修回日期: 2017-10-30
接受日期: 2017-11-04
在线出版日期: 2017-12-18
胃癌(gastric cancer, GC)是我国常见恶性肿瘤, 死亡率较高的原因之一是早期GC的检出率低. 胃镜新技术的应用使得早期GC的检出率增高, 早期GC中高分化腺癌占70%左右, 因此临床工作中遇到高分化腺癌的比例也随之增高. 高分化腺癌因其组织结构和细胞异型性较小, 诊断困难. 深在性囊性胃炎是一种罕见疾病, 表现为胃固有腺体位于黏膜肌层和/或黏膜下层, 易与高分化腺癌混淆. 在病理工作中应注意对两者进行鉴别.
核心提要: 胃高分化腺癌的组织结构和细胞异型性较小, 因此浸润性高分化腺癌易与深在性囊性胃炎混淆. 在病理组织学诊断中应注意从临床表现、大体形态、组织学形态、免疫组织化学、腺体个数以及浸润性等方面进行鉴别诊断.
引文著录: 夏靖媛, 纪小龙. 警惕深在性囊性胃炎可能是高分化腺癌. 世界华人消化杂志 2017; 25(35): 3089-3093
Revised: October 30, 2017
Accepted: November 4, 2017
Published online: December 18, 2017
Gastric cancer (GC) is one of the most common malignant tumors in China. The low detection rate of early GC is one of the reasons for its high mortality rate. Thanks to the application of new gastroscopy technology, the detection rate of early GC has increased. Highly differentiated adenocarcinoma accounts for about 70% of all early GC cases; however, well-differentiated adenocarcinoma is difficult to diagnose because of its non-significant structural abnormality and cellular atypia. Gastritis cystica profunda is a rare disease characterized by the presence of the gastric intrinsic gland in the muscularis mucosa and/or submucosa, which is easily confused with highly differentiated adenocarcinoma. Therefore, attention should be paid to the identification of these two different entities in the clinical work.
- Citation: Xia JY, Ji XL. Well-differentiated adenocarcinoma may be misdiagnosed as gastritis cystica profunda. Shijie Huaren Xiaohua Zazhi 2017; 25(35): 3089-3093
- URL: https://www.wjgnet.com/1009-3079/full/v25/i35/3089.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v25.i35.3089
胃癌(gastric cancer, GC)是全球最常见的消化系统肿瘤, 在我国其死亡率居恶性肿瘤第3位, 占全球GC死亡人数的1/4[1-3]. GC有多种分类方式, 在临床病理中, 多采用世界卫生组织(World Health Organization, WHO)分型, WHO分型(2010版)中根据GC腺体的组织结构和细胞异型性大小将管状腺癌分为高分化、中分化和低分化[4]. 胃中低分化腺癌的组织结构和细胞异型性较大, 容易区分诊断; 而高分化腺癌的形态特征为腺管结构明显可见基底膜, 腺管的形状及大小比较规则, 癌细胞异型性不大[5]. 因其组织结构和细胞异型性小, 临床工作中, 病理医生难将其与良性病变鉴别. 最近工作中, 遇到将胃高分化腺癌误诊为深在性囊性胃炎(gastritis cystica profunda, GCP)的案例, 因此本文对GCP和胃高分化腺癌的特征及鉴别要点的相关研究做一述评.
我国GC死亡率较高原因之一是早期胃癌(early gastric cancer, EGC)的检出率低, 只有10%左右, 而日韩国家可高达50%-70%[6]. 胃GC的预后与分期紧密相关, EGC的5年生存率可达90%, 而进展期GC的5年生存率只有10%左右[7-9], 因此如何提高EGC的检出率是国内外一直关注的热点问题. 在GC的众多检查方法中, 胃镜取活检做病理组织诊断准确率最高. 近些年胃镜技术飞速发展, 常用的窄带成像技术与放大内镜结合可观察到胃黏膜腺体表面小凹结构及黏膜浅表微血管状态, 并对病变良恶性进行判断[10-13], 超声内镜可检测胃黏膜深部结构[14,15]. 这些技术的发展使得EGC的检出率逐步提高[16], EGC是指癌组织仅局限于胃黏膜层或黏膜下层, 不论有无淋巴结转移.
根据病理组织学分型(WHO分型)[17]可以将GC分为: 乳头状腺癌、管状腺癌、黏液腺癌、印戒细胞癌、腺鳞癌、鳞状细胞癌、小细胞癌、未分化癌. GC分型中, 管状腺癌约占80%, 管状腺癌根据分化程度分为高、中、低分化, 在EGC中, 高分化腺癌可达70%以上[18]. 因此, 在临床工作中遇到的胃高分化腺癌病例也随之增多.
胃镜技术的发展使得更多的胃部疾病能够及时发现并治疗, 普通小息肉可以通过胃镜切除, 深部或严重病变可以采用内镜下黏膜切除术(endoscopic mucosal resection, EMR)、内镜下黏膜剥离术(endoscopic submucosal dissection, ESD)以及手术切除治疗[19-21]. 在临床工作中发现, ESD和手术切除的胃组织标本中, 有时可见到高分化腺癌突破黏膜肌层到达黏膜下层, 而高分化腺癌的腺体形态及大小相对规则, 细胞的异型性不大, 对此, 能否诊断为胃高分化腺癌浸润到黏膜下层, 病理医生判断困难. 尤其对于分化极好的腺癌, 其形态特征为腺腔结构较规则, 细胞异型性小, 胞浆丰富, 基底核明显可见[22-25], Ushiku等[22]分析21例术后病理诊断为伴有肠化生的分化极好的胃腺癌的形态特征, 在这21例中有18例的胃镜未被诊断为癌.
当这种高分化腺癌突破黏膜肌层到达黏膜下层时, 由于腺体结构和细胞的异型性相对较小, 仍不易做出癌的诊断, 容易误诊为良性病变, 如腺体异位、胃肠间质瘤[26]、GCP等. 近年来, 在胃组织标本的病理诊断中, 关于高分化腺癌病例中伴有GCP的文献[27-31]报道时有出现, 同时也有把早期胃癌诊断为GCP的文献[31,32]描述, 如何对两者进行鉴别区分是病理诊断需要注意的内容.
GCP组织学特征为胃黏膜腺体位于黏膜肌层和/或黏膜下层, 并伴有囊性扩张[33], 是一种非常罕见疾病, 其在GC中的发病率仅为1.5%[34]. GCP最早是在1972由Littler和Gleibermann[35]首次进行描述, 47岁男性患者, 因上腹部疼痛和黑便就诊, 18年前做过胃肠吻合术, 此次在吻合口处发现息肉状肿物进行切除, 组织学表现为胃固有腺体位于黏膜肌层和/或黏膜下层, 腺体囊性扩张, 伴有结缔组织增生及炎细胞. GCP曾命名为胃囊性息肉[35]、胃扩张性黏膜下囊肿[36]、黏膜下异位胃腺体[37]. GCP常在有胃部手术史的部位发现[38], Park等[39]首次报道了无胃部手术史的GCP, 近些年来关于其在无胃部手术史的案例报道逐渐增多[40-44]. 目前GCP病因不清, 多认为手术、缝线、炎症、黏膜缺血等均可刺激黏膜腺体导致GCP生成[45,46], 也有文献[47,48]报道EB病毒和幽门螺杆菌感染与GCP的发生密切相关. GCP临床表现无特异性, 与其他胃部疾病相似, 可表现为腹痛、腹胀、恶心、消化道出血、体质量减轻等[40], 男女发病比为3.6:1, 发病年龄中位数为60岁, 常见发生部位依次为胃体、胃底、胃窦、贲门部[34]. GCP在胃深部, 常用的普通白光内镜只能观察到胃黏膜浅表部位, 因此多采用计算机断层扫描(computed tomography, CT)和超声内镜检查辅助诊断, 其CT扫描显示为胃壁不规则隆起增厚或伴有腔内肿块[42]; 超声内镜下显示多个囊腔或黏膜下囊肿, 或是表现为胃壁大范围增厚及黏膜下层低回声区[49,50].
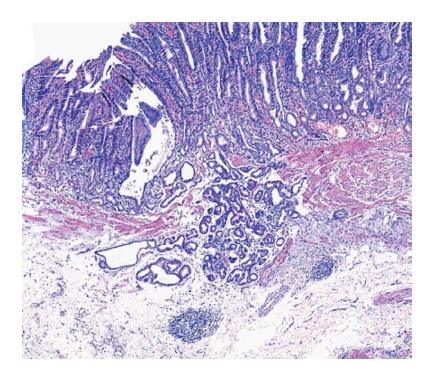
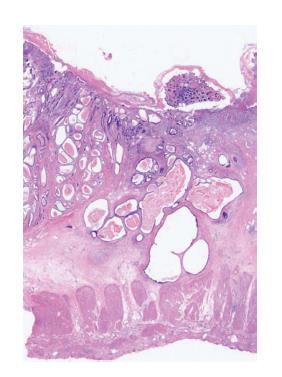
GCP的影像学检查无特异性表现, 主要诊断以组织学特征为主. GCP为胃固有腺体位于黏膜肌层和/或黏膜下层, 而常规胃镜活检只能取到黏膜层或黏膜肌层, 因此多采用EMR或ESD切除标本进行诊断. GCP中有时会伴有高分化腺癌的存在, 在病理诊断中可从以下方面进行鉴别区分: (1)临床表现: 与胃高分化腺癌相比, GCP患者发病年龄偏小, 男性偏多, 较少伴有体质量减轻、食欲不振、腹胀等症状; (2)大体表现: GCP表现为壁内肿块、质软、黏膜光滑; 胃高分化腺癌形态多样, 黏膜可呈凸起、平坦、凹陷, 呈溃疡样改变, 界限清晰[51]; (3)组织学表现: GCP表现为囊性、腺体扩张、固有层腺体无异型性、成簇腺体从固有膜连续至黏膜下层(如图1); 胃高分化腺癌腺体中单个或小簇状细胞有一定程度的异型性、核异型性, 核仁明显[52], 且表现为分隔开的不规则腺管浸润至黏膜下层(如图2); (4)免疫组织化学: 胃高分化腺癌中Ki-67、p53、p21的表达明显高于癌前病变[53,54], 因此相对于正常组织, GCP中Ki-67、p53、p21的表达虽有增高趋势[55], 仍可以作为辅助诊断的参考; (5)侵袭性: GCP多存在于黏膜下层, 而胃高分化腺癌可浸润至组织各层; (6)腺体个数: GCP腺体个数较多(3个以上), 而胃高分化腺癌腺体可单个存在.
GCP通常被认为是良性病变, 虽然也有文献[32,56]报道怀疑可能是癌前病变, 但现有文献[57]中, GCP经诊断治疗后没有出现复发和转移现象. 而胃高分化腺癌属于恶性肿瘤, 根据肿瘤大小和侵犯深度行内镜切除或手术切除, 存在复发和转移的风险. 临床上对两者的处理方式不同, 因此在病理诊断中需注意对两者进行鉴别, 以防误诊后导致治疗不足或过度.
深在性囊性胃炎(gastritis cysticaprofunda, GCP)是一种罕见疾病, 目前国内多是个案报道, 其病因不清, 多认为是一种良性病变, 预后较好. 临床病理医生对其认识不足, 容易误诊为其他疾病, 当误诊为高分化腺癌时, 两者的治疗方式及预后不同, 可导致治疗不足或过度.
GCP多发生于胃深部组织, 易与多种胃部疾病混淆. 本文详细介绍了GCP与高分化腺癌的各自特征及鉴别要点, 有助于工作中对两者进行区别认识.
本文总结GCP与高分化腺癌两者在临床表现、病理组织、免疫组织化学等不同点进行鉴别诊断.
根据GCP与高分化腺癌两者在临床表现、病理组织、免疫组织化学等不同点, 有助于病理医生鉴别区分.
本文总结了GCP与高分化腺癌的鉴别问题, 对二者的病理特征和临床表现进行了详细描述, 对临床认识这种少见疾病有很好借鉴意义.
姜春萌, 教授, 大连医科大学附属第二医院消化内科; 肖恩华, 教授, 中南大学湘雅二医院放射教研室
手稿来源: 邀请约稿
学科分类: 胃肠病学和肝病学
手稿来源地: 北京市
同行评议报告分类
A级 (优秀): A
B级 (非常好): B
C级 (良好): 0
D级 (一般): 0
E级 (差): 0
编辑: 马亚娟 电编:杜冉冉
| 1. | Nagy P, Johansson S, Molloy-Bland M. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog. 2016;8:8. [PubMed] [DOI] |
| 5. | 刘 复生. 中国肿瘤病理学分类(上). 科学技术文献出版社发行部. 2001;. |
| 7. | Gomez JM, Wang AY. Gastric intestinal metaplasia and early gastric cancer in the west: a changing paradigm. Gastroenterol Hepatol (N Y). 2014;10:369-378. [PubMed] |
| 9. | Huang Q, Fang C, Shi J, Sun Q, Wu H, Gold JS, Weber HC, Guan W, Zhang Y, Yu C. Differences in Clinicopathology of Early Gastric Carcinoma between Proximal and Distal Location in 438 Chinese Patients. Sci Rep. 2015;5:13439. [PubMed] |
| 10. | Kim KO, Ku YS. Is image-enhanced endoscopy useful for the diagnosis and treatment of gastrointestinal tumor? Clin Endosc. 2013;46:248-250. [PubMed] [DOI] |
| 11. | Hayee B, Inoue H, Sato H, Santi EG, Yoshida A, Onimaru M, Ikeda H, Kudo SE. Magnification narrow-band imaging for the diagnosis of early gastric cancer: a review of the Japanese literature for the Western endoscopist. Gastrointest Endosc. 2013;78:452-461. [PubMed] [DOI] |
| 12. | Hussain I, Ang TL. Evidence based review of the impact of image enhanced endoscopy in the diagnosis of gastric disorders. World J Gastrointest Endosc. 2016;8:741-755. [PubMed] [DOI] |
| 13. | 郑 洪伟, 薛 会光, 杨 爱华, 刘 华, 鞠 辉, 刘 希双. 窄带成像技术联合放大内镜与胃镜活检诊断早期胃癌的价值比较. 世界华人消化杂志. 2015;23:3917-3922. [DOI] |
| 16. | 李 国华, 陈 幼祥, 郭 贵海, 周 小江, 廖 旺娣, 舒 徐, 刘 志坚, 李 弼民, 祝 荫, 吕 农华. 613例早期胃癌的临床病理特点分析. 江西医药. 2014;793-795. [DOI] |
| 19. | 徐 国良, 罗 广裕, 林 世永, 高 晓燕, 李 茵, 单 宏波, 张 蓉, 黎 建军, 贺 龙君, 王 国宝. 内镜下黏膜切除术及内镜黏膜下剥离术治疗上消化道早期癌及癌前病变. 中国内镜杂志. 2010;16:1013-1016. |
| 20. | Ning B, Abdelfatah MM, Othman MO. Endoscopic submucosal dissection and endoscopic mucosal resection for early stage esophageal cancer. Ann Cardiothorac Surg. 2017;6:88-98. [PubMed] [DOI] |
| 21. | Ono H, Yao K, Fujishiro M, Oda I, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Ichinose M. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016;28:3-15. [PubMed] [DOI] |
| 22. | Ushiku T, Arnason T, Ban S, Hishima T, Shimizu M, Fukayama M, Lauwers GY. Very well-differentiated gastric carcinoma of intestinal type: analysis of diagnostic criteria. Mod Pathol. 2013;26:1620-1631. [PubMed] [DOI] |
| 23. | Yao T, Utsunomiya T, Oya M, Nishiyama K, Tsuneyoshi M. Extremely well-differentiated adenocarcinoma of the stomach: clinicopathological and immunohistochemical features. World J Gastroenterol. 2006;12:2510-2516. [PubMed] [DOI] |
| 24. | Miyaoka Y, Izumi D, Mikami H, Yasaki T, Morito Y, Imaoka H, Fujishiro H, Kouge N, Imaoka T, Onuma H. A Case Report of an Extremely Well Differentiated Gastric Adenocarcinoma of the Fundic Gland Type Successfully Treated with ESD. Gastroenterol Endosc. 2011;53:1778-1785. |
| 25. | McFarland S, Manivel CJ, Ramaswamy A, Mesa H. Gastric-type extremely well-differentiated adenocarcinoma arising in the blind pouch of a bypassed stomach, presenting as colonic pseudo-obstruction. Ann Gastroenterol. 2015;28:499-501. [PubMed] |
| 26. | Carvalho JR, Quadros AC, Meireles L, Alves I, Moura Dos Santos P, Serejo F, Ferreira C, Freire JP, Velosa J. Gastritis cystica profunda mimicking a GIST - A diagnostic challenge. Gastroenterol Hepatol. 2017; Sep 18 [Epub ahead of print]. [PubMed] [DOI] |
| 27. | Yoon JB, Lee BE, Kim DH, Park DY, Jeon HK, Baek DH, Kim GH, Song GA. A Rare Case of Early Gastric Cancer Combined with Underlying Heterotopic Pancreas. Clin Endosc. 2017; Aug 31 [Epub ahead of print]. [PubMed] [DOI] |
| 28. | Lee SJ, Park JK, Seo HI, Han KH, Kim YD, Jeong WJ, Cheon GJ, Eom DW. A case of gastric inverted hyperplastic polyp found with gastritis cystica profunda and early gastric cancer. Clin Endosc. 2013;46:568-571. [PubMed] [DOI] |
| 29. | Ogasawara N, Noda H, Kondo Y, Yoshimine T, Sugiyama T, Kimura M, Inoue S, Takahashi E, Sasaki M, Kasugai K. A case of early gastric cancer arising from gastritis cystica profunda treated by endoscopic submucosal dissection. Case Rep Gastroenterol. 2014;8:270-275. [PubMed] [DOI] |
| 30. | Butt MO, Luck NH, Hassan SM, Abbas Z, Mubarak M. Gastritis profunda cystica presenting as gastric outlet obstruction and mimicking cancer: A case report. J Transl Int Med. 2015;3:35-37. [PubMed] [DOI] |
| 32. | Moon SY, Kim KO, Park SH, Yoo KS, Park CH, Kim JH, Park CK, Jun SY. Gastritis cystica profunda accompanied by multiple early gastric cancers. Korean J Gastroenterol. 2010;55:325-330. [PubMed] |
| 33. | McCurdy KR, Parmar K, de Melo SW. Gastritis Cystica Profunda: A Deeper Problem. ACG Case Rep J. 2016;3:e125. [PubMed] [DOI] |
| 34. | Laratta JL, Buhtoiarova TN, Sparber LS, Chamberlain RS. Gastritis Cystica Profunda: A Rare Gastric Tumor Masquerading as a Malignancy. Surg Sci. 2012;3:158-164. [DOI] |
| 35. | Littler ER, Gleibermann E. Gastritis cystica polyposa. Gastric mucosal prolapse at gastroenterostomy site, with cystic and infiltrative epithelial hyperplasia. Cancer. 1972;29:205-209. [PubMed] |
| 36. | Iwanaga T, Koyama H, Takahashi Y, Taniguchi H, Wada A. Diffuse submucosal cysts and carcinoma of the stomach. Cancer. 1975;36:606-614. [PubMed] [DOI] |
| 37. | Yamagiwa H, Matsuzaki O, Ishihara A, Yoshimura H. Heterotopic gastric glands in the submucosa of the stomach. Acta Pathol Jpn. 1979;29:347-350. [PubMed] |
| 38. | Wu MT, Pan HB, Lai PH, Chang JM, Tsai SH, Wu CW. CT of gastritis cystica polyposa. Abdom Imaging. 1994;19:8-10. [PubMed] |
| 39. | Park JS, Myung SJ, Jung HY, Yang SK, Hong WS, Kim JH, Kang GH, Ha HK, Min YI. Endoscopic treatment of gastritis cystica polyposa found in an unoperated stomach. Gastrointest Endosc. 2001;54:101-103. [PubMed] [DOI] |
| 41. | Yu XF, Guo LW, Chen ST, Teng LS. Gastritis cystica profunda in a previously unoperated stomach: a case report. World J Gastroenterol. 2015;21:3759-3762. [PubMed] [DOI] |
| 44. | Béchade D, Desramé J, Algayres JP. Gastritis cystica profunda in a patient with no history of gastric surgery. Endoscopy. 2007;39 Suppl 1:E80-E81. [PubMed] [DOI] |
| 45. | Machicado J, Shroff J, Quesada A, Jelinek K, Spinn MP, Scott LD, Thosani N. Gastritis cystica profunda: Endoscopic ultrasound findings and review of the literature. Endosc Ultrasound. 2014;3:131-134. [PubMed] [DOI] |
| 46. | Lee TH, Lee JS, Jin SY. Gastritis cystica profunda with a long stalk. Gastrointest Endosc. 2013;77:821-822; discussion 822. [PubMed] [DOI] |
| 47. | Wiedemann T, Loell E, Mueller S, Stoeckelhuber M, Stolte M, Haas R, Rieder G. Helicobacter pylori cag-Pathogenicity island-dependent early immunological response triggers later precancerous gastric changes in Mongolian gerbils. PLoS One. 2009;4:e4754. [PubMed] [DOI] |
| 48. | Kim L, Kim JM, Hur YS, Shin YW, Park IS, Choi SJ, Han JY, Chu YC, Kim KH. Extended gastritis cystica profunda associated with Epstein-Barr virus-positive dysplasia and carcinoma with lymphoid stroma. Pathol Int. 2012;62:351-355. [PubMed] [DOI] |
| 49. | 程 捷瑶, 吴 晰, 杨 爱明, 邹 龙, 姚 方, 郭 涛, 伍 东升, 冯 云路, 蒋 青伟, 周 炜洵. 超声内镜对浅表胃癌诊断及治疗决策的影响. 中华消化内镜杂志. 2016;33:663-666. [DOI] |
| 50. | Okada M, Iizuka Y, Oh K, Murayama H, Maekawa T. Gastritis cystica profunda presenting as giant gastric mucosal folds: the role of endoscopic ultrasonography and mucosectomy in the diagnostic work-up. Gastrointest Endosc. 1994;40:640-644. [PubMed] [DOI] |
| 51. | Ang TL, Khor CJ, Gotoda T. Diagnosis and endoscopic resection of early gastric cancer. Singapore Med J. 2010;51:93-100. [PubMed] |
| 52. | Srivastava A, Lauwers GY. Gastric epithelial dysplasia: the Western perspective. Dig Liver Dis. 2008;40:641-649. [PubMed] [DOI] |
| 55. | Mitomi H, Iwabuchi K, Amemiya A, Kaneda G, Adachi K, Asao T. Immunohistochemical analysis of a case of gastritis cystica profunda associated with carcinoma development. Scand J Gastroenterol. 1998;33:1226-1229. [PubMed] |
| 56. | Tsuji T, Iwahashi M, Nakamori M, Ueda K, Ishida K, Naka T, Ojima T, Akamatsu H, Yamaue H. Multiple early gastric cancer with gastritis cystica profunda showing various histological types. Hepatogastroenterology. 2008;55:1150-1152. [PubMed] |
| 57. | National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, "Surveillance, Epidemiology, and End Results (SEER) Program Research Data (1973-2008)". 2012. Available from: http://www.seer.cancer.gov/. |