Li YL, Liu B, Wang WJ, Wang YZ, Li Z. Curcumin inhibits MHCC97H liver cancer cells by activating ROS/TLR-4/caspase signaling pathway. Asian Pac J Cancer Prev. 2014;15:2329-2334. [PubMed] |
| 26. | Ren B, Luo S, Tian X, Jiang Z, Zou G, Xu F, Yin T, Huang Y, Liu J. Curcumin inhibits liver cancer by inhibiting DAMP molecule HSP70 and TLR4 signaling. Oncol Rep. 2018;40:895-901. [PubMed] [DOI] |
| 27. | Elmansi AM, El-Karef AA, Shishtawy MMEl, Eissa LA. Hepatoprotective Effect of Curcumin on Hepatocellular Carcinoma Through Autophagic and Apoptic Pathways. Ann Hepatol. 2017;16:607-618. [PubMed] [DOI] |
| 28. | Hu P, Ke C, Guo X, Ren P, Tong Y, Luo S, He Y, Wei Z, Cheng B, Li R, Luo J, Meng Z. Both glypican-3/Wnt/β-catenin signaling pathway and autophagy contributed to the inhibitory effect of curcumin on hepatocellular carcinoma. Dig Liver Dis. 2019;51:120-126. [PubMed] [DOI] |
| 29. | Chang M, Wu M, Li H. Curcumin combined with glycyrrhetinic acid inhibits the development of hepatocellular carcinoma cells by down-regulating the PTEN/PI3K/AKT signalling pathway. Am J Transl Res. 2017;9:5567-5575. [PubMed] |
| 30. | Zhang Z, Luo D, Xie J, Lin G, Zhou J, Liu W, Li H, Yi T, Su Z, Chen J. Octahydrocurcumin, a final hydrogenated metabolite of curcumin, possesses superior anti-tumor activity through induction of cellular apoptosis. Food Funct. 2018;9:2005-2014. [PubMed] [DOI] |
| 31. | Wang D, Veena MS, Stevenson K, Tang C, Ho B, Suh JD, Duarte VM, Faull KF, Mehta K, Srivatsan ES, Wang MB. Liposome-encapsulated curcumin suppresses growth of head and neck squamous cell carcinoma in vitro and in xenografts through the inhibition of nuclear factor kappaB by an AKT-independent pathway. Clin Cancer Res. 2008;14:6228-6236. [PubMed] [DOI] |
| 32. | Lin LI, Ke YF, Ko YC, Lin JK. Curcumin inhibits SK-Hep-1 hepatocellular carcinoma cell invasion in vitro and suppresses matrix metalloproteinase-9 secretion. Oncology. 1998;55:349-353. [PubMed] [DOI] |
| 33. | Wang L, Han L, Tao Z, Zhu Z, Han L, Yang Z, Wang H, Dai D, Wu L, Yuan Z, Chen T. The curcumin derivative WZ35 activates ROS-dependent JNK to suppress hepatocellular carcinoma metastasis. Food Funct. 2018;9:2970-2978. [PubMed] [DOI] |
| 34. | Zhao R, Tin L, Zhang Y, Wu Y, Jin Y, Jin X, Zhang F, Li X. EF24 Suppresses Invasion and Migration of Hepatocellular Carcinoma Cells In Vitro via Inhibiting the Phosphorylation of Src. Biomed Res Int. 2016;2016:8569684. [PubMed] [DOI] |
| 35. | Cao MT, Liu HF, Liu ZG, Xiao P, Chen JJ, Tan Y, Jiang XX, Jiang ZC, Qiu Y, Huang HJ, Zhang QG, Jiang GM. Curcumin downregulates the expression of Snail via suppressing Smad2 pathway to inhibit TGF-β1-induced epithelial-mesenchymal transitions in hepatoma cells. Oncotarget. 2017;8:108498-108508. [PubMed] [DOI] |
| 36. | Coker-Gurkan A, Bulut D, Genc R, Arisan ED, Obakan-Yerlikaya P, Palavan-Unsal N. Curcumin prevented human autocrine growth hormone (GH) signaling mediated NF-κB activation and miR-183-96-182 cluster stimulated epithelial mesenchymal transition in T47D breast cancer cells. Mol Biol Rep. 2019;46:355-369. [PubMed] [DOI] |
| 37. | Li W, Jiang Z, Xiao X, Wang Z, Wu Z, Ma Q, Cao L. Curcumin inhibits superoxide dismutase-induced epithelial-to-mesenchymal transition via the PI3K/Akt/NF-κB pathway in pancreatic cancer cells. Int J Oncol. 2018;. [PubMed] [DOI] |
| 38. | Jiao D, Wang J, Lu W, Tang X, Chen J, Mou H, Chen QY. Curcumin inhibited HGF-induced EMT and angiogenesis through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways in lung cancer. Mol Ther Oncolytics. 2016;3:16018. [PubMed] [DOI] |
| 39. | Zendehdel E, Abdollahi E, Momtazi-Borojeni AA, Korani M, Alavizadeh SH, Sahebkar A. The molecular mechanisms of curcumin's inhibitory effects on cancer stem cells. J Cell Biochem. 2019;120:4739-4747. [PubMed] [DOI] |
| 40. | Wang J, Wang C, Bu G. Curcumin inhibits the growth of liver cancer stem cells through the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway. Exp Ther Med. 2018;15:3650-3658. [PubMed] [DOI] |
| 41. | Marquardt JU, Gomez-Quiroz L, Arreguin Camacho LO, Pinna F, Lee YH, Kitade M, Domínguez MP, Castven D, Breuhahn K, Conner EA, Galle PR, Andersen JB, Factor VM, Thorgeirsson SS. Curcumin effectively inhibits oncogenic NF-κB signaling and restrains stemness features in liver cancer. J Hepatol. 2015;63:661-669. [PubMed] [DOI] |
| 42. | Tsai CF, Hsieh TH, Lee JN, Hsu CY, Wang YC, Kuo KK, Wu HL, Chiu CC, Tsai EM, Kuo PL. Curcumin Suppresses Phthalate-Induced Metastasis and the Proportion of Cancer Stem Cell (CSC)-like Cells via the Inhibition of AhR/ERK/SK1 Signaling in Hepatocellular Carcinoma. J Agric Food Chem. 2015;63:10388-10398. [PubMed] [DOI] |
| 43. | Fu X, Yang Y, Li X, Lai H, Huang Y, He L, Zheng W, Chen T. RGD peptide-conjugated selenium nanoparticles: antiangiogenesis by suppressing VEGF-VEGFR2-ERK/AKT pathway. Nanomedicine. 2016;12:1627-1639. [PubMed] [DOI] |
| 44. | Lee WS, Pyun BJ, Kim SW, Shim SR, Nam JR, Yoo JY, Jin Y, Jin J, Kwon YG, Yun CO, Nam DH, Oh K, Lee DS, Lee SH, Yoo JS. TTAC-0001, a human monoclonal antibody targeting VEGFR-2/KDR, blocks tumor angiogenesis. MAbs. 2015;7:957-968. [PubMed] [DOI] |
| 45. | Wang F, He Z, Dai W, Li Q, Liu X, Zhang Z, Zhai D, Chen J, Chen W. The role of the vascular endothelial growth factor/vascular endothelial growth factor receptors axis mediated angiogenesis in curcumin-loaded nanostructured lipid carriers induced human HepG2 cells apoptosis. J Cancer Res Ther. 2015;11:597-605. [PubMed] [DOI] |
| 46. | Chiablaem K, Lirdprapamongkol K, Keeratichamroen S, Surarit R, Svasti J. Curcumin suppresses vasculogenic mimicry capacity of hepatocellular carcinoma cells through STAT3 and PI3K/AKT inhibition. Anticancer Res. 2014;34:1857-1864. [PubMed] |
| 47. | Tang D, Zhang S, Shi X, Wu J, Yin G, Tan X, Liu F, Wu X, Du X. Combination of Astragali Polysaccharide and Curcumin Improves the Morphological Structure of Tumor Vessels and Induces Tumor Vascular Normalization to Inhibit the Growth of Hepatocellular Carcinoma. Integr Cancer Ther. 2019;18:1534735418824408. [PubMed] [DOI] |
| 48. | Jin H, Lian N, Zhang F, Chen L, Chen Q, Lu C, Bian M, Shao J, Wu L, Zheng S. Activation of PPARγ/P53 signaling is required for curcumin to induce hepatic stellate cell senescence. Cell Death Dis. 2016;7:e2189. [PubMed] [DOI] |
| 49. | Tang Y. Curcumin targets multiple pathways to halt hepatic stellate cell activation: updated mechanisms in vitro and in vivo. Dig Dis Sci. 2015;60:1554-1564. [PubMed] [DOI] |
| 50. | Shao S, Duan W, Xu Q, Li X, Han L, Li W, Zhang D, Wang Z, Lei J. Curcumin Suppresses Hepatic Stellate Cell-Induced Hepatocarcinoma Angiogenesis and Invasion through Downregulating CTGF. Oxid Med Cell Longev. 2019;2019:8148510. [PubMed] [DOI] |
| 51. | Jiang Y, Tang H, Wang Z, Sun Y, Meng W, Wang G, Li H, Yi S, Wang G, Yang Y, Chen G. Two Nomograms to Select Hepatocellular Carcinoma Patients with Macroscopic Vascular Invasion for Hepatic Resection. J Cancer. 2018;9:3287-3294. [PubMed] [DOI] |
| 52. | Wang F, Lv H, Li Y, Han T, Liu H, Jia K, Liu F, Gao Y, Wang F. Complete cure of a patient with HBV-associated hepatocellular carcinoma with lung metastasis using interferon and survival up to 108 months: A case report and literature review. Oncol Lett. 2018;16:2979-2988. [PubMed] [DOI] |
| 53. | Zhang M, Wang D, Liu H, Li H. Tenofovir decrease hepatocellular carcinoma recurrence in chronic hepatitis B patients after liver resection. Infect Agent Cancer. 2018;13:19. [PubMed] [DOI] |
| 54. | Wei ZQ, Zhang YH, Ke CZ, Chen HX, Ren P, He YL, Hu P, Ma DQ, Luo J, Meng ZJ. Curcumin inhibits hepatitis B virus infection by down-regulating cccDNA-bound histone acetylation. World J Gastroenterol. 2017;23:6252-6260. [PubMed] [DOI] |
| 55. | Rechtman MM, Har-Noy O, Bar-Yishay I, Fishman S, Adamovich Y, Shaul Y, Halpern Z, Shlomai A. Curcumin inhibits hepatitis B virus via down-regulation of the metabolic coactivator PGC-1alpha. FEBS Lett. 2010;584:2485-2490. [PubMed] [DOI] |
| 56. | Cheng Y, Zhao P, Wu S, Yang T, Chen Y, Zhang X, He C, Zheng C, Li K, Ma X, Xiang G. Cisplatin and curcumin co-loaded nano-liposomes for the treatment of hepatocellular carcinoma. Int J Pharm. 2018;545:261-273. [PubMed] [DOI] |
| 57. | Zhang J, Li J, Shi Z, Yang Y, Xie X, Lee SM, Wang Y, Leong KW, Chen M. pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities. Acta Biomater. 2017;58:349-364. [PubMed] [DOI] |
| 58. | Zhao X, Chen Q, Li Y, Tang H, Liu W, Yang X. Doxorubicin and curcumin co-delivery by lipid nanoparticles for enhanced treatment of diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur J Pharm Biopharm. 2015;93:27-36. [PubMed] [DOI] |
| 59. | Farhood B, Mortezaee K, Goradel NH, Khanlarkhani N, Salehi E, Nashtaei MS, Najafi M, Sahebkar A. Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J Cell Physiol. 2019;234:5728-5740. [PubMed] [DOI] |