修回日期: 2018-06-14
接受日期: 2018-07-08
在线出版日期: 2018-08-18
转移是影响肿瘤患者治疗效果与预后的最大障碍, 揭示肿瘤转移机制对肿瘤诊疗水平提高具有重大意义. 以往转移机制的研究多集中在原发瘤中癌细胞与间质细胞相互作用, 而近年研究显示, 原发瘤癌细胞也可释放一些活性物质随血循环到达靶器官, 招募骨髓衍生细胞并与靶器官组织固有细胞相互作用, 对靶器官组织微环境进行改造重塑, 加速预转移龛形成, 以利于转移在靶器官中的实现. 其中, 癌细胞来源的外泌体在介导原发瘤及远端靶器官预转移龛形成中发挥重要"桥梁"作用, 本文就外泌体与肿瘤预转移龛形成的最新进展进行总结阐述.
核心提要: 肿瘤细胞来源外泌体在介导原发瘤及远端靶器官预转移龛形成中发挥重要"桥梁"作用, 本文就外泌体与肿瘤预转移龛形成的最新进展进行总结阐述.
引文著录: 邢晓侠, 吴思凡, 崔杰峰. 外泌体与肿瘤预转移龛形成的新进展. 世界华人消化杂志 2018; 26(23): 1390-1395
Revised: June 14, 2018
Accepted: July 8, 2018
Published online: August 18, 2018
Metastasis is the biggest obstacle to improving the treatment outcome and prognosis of tumor patients. A better understanding of tumor metastasis mechanism is of great significance to improve cancer diagnosis and treatment levels. Previous studies on metastasis mechanism mainly focus on the interaction between cancer cells and stroma cells in primary tumors. Currently, some studies reveal that soluble factors derived from primary tumor cells reach target organs via systemic circulation and recruit bone marrow-derived cells (BMDCs). The recruited BMDCs interact with intrinsic cells to remodel the matrix microenvironment, ultimately facilitating the formation of pre-metastatic niche and the implementation of tumor metastasis in the target organ. Among them, cancer cell-secreted exosomes serve as an important bridge mediator to link primary tumor and pre-metastatic niche at distant target organ. This article reviews the latest discoveries on exosomes and their effect on pre-metastatic niche of tumor.
- Citation: Xing XX, Wu SF, Cui JF. Role of tumor-derived exosomes in facilitating pre-metastatic niche formation. Shijie Huaren Xiaohua Zazhi 2018; 26(23): 1390-1395
- URL: https://www.wjgnet.com/1009-3079/full/v26/i23/1390.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v26.i23.1390
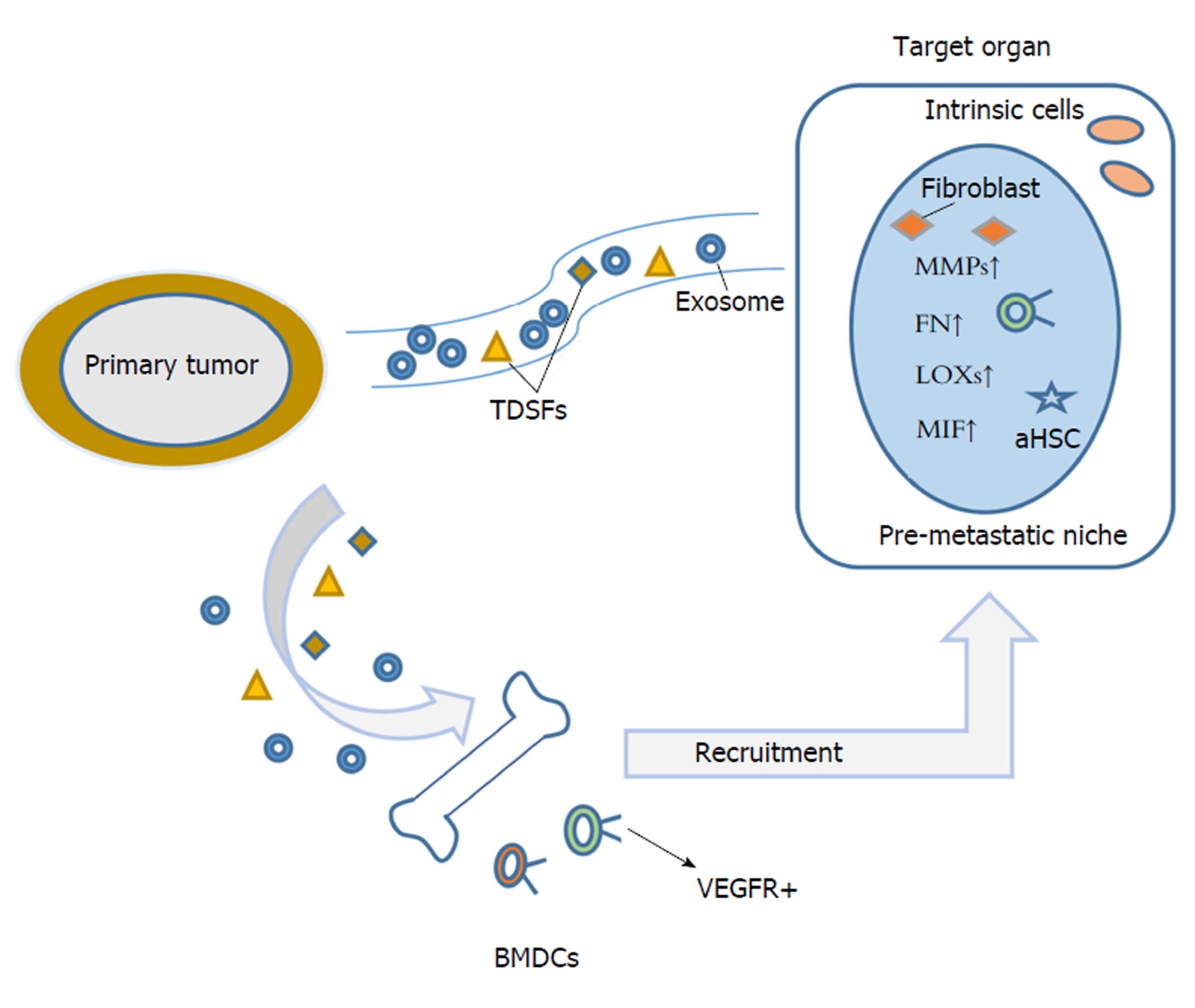
在认识肿瘤转移的历史进程中, 出现过三个具有里程碑意义的经典学说, 包括Paget的"种子土壤学说"[1], Ewing的"解剖机制学说"[2], 以及Bross和Blumenson[3]的"转移瀑布学说". 上述三个学说分别从转移非随机性, 转移途径, 转移的多因素、多阶段三个不同角度对实体肿瘤转移进行解释, 由于三种转移理论缺乏将原发瘤和远端靶器官转移灶直接联系起来的"桥梁", 均未能较好地回答原发瘤是如何参与、影响远端转移靶器官选择以及远端转移灶形成. Kaplan等人[4]的"预转移龛"学说的提出, 较好地汇集融合了上述三种转移理论, 在原发瘤与远端转移灶之间架起了一座联系的"桥梁", 也为肿瘤的远端组织转移倾向研究提供了一种崭新的思路. 近年来, 外泌体在肿瘤转移中的作用越来越凸显, 外泌体介导的肿瘤细胞、基质细胞间"交叉对话"促进肿瘤侵袭转移报道不断增多[5-8]. 另一方面, 来源于原发瘤的外泌体可随体循环到达靶器官, 招募骨髓衍生细胞与靶器官组织固有细胞相互作用, 重塑改造远端靶器官组织微环境, 加速预转移龛形成, 显示外泌体在连接原发瘤及远端靶器官预转移龛形成中所起的重要"桥梁"作用[9].
转移是影响肿瘤患者治疗效果与预后的重要障碍. 恶性肿瘤细胞从原发瘤转移扩散至远处靶器官是一个涉及多基因、多因素、多步骤复杂分子病理事件. 一般将转移过程分为癌细胞从原发瘤脱落、局部侵犯、渗入血管、在循环中逃避免疫攻击、移出血管、靶器官增殖及转移灶形成等几个主要步骤[10,11]. 前期肿瘤转移研究多聚焦原发瘤及转移早、中期阶段的分子病理改变, 对转移后期靶器官选择、转移灶形成等分子事件的关注却明显不足. 近来研究提示, 癌细胞从原发部位脱落播散对肿瘤转移的实现, 条件并不充分, 而在远端靶器官组织形成适宜游离癌细胞定植的土壤微环境(龛), 对转移最终的实现将十分必要. 预转移龛概念提出凸显了肿瘤细胞定植转移靶器官前, 靶器官组织在原发瘤来源可溶因子、外泌体诱导作用下, 发生系列细胞、分子事件, 包括骨髓衍生细胞(bone marrow-derived cells, BMDCs)募集, 募集的BMDCs与固有细胞互作, 基质土壤重塑, 免疫抑制微环境等[12-14], 上述系列改变对肿瘤转移后期靶器官选择、肿瘤细胞定植及转移灶形成均具有的重要意义, 同时也是肿瘤转移在靶器官实现的重要节点和限速步骤. 组织转移倾向是实体瘤转移的显著特征, 但鉴于靶向转移同时关联到原发瘤与转移灶, 在体内外对其模拟的转移模型的构建及检测均要求较高, 也致使其确切分子机制依然大多未明. 肿瘤组织转移倾向研究先前多局限于肿瘤细胞自身基因、趋化因子受体、黏附分子等表达[15-17], 此外, 浸润肿瘤细胞在靶器官分泌胞外糖蛋白如tenascin C[18], 靶组织基质蛋白periostin高表达[19], 也影响肿瘤细胞靶向转移及其在靶器官的定植生存. 肿瘤预转移龛聚焦肿瘤细胞定植靶器官前的细胞分子事件, 依托预转移龛理论, 越来越多的肿瘤来源的可溶性因子(如LOX, LOXL2等), 以及外泌体内含物(ITGαυβ5, ITGα6β4, ITGα6β1等)被相继发现在靶向特异脏器的重要作用[20,21], 为肿瘤靶向转移的解析提供了全新的角度.
正常细胞和病变细胞均可分泌外泌体[22,23], 肿瘤细胞一般比正常细胞分泌更多的外泌体[24]. 外泌体富含其来源细胞所含的生物活性分子, 包括四次跨膜蛋白(CD9, CD63和CD81), 热休克蛋白(Hsp60的, HSP70和HSP90)以及脂质(鞘磷脂、胆固醇、神经酰胺), 来自抗原呈递细胞的MHC-II, MVB(multivesicular bodies)合成相关蛋白(Alix, TSG101)等[25-28]. 此外, 外泌体也含小RNA, 包括vaultRNA, mRNA, tRNA和miRNA[29,30]. 外泌体内含物因生理、病理及来源细胞类型的不同而不同. 加之细胞外泌体分泌时, 其内含物组分存在分选, 外泌体内含物组成也不同于其来源细胞[31], 上述差异的机制可能与运输所需内吞体分选转运复合体(endosomal-sorting complexes required for transport , ESCRT)有关[32]. 另外泌体中miRNA分选也可能受hnRNP, 尤其是hnRNPA2B1调节[33]. 肿瘤细胞从转移起始到靶器官定植、转移灶形成整个转移过程中, 均存在外泌体的参与[34]. 但多数研究集中在原发瘤肿瘤细胞与周边基质细胞外泌体摄取、互作, 进而调控肿瘤侵袭转移. Fang等[35]报道, 肝癌细胞来源外泌体miR-1247-3p直接靶向作用于β-1, 4-半乳糖基转移酶Ⅲ, 导致成纤维细胞中β1-整合素-NF-κB信号通路的活化, 使成纤维细胞转化为癌症相关成纤维细胞(CAFs), 活化的CAFs通过促炎细胞因子分泌如IL-6和IL-8进一步促进癌症进展. Cooks等[36]报道, TP53基因突变的结肠癌细胞释放富含miR-1246的外泌体, 外泌体被周边巨噬细胞摄入后, 对巨噬细胞进行重编程. 将M2巨噬细胞与TP53基因突变的HCT116细胞进行共培养, 再将共培养所得M2巨噬细胞与新鲜肿瘤细胞混合后皮下种植到NOD-SCID小鼠体内, 发现与对照组相比, 重编后的巨噬细胞可促进肿瘤进展及转移, 进一步研究显示, 重编后的巨噬细胞IL-10、MMPs、TGF-β分泌量增多, 从而促进肿瘤的进展. Cassandra等[37]发现, 用含有重组人转化生长因子-β(rhTGFβ)或膀胱癌细胞来源外泌体的培养基培养正常成纤维细胞, 可增加成纤维细胞中αSMA的表达量, 且经肿瘤来源的外泌体处理的成纤维细胞中FAP和Galectin蛋白的表达量也显著增加. CAFs有侵入性生长和转移的特性, 可促进肿瘤进展和转移, 而癌细胞来源的外泌体含有TGFβ, 可激活SMAD通路, 触发成纤维细胞分化成CAF. Sanchez等[38]报道, 将前列腺癌细胞来源外泌体miRNA转染到前列腺正常成纤维细胞后, 细胞的迁移能力增强, 其MMPs以及RANKL表达均上调, 而两者高表达与肿瘤侵袭转移有关. 由此不难发现, 肿瘤细胞通过外泌体与基质细胞相互作用, 改变肿瘤微环境, 利于肿瘤进展和转移.
充足血供, 血管通透性改变及新血管生成是肿瘤生长及转移常见病理改变[39], 外泌体可刺激血管生成并增加血管通透性, 使肿瘤细胞更易转移到远端靶器官. Fang等[40]将可稳定表达miR-103的肝癌细胞种植于雄性裸鼠肝左叶后, 发现肿瘤血管通透性、外泌体miR-103水平、血循环中肿瘤细胞数目及肝和肺转移率均明显增加. 进一步分析发现, 肝癌细胞来源外泌体miR-103进入内皮细胞, 可抑制血管内皮细胞钙黏蛋白、p120-连环蛋白和紧密连接蛋白(Zonula occludens-1, ZO-1)表达, 减弱内皮连接完整性并促进肿瘤转移. 转移性乳腺癌细胞的外泌体miR-105, 可下调ZO-1, 也可有效破坏内皮细胞间紧密连接、增加血管通透性, 促进癌细胞渗出并迁移至远处靶器官[41]. Yukawa等[42]利用血管成像技术观察HepG2外泌体对人脐静脉内皮细胞(human umbilical vein endothelial cells, HUVEC)管腔形成影响, 发现HepG2外泌体可表达HSP70及多种miRNAs, 参与HUVEC的管腔形成. Lee等[43]报道, EIF3C过表达的肝癌细胞条件培养液与HUVEC细胞混合, HUVEC更易形成管腔, 经透射电镜和纳米颗粒跟踪分析证实, 上述条件培养液中外泌体含量增加, 将富含EIF3C的肝癌细胞外泌体与Huh7共同种植于裸鼠皮下, 可促进肿瘤生长, 此外, 免疫组化显示, 增长的肝癌组织也会导致EIF3C及内皮标志物CD31表达增加. 进一步分析显示, EIF3C可活化S100A11, 参与血管生成.
除原发瘤分泌的可溶性因子包括LOX, LOXL2, VEGF, PIGF等可诱导远端靶器官预转移龛形成外[44,45], 原发瘤来源的外泌体也可通过多种方式影响预转移龛形成, 加速转移最终实现(图1). 肿瘤通常由许多基因型和表型不同亚克隆组成[46], 不同的亚克隆可通过外泌体将其恶性特征转移到靶器官中其他细胞. Yu等[47]报道, 低转移胰腺癌细胞Panc02摄取来自高转移胰腺癌细胞Panc02-H7外泌体, 其迁移侵袭能力明显增加, 此外高转移胰腺癌细胞外泌体可诱导小鼠肝预转移龛形成,在肝预转移龛发生部位检测到预转移龛的典型细胞分子改变包括CD11b+CD45+骨髓来源细胞, SMA+的活化肝星状细胞, fibronecin, S100A8, S100A9的上调表达. Hoshino等[21]发现, 来源于不同组织转移倾向肿瘤细胞外泌体呈现不同整合素表达谱, 且优先与被转移靶器官固有细胞融合吸收, 表达ITGαυβ5外泌体与Kupffer细胞结合, 介导肝转移, 而表达ITGα6β4和ITGα6β1外泌体结合肺成纤维细胞和上皮细胞, 控制肺转移. 吸收的特定整合素可显著上调靶器官固有细胞炎性因子S100基因表达并激活Src磷酸化, 启动预转移龛形成, 决定肿瘤细胞组织转移倾向. 胰腺导管腺癌(pancreatic ductal adenocarcinoma, PDCA)细胞来源的外泌体也可运载巨噬细胞迁移抑制因子(macrophage migration inhibitory factor, MIF)到达肝脏, 诱导肝脏Kupffer细胞产生TGF-β并上调肝星状细胞纤连蛋白(fibronectin, FN)表达量, FN的沉积可促进骨髓衍生巨噬细胞和嗜中性粒细胞募集到肝脏, 促进肝预转移龛形成[48]. 除肿瘤细胞外泌体中蛋白参与预转移龛诱导外, 外泌体所携带的miRNA和RNA也参与调控预转移龛形成. 将转移性大鼠胰腺癌细胞BSp73ASML来源的外泌体皮下注射到大鼠体内, 发现外泌体优先选择被大鼠淋巴结基质细胞(LnSTR)和肺成纤维细胞摄取, ASML来源外泌体miR-494和miR-542-3p靶向作用于钙黏蛋白-17(cdh17), 将这两种miRNA转染到LnSTR细胞或将上述外泌体与LnSTR细胞共培养, 可下调cdh17表达, 同时上调基质金属蛋白酶表达. 外泌体通过转移性miRNA影响预转移龛处的基质细胞, 适应肿瘤细胞转移需求, 进而为肿瘤细胞的定植创造有利条件[49]. Liu等[50]研究显示, 将肺癌细胞或黑色素瘤细胞皮下接种于Toll样受体3(toll-like receptor 3, TLR3)敲除的小鼠, 与野生型小鼠相比, 其肺转移率显著减少, 分析显示肿瘤来源外泌体RNAs激活肺泡上皮TLR3, 从而诱导肺中趋化因子分泌并募集嗜中性粒细胞, 促进肺转预转移龛形成. 此外, Plebanek等[51]研究发现, 与未处理对照组比, 经转移性黑色素瘤来源外泌体预处理的裸鼠肺转移率显著增加. 而相同条件下, 经尾静脉分别注射来自转移性及非转移性黑色素瘤外泌体(ExoM及ExoNM), 非转移组肺转移率明显降低, 造成上述差异的原因可能与ExoNM引起Ly6Clow亚群显着增加有关, 该细胞亚群存在巡逻单核细胞(patrolling monocytes, PMo), 有抗转移功能[52]. 肿瘤细胞在转移生长期间对能量需求很大, 能量代谢对肿瘤细胞的生长和快速增殖十分重要. Fong等[53]发现, 乳腺癌细胞在转移前阶段即可影响远端器官脑和肺对葡萄糖的摄取,乳腺癌细胞外泌体将miR-122运输到远端靶器官, 影响预转移龛处细胞的代谢. 将外泌体静脉注射到NSG小鼠体内后, 小鼠肺成纤维细胞及脑星形胶质细胞可摄取上述外泌体, 降低丙酮酸激酶和GLUT1表达. 进一步分析发现乳腺癌细胞外泌体miR-122, 可下调丙酮酸激酶, 并抑制预转移龛处正常细胞对葡萄糖的摄取, 影响细胞能量代谢. 肿瘤细胞通常对能量需求较大, 而外泌体miR-122通过对靶器官内正常细胞能量代谢重编程, 抑制正常细胞营养利用, 从而为即将转移到靶器官的肿瘤细胞创造有利条件. 因此, 原发瘤通过外泌体与靶细胞进行信息交流, 赋予远端靶器官中靶细胞一些转移恶性特征, 加速预转移龛形成, 为肿瘤转移实现奠定了"土壤"基础.
尽管外泌体介导肿瘤预转移龛形成的研究取得一些进展, 其参与肿瘤预转移龛调控机制研究依然存在不少挑战. 如肿瘤细胞分泌外泌体内含物种类中如蛋白、mRNA、miRNA等, 何种成分在诱导靶器官预转移龛形成中权重最大?其是否与TDSFs存在联合诱导加速预转移龛形成; 外泌体内含物组成与其来源肿瘤细胞存在差异, 外泌体内含物分选机制对预转移龛形成是否至关重要; 界定预转移龛形成的细胞特征、分子特征尚需标准化, 体内示踪预转移龛形成的影像标准尚需统一; 现有的一些外泌体分离方法, 所获外泌体纯度差异大, 含一些非外泌体物质干扰, 对实验结果可能产生影响[54]. 此外, 预转移龛在肿瘤患者体内尚未被证实并形成检测分析的方法, 其临床应用受到限制. 相信随着技术手段的进步和新型动物模型相继建立, 必将有力地推动原发瘤来源外泌体介导预转移龛形成作用机制的阐明.
学科分类: 胃肠病学和肝病学
手稿来源地: 上海市
同行评议报告分类
A级 (优秀): 0
B级 (非常好): B, B
C级 (良好): 0
D级 (一般): 0
E级 (差): 0
编辑: 崔丽君 电编:张砚梁
| 1. | Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989;8:98-101. [PubMed] |
| 2. | Stevens AR, Ewing J. Adenocarcinoma of the testis in the adult. Ann Surg. 1928;88:1074-1078. [PubMed] [DOI] |
| 3. | Bross IDJ, Blumenson LE. Metastatic sites that produce generalized cancer: identification and kinetics of generalizing sites. Fundamental aspects of metastasis. Amsterdam: North-Holland Publishing Company 1976; 359-375. |
| 4. | Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820-827. [PubMed] [DOI] |
| 5. | Chen L, Guo P, He Y, Chen Z, Chen L, Luo Y, Qi L, Liu Y, Wu Q, Cui Y. HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death Dis. 2018;9:513. [PubMed] [DOI] |
| 6. | Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 2018;. [PubMed] [DOI] |
| 7. | Chen Y, Zeng C, Zhan Y, Wang H, Jiang X, Li W. Aberrant low expression of p85α in stromal fibroblasts promotes breast cancer cell metastasis through exosome-mediated paracrine Wnt10b. Oncogene. 2017;36:4692-4705. [PubMed] [DOI] |
| 8. | Melzer C, von der Ohe J, Hass R. Concise Review: Crosstalk of Mesenchymal Stroma/Stem-Like Cells with Cancer Cells Provides Therapeutic Potential. Stem Cells. 2018;36:951-968. [PubMed] [DOI] |
| 9. | Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679-695. [PubMed] [DOI] |
| 10. | Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274-284. [PubMed] [DOI] |
| 11. | Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275-292. [PubMed] [DOI] |
| 13. | Steeg PS. Targeting metastasis. Nat Rev Cancer. 2016;16:201-218. [PubMed] [DOI] |
| 14. | Aguado BA, Bushnell GG, Rao SS, Jeruss JS, Shea LD. Engineering the pre-metastatic niche. Nat Biomed Eng. 2017;1. [PubMed] [DOI] |
| 15. | Lawler K, Papouli E, Naceur-Lombardelli C, Mera A, Ougham K, Tutt A, Kimbung S, Hedenfalk I, Zhan J, Zhang H. Gene expression modules in primary breast cancers as risk factors for organotropic patterns of first metastatic spread: a case control study. Breast Cancer Res. 2017;19:113. [PubMed] [DOI] |
| 16. | Mauri FA, Pinato DJ, Trivedi P, Sharma R, Shiner RJ. Isogeneic comparison of primary and metastatic lung cancer identifies CX3CR1 as a molecular determinant of site-specific metastatic diffusion. Oncol Rep. 2012;28:647-653. [PubMed] [DOI] |
| 17. | Satcher RL, Pan T, Cheng CJ, Lee YC, Lin SC, Yu G, Li X, Hoang AG, Tamboli P, Jonasch E. Cadherin-11 in renal cell carcinoma bone metastasis. PLoS One. 2014;9:e89880. [PubMed] [DOI] |
| 18. | Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, Massagué J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;17:867-874. [PubMed] [DOI] |
| 19. | Fukuda K, Sugihara E, Ohta S, Izuhara K, Funakoshi T, Amagai M, Saya H. Periostin is a key niche component for wound metastasis of melanoma. PLos One. 2015;10:e0129704. [PubMed] [DOI] |
| 20. | Cox TR, Rumney RMH, Schoof EM, Perryman L, Høye AM, Agrawal A, Bird D, Latif NA, Forrest H, Evans HR. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522:106-110. [PubMed] [DOI] |
| 21. | Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329-335. [PubMed] [DOI] |
| 22. | Munagala R, Aqil F, Gupta RC. Exosomal miRNAs as biomarkers of recurrent lung cancer. Tumour Biol. 2016;37:10703-10714. [PubMed] [DOI] |
| 23. | Pant S, Hilton H, Burczynski ME. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol. 2012;83:1484-1494. [PubMed] [DOI] |
| 24. | Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126:1208-1215. [PubMed] [DOI] |
| 25. | Michael A, Bajracharya SD, Yuen PS, Zhou H, Star RA, Illei GG, Alevizos I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16:34-38. [PubMed] [DOI] |
| 26. | Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63-70. [PubMed] |
| 27. | Yoshioka Y, Konishi Y, Kosaka N, Katsuda T, Kato T, Ochiya T. Comparative marker analysis of extracellular vesicles in different human cancer types. J Extracell Vesicles. 2013;2. [PubMed] [DOI] |
| 28. | Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569-579. [PubMed] [DOI] |
| 29. | Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654-659. [PubMed] [DOI] |
| 30. | Nolte-'t Hoen EN, Buermans HP, Waasdorp M, Stoorvogel W, Wauben MH, 't Hoen PA. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40:9272-9285. [PubMed] [DOI] |
| 31. | Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W. Exosomes in cancer: small particle, big player. J Hematol Oncol. 2015;8:83. [PubMed] [DOI] |
| 32. | Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Théry C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553-5565. [PubMed] [DOI] |
| 33. | Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M, Sánchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. [PubMed] [DOI] |
| 34. | Steinbichler TB, Dudás J, Riechelmann H, Skvortsova II. The role of exosomes in cancer metastasis. Semin Cancer Biol. 2017;44:170-181. [PubMed] [DOI] |
| 35. | Fang T, Lv H, Lv G, Li T, Wang C, Han Q, Yu L, Su B, Guo L, Huang S. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun. 2018;9:191. [PubMed] [DOI] |
| 36. | Cooks T, Pateras IS, Jenkins LM, Patel KM, Robles AI, Morris J, Forshew T, Appella E, Gorgoulis VG, Harris CC. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat Commun. 2018;9:771. [PubMed] [DOI] |
| 37. | Ringuette Goulet C, Bernard G, Tremblay S, Chabaud S, Bolduc S, Pouliot F. Exosomes Induce Fibroblast Differentiation into Cancer-Associated Fibroblasts through TGFβ Signaling. Mol Cancer Res. 2018;. [PubMed] [DOI] |
| 38. | Sánchez CA, Andahur EI, Valenzuela R, Castellón EA, Fullá JA, Ramos CG, Triviño JC. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget. 2016;7:3993-4008. [PubMed] [DOI] |
| 39. | Hughes CC. Endothelial-stromal interactions in angiogenesis. Curr Opin Hematol. 2008;15:204-209. [PubMed] [DOI] |
| 40. | Fang JH, Zhang ZJ, Shang LR, Luo YW, Lin Y, Yuan Y, Zhuang SM. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology. 2018;. [PubMed] [DOI] |
| 41. | Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O'Connor ST, Chin AR. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501-515. [PubMed] [DOI] |
| 42. | Yukawa H, Suzuki K, Aoki K, Arimoto T, Yasui T, Kaji N, Ishikawa T, Ochiya T, Baba Y. Imaging of angiogenesis of human umbilical vein endothelial cells by uptake of exosomes secreted from hepatocellular carcinoma cells. Sci Rep. 2018;8:6765. [PubMed] [DOI] |
| 43. | Lee HY, Chen CK, Ho CM, Lee SS, Chang CY, Chen KJ, Jou YS. EIF3C-enhanced exosome secretion promotes angiogenesis and tumorigenesis of human hepatocellular carcinoma. Oncotarget. 2018;9:13193-13205. [PubMed] [DOI] |
| 44. | Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35-44. [PubMed] [DOI] |
| 45. | Wu S, Zheng Q, Xing X, Dong Y, Wang Y, You Y, Chen R, Hu C, Chen J, Gao D. Matrix stiffness-upregulated LOXL2 promotes fibronectin production, MMP9 and CXCL12 expression and BMDCs recruitment to assist pre-metastatic niche formation. J Exp Clin Cancer Res. 2018;37:99. [PubMed] [DOI] |
| 46. | Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328-337. [PubMed] [DOI] |
| 47. | Yu Z, Zhao S, Ren L, Wang L, Chen Z, Hoffman RM, Zhou J. Pancreatic cancer-derived exosomes promote tumor metastasis and liver pre-metastatic niche formation. Oncotarget. 2017;8:63461-63483. [PubMed] [DOI] |
| 48. | Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816-826. [PubMed] [DOI] |
| 49. | Rana S, Malinowska K, Zöller M. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia. 2013;15:281-295. [PubMed] |
| 50. | Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z, Zhang X, Huang B, Xu X, Zheng J, Cao X. Tumor Exosomal RNAs Promote Lung Pre-metastatic Niche Formation by Activating Alveolar Epithelial TLR3 to Recruit Neutrophils. Cancer Cell. 2016;30:243-256. [PubMed] [DOI] |
| 51. | Plebanek MP, Angeloni NL, Vinokour E, Li J, Henkin A, Martinez-Marin D, Filleur S, Bhowmick R, Henkin J, Miller SD. Pre-metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche. Nat Commun. 2017;8:1319. [PubMed] [DOI] |
| 52. | Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, Herrley E, Rasquinha N, McArdle S, Wu R. Patrolling monocytes control tumor metastasis to the lung. Science. 2015;350:985-990. [PubMed] [DOI] |
| 53. | Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, Chow A, O'Connor ST, Li S, Chin AR. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17:183-194. [PubMed] [DOI] |
| 54. | Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A, Möller A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031. [PubMed] [DOI] |