修回日期: 2018-01-20
接受日期: 2018-01-29
在线出版日期: 2018-02-28
比较年龄、胆红素、INR、肌酸肝(age, bilirubin, INR, creatinine, ABIC), Maddrey's判别式函数(discriminant function, MDF), 终末期肝病模型(model for end-stage liver disease, MELD), 慢性肝衰竭-序贯器官衰竭(chronic liver failure-sequential organ failure assessment, CLIF-SOFA)评分, Child-Turcotte-Pugh (CTP)五种评分系统对酒精相关慢加急性肝衰竭(acute-on-chronic liver failure, ACLF)患者短期预后的预测价值.
本研究回顾性收集并分析了从2005-08/2017-06在天津市第三中心医院住院的肝衰竭患者462例, 并根据诊断标准和排除标准, 最终纳入酒精相关的ACLF患者152例. 其中入院时仅符合亚太肝脏研究学会标准而不符合欧洲肝脏研究学会-慢性肝衰竭(European Association for the Study of the Liver-Chronic Liver Failure, EASL-CLIF)标准的归为A组, 符合EASL-CLIF标准的归为B组, 采用受试者工作特征曲线(receiver operator characteristic curve, ROC)下面积(area under the curve, AUC)分别评估五种评分系统对两组患者28 d预后的预测价值.
A、B两组患者28 d死亡率分别为19%和50%, 差异有统计学意义(P = 0.002). 在A组患者中, CLIF-SOFA评分预测28 d死亡率的ROC曲线下面积(AUC)最大为0.889, 随后依次为MELD(0.761)、MDF(0.738)、ABIC(0.718)和CTP(0.671), CTP与其他四种评分模型相比有统计学差异. B组患者中, CLIF-SOFA评分预测28 d死亡率的ROC曲线下面积(AUC)最大为0.916, 随后依次为MELD(0.804)、MDF(0.770)、ABIC(0.729)和CTP(0.647), CLIF-SOFA与其他四种评分模型相比以及CTP和其他4种评分模型相比均有统计学差异.
五种评分系统均能预测A、B两组患者短期预后. 但无论A组和B组, CLIF-SOFA评分对28 d死亡率的预测能力均优于其他评分系统.
核心提要: 目前国内外出现多种慢加急性肝衰竭(acute-on-chronic liver failure, ACLF)诊断标准, 且酒精相关的ACLF发生率逐年升高, 本研究主要比较了符合不同标准酒精相关ACLF患者的预后情况.
引文著录: 席蓉蓉, 韩涛, 吕佳昱, 蔡均均. 比较不同评分模型对酒精相关慢加急性肝衰竭患者短期预后的评估. 世界华人消化杂志 2018; 26(6): 365-372
Revised: January 20, 2018
Accepted: January 29, 2018
Published online: February 28, 2018
To compare the performance of age, bilirubin, INR, and creatinine (ABIC), Maddrey's discriminant function (MDF), model for end-stage liver disease (MELD), chronic liver failure-sequential organ failure assessment (CLIF-SOFA), and Child-Turcotte-Pugh (CTP) in predicting short-term mortality in patients with alcohol-related acute-on-chronic liver failure (ACLF).
There were 462 consecutive patients with live failure treated from August 2005 to June 2017 at Tianjin Third Central Hospital, of whom 152 with alcohol-related ACLF were finally enrolled in this study according to the inclusion criteria and exclusion criteria. We divided patients into either group A or group B. Patients in group A met the criteria of Asian Pacific Association for the Study of the Liver but did not met the criteria of European Association for the Study of the Liver-Chronic Liver Failure (EASL-CLIF), and patients in group B met the criteria of EASL-CLIF on admission. The performance of different scoring models in predicting short-term mortality was assessed using the area under the receiver operating characteristic curve (AUC-ROC).
The 28-d mortality rate was 19% in group A and 50% in group B (P = 0.002). In group A, the AUC of CLIF-SOFA for predicting the 28-d mortality was highest (0.889), followed by MELD (0.761), MDF (0.738), ABIC (0.718), and CTP (0.671), and there was a significant difference between CTP and the others. In group B, the AUC of CLIF-SOFA was 0.916, followed by MELD (0.804), MDF (0.770), ABIC (0.729), and CTP (0.647), and there was a significant difference between CLIF-SOFA and the others and between CTP and the others.
The five scoring systems could all predict the short-term prognosis of the two groups of patients. However, CLIF-SOFA performs well compared to the others, regardless of patients in group A or group B.
- Citation: Xi RR, Han T, Lv JY, Cai JJ. Comparison of five different scoring models for predicting short-term mortality in patients with alcohol-related acute-on-chronic liver failure. Shijie Huaren Xiaohua Zazhi 2018; 26(6): 365-372
- URL: https://www.wjgnet.com/1009-3079/full/v26/i6/365.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v26.i6.365
慢加急性肝衰竭(acute-on-chronic liver failure, ACLF)是在慢性肝病基础上出现急性肝功能失代偿, 导致患者出现以凝血功能障碍、黄疸、肝性脑病、腹水等为主要表现的威胁生命的临床综合征[1]. 近来, 酒精相关ACLF患者比例逐年上升, 占临床所有ACLF病例的25%[2]. 酒精相关ACLF患者发生多器官衰竭的比例更高, 其28 d死亡率高达50%[3]. 因此, 临床迫切需要一种可靠的预后评分系统评估疾病的严重程度和短期预后, 这在终末期肝病患者管理中尤为非常重要. 本文旨在比较不同评分模型对不同严重程度酒精相关ACLF患者预后的预测价值.
本研究回顾性收集并分析了从2005-08/2017-06在天津市第三中心医院住院的肝衰竭患者462例, 并根据诊断标准和排除标准, 最终纳入酒精相关的ACLF患者152例. 其中入院时仅符合亚太肝脏研究学会(Asian Pacific Association for the Study of the Liver, APASL)标准[4]而不符合欧洲肝脏研究学会(European Association for the Study of the Liver, EASL)标准[5]的归为A组(n = 90例), 符合EASL标准的归为B组(n = 64例). 排除标准: (1)合并病毒性肝炎; (2)自身免疫性肝病; (3)肝癌或其他恶性肿瘤; (4)肝脏或其他器官移植史; (5)HIV感染者; (6)合并严重其他脏器疾病如肾功能衰竭, 心力衰竭等.
通过病历收集患者入院24 h内的临床与实验室指标并随访至28 d, 分别计算患者MELD[6]、ABIC[7]、MDF[8]、Chronic Liver Failure(CLIF)-SOFA[4]、CTP[9]分值, 各评分系统具体计算方式见表1. 采用受试者工作特征曲线(receiver operating characteristic, ROC)评估五种评分模型对A组和B组患者28 d预后的预测价值.
| 评分系统 | 评分标准 | ||||
| MELD[6] | 公式: 3.78 ln[bilirubin (mg/dL)]+11.20ln(INR)+9.57ln[creatinine(mg/dL)]+6.43 | ||||
| ABIC[7] | 公式: (age×0.1)+[serum bilirubin (mg/dL)×0.08]+[serum creatinine (mg/dL)×0.3]+(INR×0.8) | ||||
| MDF[8] | 公式: 4.6 (patient's PT-reference PT)+totalbilirubin (mg/dL) | ||||
| CLIF-SOFA[4] | 0分 | 1分 | 2分 | 3分 | 4分 |
| 肝脏(总胆红, mg/dL) | <1.2 | ≥1.2-<2.0 | ≥2.0-<6.0 | ≥6.0-<12.0 | ≥12.0 |
| 肾脏(肌酐, mg/dL) | <1.2 | ≥1.2-<2.0 | ≥2.0-<3.5 | ≥3.5-<5.0 | ≥5.0 |
| 神经(肝性脑病分级) | 无 | Ⅰ | Ⅱ | Ⅲ | Ⅳ |
| 凝血功能(国际标准化比值) | <1.1 | ≥1.1-<1.25 | ≥1.25-<1.5 | ≥1.5-<2.5 | ≥2.5或血小板≤20×109/L |
| 循环[平均动脉压(mmHg) 治疗用药[μg/(kg·min)] | ≥70 | <70 | 多巴胺≤5或多巴酚丁胺或特利加压素 | 多巴胺>5或肾上腺素≤0.1或去甲肾上腺≤0.1 | 多巴胺>15或肾上腺素>0.1或去甲肾上腺素>0.1 |
| 呼吸(PaO2/FiO2或SpO2/FiO2) | >400>512 | >300-≤400>357-≤512 | >200-≤300>214-≤357 | >100-≤200>89-≤214 | ≤100≤89 |
| CTP评分[9] | 1分 | 2分 | 3分 | ||
| 肝性脑病(级) | 无 | Ⅰ-Ⅱ级 | Ⅲ-Ⅳ级 | ||
| 腹水 | 无 | 轻度 | 中到重度 | ||
| 总胆红素(μmol/L) | <34 | 34-51 | >51 | ||
| 凝血酶原时间延长(s) | <4 | 4-6 | >6 | ||
| 血白蛋白(g/L) | >35 | 28-35 | <28 | ||
统计学处理 所有数据均采用SPSS 23.0软件进行统计学分析. 满足正态分布计量资料以mean±SD表示, 两组间比较采用t检验. 非正态计量资料以中位数(M)和四分位间距(QR)表示, 两组间比较采用Mann-Whitney U检验. 计数资料采用数量及百分数表示, 两组间比较选用χ2检验和Fisher's精确检验. 采用ROC曲线评估五种评分预测ACLF患者28 d死亡率的准确性. 采用Kaplan-Meier生存分析法评估患者的预后情况, 两组生存率的比较采用Log-rank检验. P<0.05为差异有统计学意义.
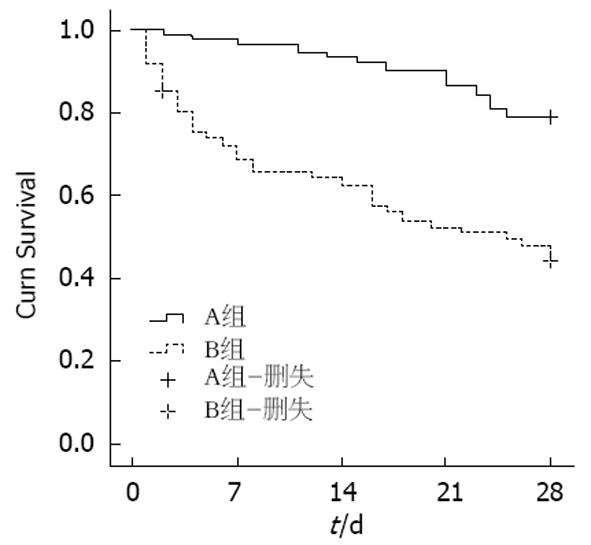
A组和B组患者28 d死亡率分别是19%和50%, 差异有统计学意义(Z = 23.768, P = 0.002)(图1). 两组间白细胞、凝血酶原时间、INR、谷丙转氨酶、总胆红素、γ-谷氨酰转肽酶、肌酐、血尿素氮、血小板及五种评分有统计学差异(P<0.01), 而年龄、性别、Na+无统计学差异(P>0.05)(表2).
| 项目 | A组 (n = 90) | B组 (n = 64) | t/U/χ2 | P值 |
| 年龄 (岁) | 49.74 ± 8.99 | 48.94 ± 8.97 | 0.542 | 0.580 |
| 性别 (男) | 88 (97.8) | 60 (96.8) | -2.183 | 0.670 |
| WBC (×109/L) | 8.3 (5.3,11.8) | 9.6 (6.2,15.2) | -2.165 | 0.000 |
| PLT (×109/L) | 92.5 (64.5, 151.3) | 59.0 (36.5, 87.2) | 3.946 | 0.000 |
| PT (s) | 21.7 (20.0, 24.5) | 29.8 (25.0, 37.1) | -7.079 | 0.000 |
| INR | 2.02 ± 0.42 | 3.32 ± 1.62 | -7.244 | 0.000 |
| Na+(mmol/L) | 131.8 (127.5, 136.0) | 131.3 (123.9, 134.7) | 1.955 | 0.067 |
| ALT (U/L) | 34.0 (22.0, 51.5) | 36.0 (25.0, 58.0) | -0.321 | 0.000 |
| GGT (U/L) | 150.5 (58.5, 301.0) | 63.5 (31.7, 126.5) | 3.543 | 0.000 |
| TBIL (mg/dL) | 11.0 (6.7, 18.3) | 12.8 (8.5, 19.8) | -1.316 | 0.000 |
| Cr (mg/dL) | 0.75 ± 0.30 | 1.76 ± 1.56 | -5.969 | 0.000 |
| CTP | 11.82 ± 1.19 | 12.45 ± 1.59 | -2.799 | 0.009 |
| MDF | 46.06 ± 16.43 | 92.62 ± 58.35 | -7.177 | 0.000 |
| MELD | 21.44 ± 5.94 | 30.94 ± 9.65 | -7.510 | 0.000 |
| ABIC | 7.78 ± 1.07 | 9.28 ± 2.29 | -5.704 | 0.000 |
| CLIF-SOFA | 6.64 ± 0.81 | 9.45 ± 2.04 | -11.794 | 0.000 |
| 28 d死亡率 n (%) | 17 (19) | 31 (50) | 13.768 | 0.002 |
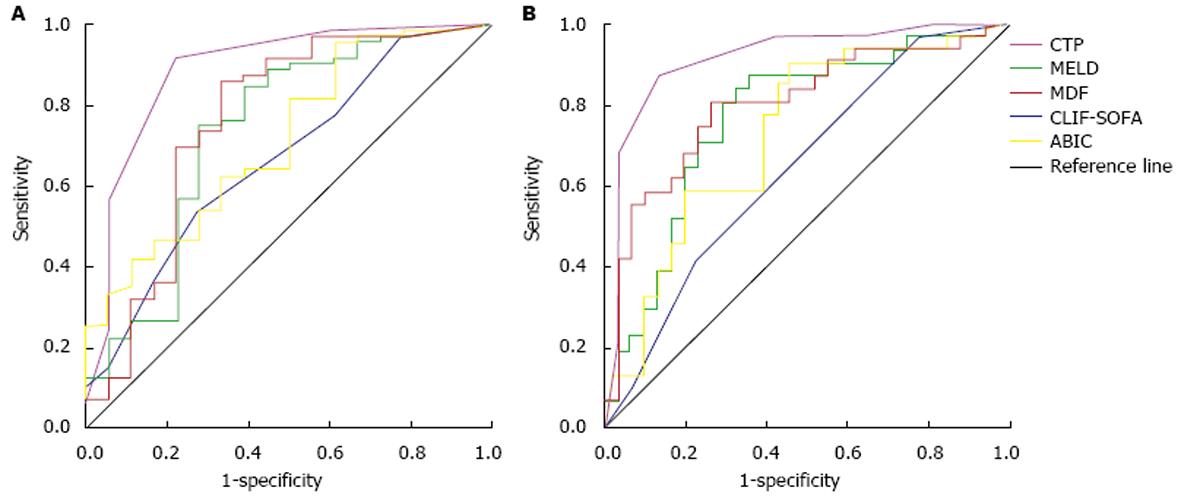
ROC曲线显示, 五种评分模型对A组和B组预后均有较好的预测价值(表3, 4, 图2). A组中评分模型依据曲线下面积AUC从大到小分别为CLIF-SOFA(0.889), MELD(0.761), MDF(0.738), ABIC(0.718), CTP(0.671); CTP评分AUC与CLIF-SOFA、MELD、MDF、ABIC评分AUC相比有统计学差异(Z = 2.768、0.806、1.035、0.466, P<0.05); CLIF-SOFA、MELD、MDF、ABIC四种评分模型两两相比无统计学差异. B组中五种评分模型依据曲线下面积AUC从大到小分别是CLIF-SOFA(0.916)、MELD(0.804)、MDF(0.770)、ABIC(0.729)、CTP(0.647); CLIF-SOFA评分AUC与ABIC、MDF、MELD、CTP评分AUC之间有统计学差异(Z = 2.468、1.801、2.180、3.453, P<0.05); CTP评分AUC与ABIC、MDF、MELD评分AUC相比有统计学差异(Z = 0.714、1.742、1.414, P<0.05); MELD、MDF、ABIC三种评分模型两两相比无统计学差异.
随着酗酒成为全球性问题, 酒精相关的ACLF近年来呈上升趋势[4]. 酒精相关ACLF是在慢性肝病或肝硬化基础上, 肝内外损伤因素急性打击导致的急性肝功能失代偿, 伴多器官衰竭及短期高死亡率的临床综合征. 目前肝移植是其最有效的治疗手段, 由于肝源缺乏、移植排斥反应、医疗费用昂贵等, 限制了其在临床上广泛应用[10,11]. 因此准确评估患者病情及其预后, 有可能逆转病情或者阻止其向更高级别ACLF发展.
在不同地区, ACLF患者的肝脏基础疾病、诱发因素和临床特征有很大差异, 目前国内外出现了多达十三种关于ACLF的定义[12], 比较认可的ACLF诊断标准有东方的APASL标准和西方的EASL-CLIF标准, 而符合不同标准的患者, 其预后不同. 本研究发现, 与A组相比, B组死亡率更高, 一方面: 这可能与在B组诊断标准中, 胆红素、凝血功能等各项指标的界定水平较A组高有关, 另一方面在患者发展为ACLF的过程中, 伴随大量的肝细胞破坏, 对于A组患者, 其处于疾病早期, 肝脏损伤程度轻, 如果及时采取措施, 可极大逆转病情, 改善预后[13-15]. 但对于B组患者, 疾病进展迅速, 肝脏损害严重甚至会发生全身多器官衰竭[16,17], 这可能与其死亡率较A组高有关.
本研究中, B组五种评分模型分值均高于A组, 提示病情较A组重, 可能由于B组处于ACLF晚期阶段, 出现了免疫麻痹. 酒精肝硬化基础上发生ACLF的初始阶段会出现促炎反应, 但随后会发生长时间免疫麻痹, 使得患者易于继发感染, 继而导致脓毒症及多器官衰竭, 预后较差.
五种评分系统在判断患者28 d预后准确性方面, ROC曲线下面积AUC越大, 预估准确性越高. 长期大量饮酒会导致酒精性脂肪肝, 酒精性肝炎, 酒精性肝硬化甚至发生酒精性肝癌. 重症酒精型肝炎是酒精性肝炎的严重类型, 常伴随SIRS, 而SIRS的存在可诱发患者多器官衰竭, 6 mo死亡率可达40%以上[18]. 本研究结果显示, CLIF-SOFA评分AUC在两组中均最高, 分析原因: 慢性肝病患者在急性损伤打击下, 机体会产生一系列炎症反应, 发生全身炎症反应综合征, 进而导致多器官衰竭, 这与其预后密切相关[19], 而CLIF-SOFA评分模型是在CANONIC 研究结果的基础上, 对原有的序贯器官衰竭评估(sequential organ failure assessment, SOFA)量表进行改良, 提出了评估包含了肝脏、肾脏、凝血系统、循环系统、中枢神经系统、呼吸系统六个器官(系统)功能指标, 使其能准确地预测患者预后[20]. Kim等[21]也认为CLIF-SOFA可以更好的预测酒精相关的ACLF患者的短期预后. MDF是最早被提出用于酒精性肝病患者预后评估, MELD是由Malinchoc等提出, 已有多篇文献证实其对肝衰竭预后有良好的预测价值[22-24]. 本研究中MDF和MELD在两组中均有一定的预估价值, MELD评分AUC大于MDF评分AUC, 差异无统计学意义, Dunu等[25]也认为两者预测能力相当. MDF评分系统中, 反映凝血系统功能指标采用的是PT, 其缺乏标准化, 在不同实验室之间测量结果差异很大, 而MELD评分系统中不仅使用INR代替PT值, 还包含了血清肌酐, 而血清肌酐水平与酒精性肝病的生存有密切关系[26,27], 这使MELD的临床使用价值高于MDF. ABIC评分系统在MELD的基础上增加了年龄因素, 但ABIC评分AUC低于MELD评分AUC, 这可能与年龄在两组之间无统计学差异有关. Papastergiou等[28]结果显示ABIC和MELD评分AUC(0.71 vs 0.79), 无统计学差异, 与本研究结果一致. CTP评分最早由Child和Turcotte提出, 经Pugh等修改形成的预后评估模型, 是目前判断肝病预后常用的评估系统[9]. 本文中CTP在两组中预测价值不如MELD、ABIC、MDF评分系统, 与既往研究结果一致[29], 这可能与其包含了腹水, 肝性脑病等主观性较强的指标和反应病情严重程度范围较窄有关(只包含了有反映肝功能和中枢神经系统功能的指标).
总之, 五种评分系统对A、B两组短期预后均有一定的预测价值, 相比之下, 在两组中, CLIF-SOFA预测能力均优于其他评分系统, 尤其是对B组患者预后的预测. 由于本实验纳入病例数较少, 有待进一步扩大样本量, 对不同评分模型评估酒精相关ACLF患者短期预后的预测价值加以证实.
目前国内外存在多种慢加急性肝衰竭(acute-on-chronic liver failure, ACLF)诊断标准, 酒精因素引起的ACLF逐年上升, 其具有病情进展迅速, 短期高死亡率等特点, 而符合不同诊断标准的患者其预后不同.
随着对ACLF认识的不断加深, 东西方出现了不同的诊断标准, 对于符合不同标准的酒精相关ACLF患者预后的研究需要进一步加深.
通过比较符合不同诊断标准酒精相关的ACLF短期预后, 加深对东西方不同标准的认识, 实现对患者早诊断, 早治疗, 最终改善其预后.
本文将酒精相关的ACLF患者根据东方的亚太肝脏研究学会(Asian Pacific Association for the Study of the Liver, APASL)标准和西方的EASL-CLIF标准进行分组分析, 采用受试者工作特征曲线下面积, 分析比较不同评分模型对符合不同标准患者预后的预测价值.
本研究结果显示对符合不同标准的患者, CLIF-SOFA的曲线下面积均最高, 可进一步研究和扩大CLIF-SOFA在临床上的应用. 通过对不同标准的比较, 从而提高临床医生对东西方标准差异的认识和理解, 推动国内外ACLF相关协作研究, 以望早日达成诊治共识, 最终降低患者死亡率.
通过本研究发现五种评分模型对两组均有一定的预测价值, 相比较而言, CLIF-SOFA的预测能力更强, 不管是符合APASL标准还是符合EASL-CLLF标准的患者. 因此在未来临床实践中, 应用CLIF-SOFA评分模型结合患者的临床表现等评估患者预后, 能够有效指导临床医生进一步采取适当的措施.
本课题组曾动态观察ACLF患者住院期间CTP评分, MELD评分, 以及CLIF-SOFA评分变化情况, 研究显示其均能够有效反映病情的进展及预后情况. 因此我们下一步将采用动态评分模型评估酒精相关的ACLF患者预后情况.
学科分类: 胃肠病学和肝病学
手稿来源地: 天津市
同行评议报告分类
A级 (优秀): 0
B级 (非常好): 0
C级 (良好): C, C
D级 (一般): 0
E级 (差): 0
编辑: 马亚娟 电编:闫晋利
| 1. | Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, Arroyo V, Kamath PS. Acute-on chronic liver failure. J Hepatol. 2012;57:1336-1348. [PubMed] [DOI] |
| 2. | Hernaez R, Solà E, Moreau R, Ginès P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541-553. [PubMed] [DOI] |
| 3. | Mehta G, Mookerjee RP, Sharma V, Jalan R. Systemic inflammation is associated with increased intrahepatic resistance and mortality in alcohol-related acute-on-chronic liver failure. Liver Int. 2015;35:724-734. [PubMed] [DOI] |
| 4. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1-1437.e9. [PubMed] [DOI] |
| 5. | Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC, Chawla YK, Dokmeci AK, Garg H, Ghazinyan H. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453-471. [PubMed] [DOI] |
| 6. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [PubMed] [DOI] |
| 7. | Dominguez M, Rincón D, Abraldes JG, Miquel R, Colmenero J, Bellot P, García-Pagán JC, Fernández R, Moreno M, Bañares R. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747-2756. [PubMed] [DOI] |
| 8. | Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193-199. [PubMed] |
| 9. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] |
| 10. | Shakil AO, Dvorchik I, Fung JJ, Rakela J. Liver transplantation for acute liver failure: outcome analysis. J Viral Hepat. 1997;4 Suppl 1:107-110. [PubMed] |
| 11. | Olson JC, Kamath PS. Acute-on-chronic liver failure: concept, natural history, and prognosis. Curr Opin Crit Care. 2011;17:165-169. [PubMed] [DOI] |
| 12. | Wlodzimirow KA, Eslami S, Abu-Hanna A, Nieuwoudt M, Chamuleau RA. A systematic review on prognostic indicators of acute on chronic liver failure and their predictive value for mortality. Liver Int. 2013;33:40-52. [PubMed] [DOI] |
| 14. | Cai J, Han T, Zhou J, Nie C, Li Y, Han L, Zhang Y. Comparison of different criteria to evaluate acute kidney injury and determine short-term prognosis of patients with acute-on-chronic liver failure. Zhonghua Ganzangbing Zazhi. 2015;23:684-687. [PubMed] |
| 15. | Laleman W, Verbeke L, Meersseman P, Wauters J, van Pelt J, Cassiman D, Wilmer A, Verslype C, Nevens F. Acute-on-chronic liver failure: current concepts on definition, pathogenesis, clinical manifestations and potential therapeutic interventions. Expert Rev Gastroenterol Hepatol. 2011;5:523-537; quiz 537. [PubMed] [DOI] |
| 16. | Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42 Suppl:S100-S107. [PubMed] [DOI] |
| 17. | Lee YH, Hsu CY, Hsia CY, Huang YH, Su CW, Lin HC, Loong CC, Chiou YY, Huo TI. Defining the severity of liver dysfunction in patients with hepatocellular carcinoma by the model for end-stage liver disease-derived systems. Dig Liver Dis. 2012;44:868-874. [PubMed] [DOI] |
| 19. | Mitchell MC, Friedman LS, McClain CJ. Medical Management of Severe Alcoholic Hepatitis: Expert Review from the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol. 2017;15:5-12. [PubMed] [DOI] |
| 20. | Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, Levesque E, Durand F, Angeli P, Caraceni P. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038-1047. [PubMed] [DOI] |
| 21. | Kim HY, Kim CW, Kim TY, Song DS, Sinn DH, Yoon EL, Jung YK, Suk KT, Lee SS, Lee CH. Assessment of scoring systems for acute-on-chronic liver failure at predicting short-term mortality in patients with alcoholic hepatitis. World J Gastroenterol. 2016;22:9205-9213. [PubMed] [DOI] |
| 22. | Yu JW, Sun LJ, Zhao YH, Li SC. Prediction value of model for end-stage liver disease scoring system on prognosis in patients with acute-on-chronic hepatitis B liver failure after plasma exchange and lamivudine treatment. J Gastroenterol Hepatol. 2008;23:1242-1249. [PubMed] [DOI] |
| 23. | Yu JW, Wang GQ, Li SC. Prediction of the prognosis in patients with acute-on-chronic hepatitis using the MELD scoring system. J Gastroenterol Hepatol. 2006;21:1519-1524. [PubMed] [DOI] |
| 24. | Chen W, You J, Chen J, Zheng Q, Jiang JJ, Zhu YY. Modified model for end-stage liver disease improves short-term prognosis of hepatitis B virus-related acute-on-chronic liver failure. World J Gastroenterol. 2017;23:7303-7309. [PubMed] [DOI] |
| 25. | Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KV, Malinchoc M, Kamath PS, Shah V. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353-358. [PubMed] [DOI] |
| 26. | Ramond MJ, Poynard T, Rueff B, Mathurin P, Théodore C, Chaput JC, Benhamou JP. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med. 1992;326:507-512. [PubMed] [DOI] |
| 27. | Mathurin P, Mendenhall CL, Carithers RL Jr, Ramond MJ, Maddrey WC, Garstide P, Rueff B, Naveau S, Chaput JC, Poynard T. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol. 2002;36:480-487. [PubMed] |
| 28. | Papastergiou V, Tsochatzis EA, Pieri G, Thalassinos E, Dhar A, Bruno S, Karatapanis S, Luong TV, O'Beirne J, Patch D. Nine scoring models for short-term mortality in alcoholic hepatitis: cross-validation in a biopsy-proven cohort. Aliment Pharmacol Ther. 2014;39:721-732. [PubMed] [DOI] |