修回日期: 2017-08-24
接受日期: 2017-08-30
在线出版日期: 2017-11-18
肿瘤多药耐药指癌细胞对多种不同的抗癌药物的交叉性耐药, 可以是原发性的或继发性的, 耐药的机制有多种. 本文对胃癌细胞通过上调ABC转运蛋白超家族成员的表达使药物外排增加和抑制或逃避细胞凋亡而引起多药耐药以及长链非编码RNA在胃癌多药耐药形成中的作用的研究进展做了总结论述.
核心提要: 肿瘤多药耐药是肿瘤药物治疗中存在的一个严重问题, ABC转运蛋白超家族成员表达上调使药物外排增加和细胞凋亡受抑制是癌细胞耐药的两种重要机制. 新近的研究发现, 长链非编码RNA在胃癌多药耐药的调节中起着重要的作用.
引文著录: 符兆英. 胃癌多药耐药在ABC转运蛋白、细胞凋亡和长链非编码RNA方面的研究进展. 世界华人消化杂志 2017; 25(32): 2838-2850
Revised: August 24, 2017
Accepted: August 30, 2017
Published online: November 18, 2017
Cancer multidrug resistance refers to the cross resistance of cancer cells to a variety of anticancer drugs, which can be primary or secondary. Several mechanisms attribute to cancer multidrug resistance. In this paper, the recent progress in the understanding of the mechanisms of multi-drug resistance of gastric cancer cells with regard to the role of adenosine triphosphate binding cassette transporters, apoptosis, and long non-coding RNAs is reviewed.
- Citation: Fu ZY. Role of ATP-binding cassette transporters, apoptosis, and long non-coding RNAs in gastric cancer multidrug resistance. Shijie Huaren Xiaohua Zazhi 2017; 25(32): 2838-2850
- URL: https://www.wjgnet.com/1009-3079/full/v25/i32/2838.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v25.i32.2838
胃癌是全球常见的一种恶性肿瘤, 在东亚国家更为多见; 在中国, 其发病率和死亡率居所有恶性肿瘤之第2位[1,2]. 早期胃癌先行手术治疗, 术后进行化学治疗和其他辅助治疗, 而中晚期胃癌则以化疗为主要治疗手段; 但由于癌细胞往往对化疗药物产生多药耐药, 而使疗效不佳或肿瘤复发[3,4]. 癌细胞对化疗药的耐受可以是原发性的或天然性的耐受, 亦称内在性的耐受, 即有些癌细胞本身即携带有耐药的遗传特征; 也可能是继发性的或获得性的耐受, 即癌细胞在化疗药应用后被诱导产生耐药性. 多种癌症都可以产生多药耐药; 一些胃肠道肿瘤对化疗药具有内在的耐受性[5].
肿瘤多药耐药是指肿瘤一旦对某一种化疗药物产生耐药, 就可以对多种化学性质不同和作用机制不同的化疗药物产生交叉性耐药[3,4]. 已发现癌细胞可经多种机制对化疗药产生耐药, 包括上调ABC转运蛋白(ATP-binding cassette transporter, ABC transporter)超家族某些成员的表达使药物外排增加、抑制细胞凋亡或对凋亡的敏感性降低、增强DNA修复能力、改变药物作用的靶分子、促进药物在细胞内的代谢和减弱药物的毒性等[3-8]. 不同的肿瘤可以通过不同的机制耐药, 一种肿瘤可以通过数种机制耐药, 肿瘤对不同的药物可以通过相同的和/或不同的机制耐药. 本文回顾论述了ABC transporter超家族成员过表达和细胞凋亡受抑制这两种机制引起胃癌多药耐药的研究进展, 并总结了近几年来长链非编码RNA(long non-coding RNA, lncRNA)在胃癌多药耐药形成中作用的研究成果.
ABC transporter超家族是非常大的一个(膜)转运蛋白超家族, 该超家族成员的蛋白通常由多个亚基组成, 其中一个或两个为跨膜蛋白、另一个或两个为膜相关的腺嘌呤核苷三磷酸(adenosine triphosphate, ATP)酶. ATP酶利用ATP的结合和水解为多种物质的跨膜转运提供能量. 原核生物的ABC transporter有向细胞内泵入和向细胞外泵出2种类型, 而真核生物的ABC transporter只有向细胞外泵出的作用[9-13]. 人类的ABC transporter共49个成员, 分为7个亚家族, 从ABCA到ABCG. ABC transporter具有生理作用, 但也可以介导细胞对药物的耐受. 介导人类肿瘤多药耐药的ABC transporter超家族成员最主要的是ABCB1蛋白, 此外还有ABCC1/ABCC2蛋白以及ABCG2蛋白[14-19].
ABCB1(ATP-binding cassette sub-family B member 1)蛋白属ABC transporter超家族B亚家族的一个成员, 由ABCB1基因编码, 亦称P-糖蛋白(permeability glycoprotein, P-gp)或多耐药基因1(multidrug resistance gene 1, MDR1), 由12个跨膜结构域(N-端和C-端各6个)和一个大的主要位于细胞内的细胞质结构域(在N-端和C-端之间)组成, 后者有ATP结合位点[19-21]. 进入细胞内的底物从ABCB1蛋白细胞质结构域的开口处进入, 随着ATP分子与ATP结合位点的结合和ATP的水解, 底物被排出胞外; 同时, ATP水解产生的磷酸亦被释放; ATP结合位点的ADP被新的ATP分子置换, 从而可以开始下一个循环的底物泵出[19,22,23]. ABCB1蛋白能将进入细胞的外源性物质(毒物、药物等)泵出细胞外; 多种正常细胞表达ABCB1蛋白, 但在耐药的癌细胞ABCB1蛋白呈超表达[19]. ABCB1蛋白的底物非常广泛, 其中包括多种化疗药物如阿霉素、道诺霉素(柔红霉素)、长春新碱、长春花碱、依托泊苷、替尼泊苷、紫杉醇和放线菌素D等.
胃癌标本的免疫组织化学染色发现[24,25], ABCB1蛋白在胃癌细胞的细胞膜和细胞质中都有分布. Hu等[25]发现, 59份未用化疗药治疗的胃癌组织标本中, ABCB1蛋白的表达率为86.4%, 这些组织中ABCB1蛋白的表达率与胃癌的病理组织(分化程度)类型没有关联性. 该结果支持胃癌对化疗药物具有内在的或固有的耐药性的观点. 研究[24]还发现, ABCB1蛋白的表达在对阿霉素、高喜树碱等化疗药耐受的胃癌组织中显著性地比在对这些化疗药敏感的胃癌组织中高. Shi等[26]用免疫组织化学染色发现, 69份胃贲门腺癌标本中ABCB1蛋白的表达率为49.2%, 而作为对照的正常组织ABCB1蛋白的表达率为0%; 有转移的胃癌ABCB1蛋白的表达率比没有转移的更高(67.5% vs 24.1%); ABCB1蛋白的表达率与胃癌的临床病理分期相关而与胃癌的分化程度无关.
近年来的许多研究表明, 微小RNA(microRNA, miRNA)在肿瘤多药耐药上起着重要的作用; 化疗药物的应用可以引起miRNA表达的异常上调或下调并进而经不同机制导致癌细胞的耐药; 多种miRNA可以通过调节ABCB1蛋白的表达而介导癌细胞多药耐药. Lu等[27]发现, microRNA-129(miR-129)在耐顺铂的胃癌组织和细胞中呈低表达; 过表达miR-129可以减低胃癌细胞对顺铂的耐药性, 而敲低miR-129则减弱胃癌细胞对顺铂的敏感性; miR-129作用的靶分子是ABCB1蛋白; miR-129结合于ABCB1蛋白mRNA的3'末端非翻译区(3'-untranslated region, 3'-UTR)而下调其表达; 因此, 我们认为miR-129通过抑制ABCB1蛋白的表达而逆转胃癌对顺铂的耐药. 用实时定量聚合酶链反应(polymerase chain reaction, PCR)检测体外培养的胃癌传代细胞中ABCB1蛋白的表达发现, 下调miR-27a可以显著性地降低ABCB1蛋白的表达[28]. 用实时定量PCR检测胃癌组织标本中ABCB1蛋白的表达的结果显示, 与癌旁正常组织相比, miR-27a在胃癌组织中明显高表达、miR-27a的高表达与胃癌的组织病理学分级显著相关; 在胃癌传代细胞, 下调miR-27a的表达可以明显增强抗肿瘤药哌立福辛抑制细胞生长的活性, 其机制可能是下调了P-gp的表达[29]. Wang等[30]报道, miR-19a/b在多药耐药细胞系中表达上调并降低胃癌细胞对化疗药的敏感性, miR-19a/b通过增加P-gp的表达水平而加速胃癌细胞对化疗药阿霉素的排出.
Shang等[31]于2014年用高通量功能筛选技术找出11种调控胃癌多药耐药的miRNA, 其中的miR-508-5p能够最有效地逆转肿瘤多药耐药; 过表达miR-508-5p足以在体外逆转癌细胞对多种化疗药的耐药并在体内增强肿瘤对化疗的敏感性; 进一步的研究显示, miR-508-5p能够直接靶向于ABCB1基因和ZNRD1基因的3'-UTR从而抑制它们在mRNA和蛋白质水平的表达, 对ZNRD1的抑制同时导致ABCB1的降低; 以上发现提示, miR-508-5p/ZNRD1/ABCB1调节环在胃癌多药耐药的调节上起着关键性的作用. Shang等[32]于2016年又研究了miR-508-5p在耐药胃癌细胞中被下调的机制, 他们发现, miR-27b能够直接靶向细胞周期蛋白(cell cyclin G1, CCNG1)的3'-UTR, 通过抑癌基因P53而上调miR-508-5p的表达(CCNG1是一种重要的细胞周期调控因子, 能够负性调节P53蛋白的稳定性); 因此miR-27b/CCNG1/P53/miR-508-5p轴在胃癌多药耐药的形成中起着重要的作用; 通过对胃癌组织中miR-27b和miR-508-5p表达的检测, 他们还发现, miR-27b和miR-508-5p都高表达的胃癌对化疗更敏感.
ABCC1和ABCC2(ATP-binding cassette sub-family C member 1, 2)蛋白为ABC transporter超家族C亚家族的两个成员, 分别由ABCC1基因和ABCC2基因编码, 二者与多种药物的耐受相关, 又分别称为多药耐药相关蛋白(multidrug resistance-associated protein, MRP)1和2(MRP1, MRP2)[33-36]. ABCC1和ABCC2可以介导神经母细胞瘤、肺癌、乳腺癌、前列腺癌、肝癌、卵巢癌和结肠癌等[37-43]对化疗药的耐药, 也有介导胃癌耐药的报道. Chen等[44]在体外诱导分离出了对三氧化二砷耐受的胃癌细胞株SGC7901/AS, 发现该细胞株ABCB1蛋白和基因的表达升高最明显, 同时也有ABCC1和ABCC2蛋白和基因表达的升高. Yu等[45]回顾检查了119例胃癌中MRP等的表达, 结果发现MRP的表达阳性率为42.9% . 靳胜[46]用实时荧光定量PCR检测了47例胃癌标本和17例正常胃组织的MRP、脂蛋白受体相关蛋白和ABCB1基因的表达, 结果发现, 3种基因在胃癌标本中的表达均高于正常胃组织, MRP在早期胃癌中的表达显著高于进展期胃癌, 在高、中分化腺癌中的表达显著高于低分化和未分化腺癌, 在病情恶化患者中MRP表达上调30%. Hu等[25]检测了59例胃癌标本中ABCB1和MRP等的表达, 结果发现ABCB1和MRP的表达阳性率分别为86.4%和27.1%. 以上两项研究的病人在术前均未使用化疗药. Ji等[47]发现, 来自间充质干细胞的外泌体在体内和体外显著性地增加了胃癌细胞对5-氟尿嘧啶(5-fluorouracil, 5-Fu)的耐药, 其机制包括对抗凋亡和增强MDR1和MRP等蛋白的表达. Takegawa等[48]发现胃癌细胞株N87-TDMR对T-DM1的耐药是因ABCC2和ABCG2表达异常使药物外排增加引起的: N87-TDMR中ABCC2和ABCG2表达上调, 用MK571抑制ABCC2和ABCG2能够恢复药物敏感性.
ABCG2(ATP-binding cassette sub-family G member 2)蛋白属ABC transporter超家族G亚家族的一个成员, 由ABCG2基因编码, 最初是在乳腺癌耐药细胞系中发现的, 故又称为乳腺癌耐药蛋白, 后来发现ABCG2蛋白与多种肿瘤包括胃癌的耐药相关[49-58]. Wang等[59]报道, ABCG2在胃癌患者频繁地异常表达, ABCG2在胃癌标本和胃癌细胞系均表达极度上调, 并与胃癌临床病理特征和预后不良相关. 用ABCG2 siRNA转染MKN-45胃癌细胞, 能够抑制细胞增殖、阻滞细胞周期并诱导细胞凋亡. 我们还发现, ABCG2与胃癌的一种关键性启动子CRKL相关, 用ABCG2 siRNA转染MKN-45细胞能使CRKL下调, 在MKN-45细胞中过表达CRKL可以恢复siRNA转染引起的ABCG2表型改变. Zhang等[60]报道, miR-132在Lgr5阳性胃癌干细胞中表达上调, miR-132的高表达与胃癌患者对化疗药的耐药相关、与患者的预后相关. 功能分析发现, miR-132在体内外均能促进Lgr5阳性胃癌干细胞对顺铂的耐药. 用双荧光酶报告基因分析发现, SIRT1是miR-132的直接靶标. 在胃癌标本中, miR-132表达与SIRT1表达呈反相关. 进一步的研究发现, ABCG2是SIRT1的下游靶标; 过表达SIRT1可通过促进转录因子CREB的去乙酰化而下调ABCG2表达; CREB结合于ABCG2启动子而诱导ABCG2的转录. Zhao等[61]用含部分随机的4×106条siRNA的逆转录病毒文库转染胃癌SGC7901细胞寻找耐药相关基因, 得到12个耐化疗药表柔比星的细胞集落, 从这12个耐药集落中鉴定出两种基因: GAS1(growth arrest-specific 1)和PTEN(phosphatase and tensin homolog); GAS1的抑制导致SGC7901细胞对表柔比星显著的耐药和对5-Fu和顺铂的交叉性耐药. GAS1基因的被抑制导致ABCB1和ABCG2的表达上调(但没有ABCC1表达上调)使药物外排增加所以细胞耐药, 敲低ABCB1和ABCG2能部分地逆转GAS1抑制导致的细胞耐药. IMMU-132是抗体与抗肿瘤药SN-38偶联的一种抗体-药物偶联物, 在治疗时往往由于ABC transporter介导的耐药而使治疗失败. Chang等[62]研究了用ABC transporter抑制剂YHO-13351逆转耐药增强IMMU-132治疗效果的作用. 他们建立了两个耐药细胞株: 来源于乳腺癌MDA-MB-231 细胞的耐药细胞株MDA-MB-231-S120和来源于胃癌NCI-N87细胞的耐药细胞株NCI-N87-S120. 他们发现, 这两个细胞株在体外对SN-38的敏感性下降(IC50比未耐药的MDA-MB-231细胞和NCI-N87细胞分别上升约50倍), 这两株耐药细胞均发现有ABCG2表达升高但没有ABCB1表达升高, 用ABC transporter抑制剂YHO-13351处理这两株细胞可以恢复SN-38的细胞毒性, YHO-13351与IMMU-132合用能够增加NCI-N87-S120移植鼠的中位生存期.
化疗药物杀伤肿瘤细胞的重要机制, 是通过引起DNA损伤、干扰DNA合成、或阻遏有丝分裂而最终引起细胞凋亡[63,64]. 癌细胞对化疗药产生多药耐药的另一个重要机制是抑制或逃避细胞凋亡[65,66]. 细胞凋亡的始动主要有两条通路: 外源性通路和内源性通路. 外源性通路亦称死亡受体通路, 通过细胞膜上的死亡受体如Fas(First apoptosis signal)和肿瘤坏死因子(tumor necrosis factor, TNF)而启动细胞凋亡, 该通路主要活化caspase-8. 内源性通路亦称线粒体通路, 通过线粒体蛋白如SMACs(second mitochondria-derived activator of caspases)和细胞色素c向细胞浆的外溢而启动细胞凋亡, 该通路主要活化caspase-9. 活化的caspase-8和caspase-9引起caspase-3的连锁活化而最终导致细胞结构降解和细胞死亡[67-70].
多种化疗药物可通过线粒体途径诱导细胞凋亡. 研究显示, 线粒体裂解能够诱发细胞凋亡, 而线粒体融合可以抑制细胞凋亡, 前者是因为线粒体裂解促进了细胞色素c的释放; Aung等[71]发现, 在阿霉素作用下线粒体膜蛋白18(mitochondrial membrane protein 18, MTP18)可促进动力相关蛋白1在线粒体聚集并引起线粒体裂解或碎片化从而诱导胃癌细胞发生凋亡; 他们还发现, 在阿霉素治疗过程中, MTP18的表达下调, 故认为MTP18低表达是胃癌细胞通过细胞凋亡途径而耐药的一种机制. Liang等[72]报道, 紫草素(一种天然萘醌)能通过增加细胞内的活性氧而使线粒体膜去极化最终诱发细胞凋亡, 其机制除了caspase依赖性的(通过细胞色素c的释放活化caspase酶)外, 还包括caspase非依赖性的, 后者介导凋亡诱导因子和内切酶G的核转运. 紫草素能在体内和体外增加胃癌细胞对化疗药5-Fu和奥沙利铂的敏感性. Tang等[73]发现, 在对顺铂耐药的胃癌细胞中, 磷酸化的丝切蛋白呈过表达, 顺铂能使非耐药的胃癌细胞的丝切蛋白去磷酸化但不能使耐药细胞的丝切蛋白去磷酸化. 但是, 中药左金丸能诱导耐药胃癌细胞的丝切蛋白去磷酸化并促进该蛋白从细胞质进入线粒体, 最终经线粒体通路活化细胞凋亡. 向耐顺铂胃癌细胞转染丝切蛋白特异性siRNA能够抑制这一作用.
Yin等[74]报道, 用外源性凋亡通路的Fas受体基因转染, 能够逆转人胃癌SGC7901/VCR细胞的多药耐药, 其机制可能是增敏了细胞凋亡和抑制了ABCB1蛋白. Zhang等[75]报道, α-生育酚琥珀酸酯与阿霉素合用, 能增加Fas蛋白表达水平并诱导胃癌细胞凋亡. Lim等[76]研究了熊去氧胆酸对顺铂耐药细胞的作用, 结果发现, 熊去氧胆酸作用后, 死亡受体Fas转位于脂筏并诱发细胞凋亡. Li等[77]研究了Mcl-1基因沉默对耐药胃癌细胞系的作用及其机制, 结果发现, 用siRNA能够有效地沉默Mcl-1基因的表达并阻滞细胞周期进展和促进细胞凋亡从而在一定程度上逆转细胞对长春新碱、顺铂和5-Fu的耐药, 其机制是增强或减弱了Fas和Bcl-2等基因的表达. Na等[78]报道, 对TRAIL(TNF-related apoptosis-inducing ligand)耐受的胃癌细胞经信号通路特异性抑制剂环巴胺作用后, 能通过上调死亡受体DR5的表达而增加对TRAIL诱导的凋亡的敏感性; survivin具有对抗DR5的作用, 而环巴胺能减弱survivin的表达.
Bcl-2家族由进化上保守的含有Bcl-2同源结构域的多个成员组成, 在细胞凋亡的调控上发挥着非常重要的作用. 该家族的Bax是非常重要的凋亡促进蛋白、Bcl-2是非常重要的凋亡抑制蛋白. 耐药的肿瘤中Bax往往呈低表达而Bcl-2往往呈超表达, 使癌细胞对化疗药的敏感性下降并逃避凋亡.
Fan等[79]发现, 锌指蛋白家族的成员ZNF139能够通过调节Bax和Bcl-2等凋亡相关基因而介导胃癌细胞对凋亡的耐受, 用siRNA抑制ZNF139的表达能够显著下调胃癌细胞中Bcl-2和survivin的表达、显著上调Bax和caspase-3的表达, 并引起胃癌细胞的凋亡率显著升高. 周露婷等[80]用免疫组化检测了Bcl-2蛋白在胃癌组织中的表达, 结果发现, 胃癌组织中Bcl-2蛋白的阳性率为70.4%, 5-Fu、阿霉素和丝裂霉素对Bcl-2阳性表达胃癌的抑制率显著低于对Bcl-2阴性表达胃癌的抑制率, Bcl-2的阳性表达与上述3种药物的体外耐药相关, 故认为Bcl-2能介导胃癌耐药, 其高表达是胃癌产生多药耐药的一个原因. Ji等[81]发现, CD133+胃癌细胞比CD133-细胞对化疗药更耐受, 骨髓间充质干细胞(BM-MSC)能够通过下调Bax表达和上调Bcl-2表达而增加CD133+胃癌细胞的抗凋亡能力和耐药性, 其机制是BM-MSC在CD133+胃癌细胞中诱发了PI3K/Akt信号, 阻断PI3K/Akt信号能够抑制耐药形成. Zhao等[82]发现, 低氧诱导因子1α siRNA转染耐药胃癌细胞OCUM-2MD3/L-OHP后, Bcl-2等表达下调而Bax等表达上调, 提示低氧诱导因子1α是通过调节这些凋亡相关基因而介导胃癌耐药的. Xu等[83]报道, 曲克芦丁(在茶叶、咖啡、谷物和多种水果蔬菜中含有的一种黄酮类物质)与5-Fu合用能抑制Bcl-2表达和上调Bax表达并增加耐药胃癌细胞对5-Fu的敏感性. Zhao等[61]的研究发现, 胃癌SGC7901细胞对化疗药表柔比星、5-Fu和顺铂耐药的主要机制之一是GAS1基因被抑制导致Bcl-2/Bax比值升高使细胞凋亡受抑制, 抑制Bcl-2能够解除凋亡抑制.
许多种的miRNA可以通过调节Bax和Bcl-2表达而介导癌细胞耐药. Chen等[84]发现, miRNA-200c能诱导E-钙黏素的表达并进而增加SGC7901/DDP细胞对化疗药顺铂的敏感性; E-钙黏素增加癌细胞对化疗药敏感性的机制是上调Bax表达和下调Bcl-2表达并促进细胞凋亡. 智慧等[85]发现, Bcl-2基因直接受miR-125b调控, 在耐药细胞株中上调miR-125b表达能显著抑制Bcl-2蛋白表达水平, 并显著增加耐药细胞对长春新碱、阿霉素、依托泊苷和顺铂的敏感性、显著增加耐药细胞在长春新碱诱导下的凋亡. Wang等[86]研究了miR-503在胃癌对顺铂耐药中所起的作用, 结果发现, miR-503的高表达能够增加耐药细胞株SGC7901/DDP对顺铂的敏感性, miR-503的直接靶基因有胰岛素样生长因子-1受体(IGF1R)和Bcl-2, 在SGC7901/DDP细胞中增强miR-503的表达能够降低IGF1R和Bcl-2的表达从而增敏顺铂诱导的细胞凋亡. Zhuang等[87]报道, miR-143在顺铂耐药细胞株中呈低表达, 同时伴有IGF1R和Bcl-2的高表达, miR-143的靶基因也是IGF1R和Bcl-2, 增强miR-143的表达能够降低IGF1R和Bcl-2的表达而增加顺铂诱导的SGC7901/DDP细胞的凋亡, 故认为miR-143通过靶向IGF1R和Bcl-2而介导了胃癌细胞对顺铂的耐药. Wang等[30]报道, miR-19a/b在耐药胃癌细胞中表达上调, 其表达上调使胃癌对化疗药的敏感性下降, miR-19a/b除了能加快药物从细胞的排出外, 还能通过调节Bcl-2和Bax而抑制细胞凋亡, miR-19a/b通过靶向蛋白激酶B磷酸化的抑制因子PTEN而发挥作用. Wang等[88]还发现, 参与染色体分离的中心粒蛋白Shugoshin1能通过调节Bcl-2和Bax等而抑制细胞凋亡并介导胃癌细胞对阿霉素的耐药. 除上述miRNA外, miR-27a、miR-27b、miR-29、miR-34、miR-187、miR-203、miR-1271、miR-15b和miR-16也能通过靶向Bax和Bcl-2以及其他凋亡相关基因而调控胃癌多药耐药[89-92].
lncRNA指的是长度大于200 nt的lncRNA分子, 在非编码RNA转录组中占较大比例, 参与基因转录调控、转录后调控和表遗传调控[93,94]. 近几年来, lncRNA在肿瘤发生发展、肿瘤转移和肿瘤多药耐药形成中的作用被受到高度重视[95-100]. lncRNAs能通过ABC transporter和细胞凋亡等而调节胃癌多药耐药.
王颖[101]于2012年利用高通量lncRNA芯片比较了胃癌耐药细胞株SGC7901/ADR和SGC7901/VCR与亲本细胞SGC7901的lncRNA表达谱差异, 结果发现, SGC7901/ADR细胞和SGC7901/VCR细胞与SGC7901细胞lncRNA表达差异在2倍以上的分别有1499条和1420条: 其中627条为交集差异, 即在两种耐药细胞中均呈差异表达; 差异在4倍以上的有32种: 其中上调的和下调的各占16种; 差异最大的一种lncRNA(DMTF1v4)在SGC7901/ADR细胞中上调25.89倍, 在SGC7901/VCR细胞中上调21.42倍; 基因芯片差异倍数、邻近编码基因信息分析、phylop物种进化保守性评分、实时定量PCR表达水平验证等均提示, DMTF1v4可能是介导胃癌多药耐药的一种关键性lncRNA分子. Wang等[102]于2015年又用高通量lncRNA微阵列分析发现, 胃癌细胞系SGC7901及其衍生的SGC7901/VCR和SGC7901/ADR耐药细胞株总共表达27833条lncRNA, 差异表达1637条(差异倍数≥2.0): 其中638条为表达上调, 999条为表达下调; 上调最高的差异倍数为146, 下调最大的差异倍数为59. 通路分析显示, 有20条通路与lncRNA转录物的上调相关, 15条通路与lncRNA转录物的下调相关. 张哲[103]于2015年用高通量表达谱芯片筛选SGC7901/ADR和SGC7901/VCR相对于SGC7901的差异表达基因, 发现了一大批差异表达的lncRNA和miRNA分子. SGC7901/ADR细胞和SGC7901/VCR细胞相对于SGC7901细胞lncRNA分子表达上调4倍以上的分别有1811个和1571个, 其中有683个交集. SGC7901/ADR细胞和SGC7901/VCR细胞相对于SGC7901细胞lncRNA分子表达下调4倍以上的分别有2080个和1693个, 其中有885个交集. lncRNA分子在两株耐药细胞表达上调10倍以上的共有19个; 用qRT-PCR验证了从其中选出的10个lncRNA分子的表达水平, 结果显示, 所验证的分子都在两株耐药细胞中显著表达上调.
王颖[101]用高通量lncRNA芯片等技术筛选分析出胃癌耐药细胞株SGC7901/ADR和SGC7901/VCR与亲本细胞SGC7901差异表达最大、最可能介导胃癌多药耐药的lncRNA分子DMTF1v4后, 进一步研究发现, DMTF1v4在原发性胃腺癌组织中的表达水平与癌组织在体外培养时对化疗药物的敏感率呈负相关; 用siRNA下调DMTF1v4在SGC7901/ADR细胞和SGC7901/VCR细胞中的表达可以增加这些细胞对ABCB1蛋白相关化疗药物(如阿霉素、长春新碱、紫杉醇)的敏感度. 阿霉素蓄积潴留实验显示, DMTF1v4表达水平的下调能使SGC7901/ADR中阿霉素的蓄积比例增加、外排比例减少. Western blot检测显示, 下调DMTF1v4表达能使SGC7901/ADR细胞和SGC7901/VCR细胞的ABCB1蛋白表达下调, 而多药耐药相关蛋白MRP的表达没有显著变化. 裸鼠皮下异位移植瘤实验表明, DMTF1v4下调组肿瘤细胞的增殖被抑制. 在SGC7901/ADR细胞中下调DMTF1v4的表达后, ABCB1蛋白表达水平也随之显著下降. 双荧光素酶报告基因分析显示, DMTF1v4是通过增强子样作用而促进ABCB1蛋白的表达. CASC9(Cancer Susceptibility Candidate 9)是由染色体8q21.11编码的一种lncRNA, Shang等[104]用微阵列技术发现, CASC9基因在胃癌组织中的表达比正常胃癌组织高出近8倍, 进一步的研究发现, 敲低CASC9基因后可以恢复多药耐药胃癌细胞BGC823/DR和SGC7901/DR对化疗药紫杉醇和阿霉素的敏感性, 该作用与ABCB1蛋白的低表达相关. Lan等[105]研究了lncRNA ANRIL(antisense non-coding RNA in the INK4 locus)在胃癌多药耐药中的作用, 结果发现, ANRIL在对顺铂和5-Fu耐药的胃癌组织中呈高表达、在对顺铂和5-Fu耐药的胃癌细胞(BGC823/DDP和BGC823/5-Fu)中亦呈高表达, 用ANRIL siRNA转染BGC823/DDP细胞和BGC823/5-Fu细胞后再分别用顺铂和5-Fu处理, 可以降低细胞存活率和侵袭力并增加细胞凋亡率, 用IC50分析发现, ANRIL敲低或沉默的BGC823/DDP细胞和BGC823/5-Fu细胞对顺铂和5-Fu的敏感性增加, 用qRT-PCR和Western blotting检测发现, ANRIL基因敲低的BGC823/DDP细胞和BGC823/5-Fu细胞中ABCB1蛋白呈低表达, 回归分析显示, ANRIL基因的表达和ABCB1基因的表达呈正相关. MRUL(MDR-related and upregulated lncRNA)是位于ABCB1基因下游400 kb处的lncRNA基因, Wang等[106]发现, MRUL能通过增强子样作用而上调阿霉素耐药细胞SGC7901/ADR和长春新碱耐药细胞SGC7901/VCR中ABCB1基因的表达. Hang等[107]的研究发现, 在对顺铂耐药的两株胃癌细胞(SGC7901/DDP和BGC823/DDP)中, Notch 1基因呈高表达, 向不耐药的亲本细胞(SGC7901和BGC823)中转染Notch 1过表达的载体质粒, 可使细胞中Notch 1基因高度表达; 同时发现, 这两种细胞中ABCB1蛋白和ABCC1蛋白亦呈高表达, 而细胞凋亡受抑制; 经筛选发现lncRNA AK022798参与了这一过程, 用siRNA干扰AK022798的表达可上调ABCB1蛋白和ABCC1蛋白的表达并增强细胞凋亡, 故我们认为, 是Notch 1的高表达促进了AK022798的高表达从而导致了SGC7901/DDP细胞和BGC823/DDP细胞的耐药.
张哲[103]在SGC7901/ADR和SGC7901/VCR两株耐药细胞筛选出19个表达上调10倍以上的lncRNA分子, 用qRT-PCR验证显示, 其中表达升高幅度最大的两个依次是AK127463和UCA1, 但由于AK127463的干扰效果较差, 所以选取了UCA1做进一步研究. 在临床样本中检测UCA1的表达发现, UCA1在胃癌组织中的表达水平显著高于癌旁正常组织. UCA1可显著促进胃癌细胞对化疗药阿霉素、5-Fu和顺铂产生耐药, 该作用与癌细胞的抗凋亡能力增强相关; 下调UCA1表达可以逆转SGC7901/ADR细胞对化疗药的耐药性; 裸鼠移植瘤试验亦显示, 下调UCA1表达能够显著地增加胃癌细胞对化疗药的敏感性. 荧光原位杂交和胞浆胞核分离实验证实, UCA1主要分布在细胞浆, 细胞核中有少量分布, 提示UCA1主要是在转录后水平发挥其调节作用. UCA1序列中包含有let-7家族miRNA的结合位点, 其中以let-7e结合位点评分最高. Let-7e可降低UCA1双荧光素酶报告基因的荧光素信号强度, UCA1上let-7e结合位点的突变可部分抑制这一效应, 表明UCA1可与let-7e直接结合、通过竞争内源性RNA结合let-7e而发挥转录后调节作用. HMGA2是let-7e的下游靶分子. 在胃癌耐药细胞let-7e表达降低而HMGA2表达升高. 过表达let-7e或干扰HMGA2能降低阿霉素、5-Fu和顺铂对胃癌耐药细胞的IC50, 并可使药物诱导的细胞凋亡增加. Let-7e可以负调控HMGA2蛋白的表达, 说明HMGA2是Let-7e的下游靶分子. UCA1可以促进HMGA2蛋白表达, 该作用依赖于结合let-7, let-7结合位点突变可以抑制这一作用. 综上所述, lncRNA UCA1通过与let-7 miRNA家族(主要是let-7e)结合, 降低let-7对耐药相关基因HMGA2的抑制作用, 使HMGA2的表达升高, 通过抗凋亡作用而促进胃癌细胞的多药耐药. Shang等[108]研究了lncRNA UCA1对胃癌对阿霉素敏感性的影响, 结果发现, UCA1在胃癌中高表达, 沉默UCA1能够抑制Bcl-2表达并促进细胞凋亡. Zhang等[109]发现, lncRNA NEAT1(Nuclear-enriched abundant transcript 1)在阿霉素耐药胃癌细胞中表达升高, 沉默NEAT1能够增加SGC7901/ADR对阿霉素的敏感性、促进阿霉素诱导的细胞凋亡. Yan等[110]发现lncRNA HOTAIR(HOX antisense intergenic RNA)在对顺铂耐药的胃癌细胞和组织中明显上调, 过表达HOTAIR能增强细胞增殖、减低细胞凋亡, 并发现HOTAIR通过直接结合于并抑制miR-126、促进VEGFA和PIK3R2表达并活化PI3K/AKT/ABCC1通路而介导胃癌细胞对顺铂的耐药. Zhang等[111]报道了lncRNA GHET1过表达在促进胃癌多药耐药形成中的作用. 他们发现, GHET1在耐药胃癌患者和耐顺铂胃癌细胞(BGC823/DPP和SGC7901/DDP细胞系)中表达升高, 沉默GHET1能够抑制BGC823/DPP细胞和SGC7901/DDP细胞的耐药, 转染GHET1 siRNA能显著地降低这两株顺铂耐药细胞的IC50. GHET1在亲代细胞BGC823和SGC7901中过表达能够降低细胞对顺铂的敏感性并减低细胞凋亡率, qRT-PCR和western blot检测发现, GHET1的过表达下调了BGC823和SGC7901细胞中Bax表达、上调了Bcl-2表达并上调了ABCB1和ABCC1表达. Zhang等[112]还发现, lncRNA PVT-1(Plasmacytoma variant translocation 1)在顺铂耐药胃癌患者和顺铂耐药胃癌细胞BGC823/DDP和SGC7901/DDP中呈高表达, 用PVT-1 siRNA转染BGC823/DDP和SGC7901/DDP再用顺铂处理, 可见细胞存活率显著下降、细胞凋亡率显著升高; 此外, 用qRT-PCR和Western blot检测发现, PVT1的上调还能增加ABCB1和MRP等的表达.
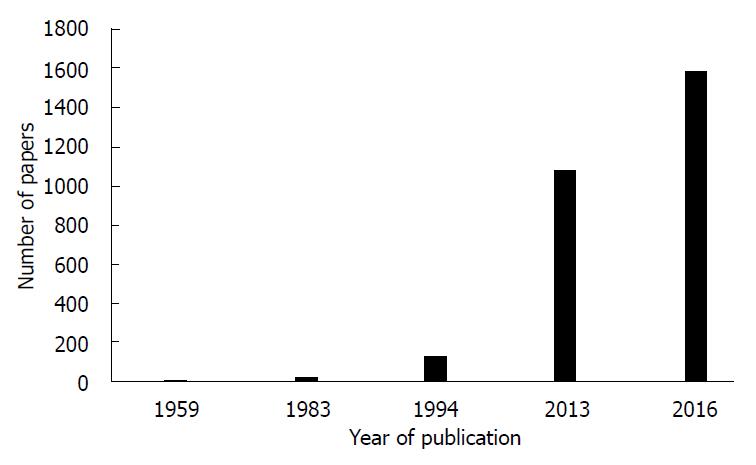
肿瘤多药耐药是肿瘤药物治疗的一大障碍. 对肿瘤对化疗药耐药的认识已经有数十年的历史[113-119](图1, 表1), 并对耐药机制提出了数种假说, 但耐药的机制迄今尚未完全明了[120]. 近几年来, lncRNA在肿瘤多药耐药形成中的调节作用被受到重视, 相关领域研究论文的数量迅速增加. 对肿瘤多药耐药机制的深入理解将有助于我们采取相应的措施应对或逆转耐药; 临床医生在选择化学治疗药物之前若能对癌细胞对相应化疗药的敏感性做一测试, 应该非常有助于合理选择药物和改善治疗效果.
| 年代范围 | 论文数范围 |
| 1956-1982 | 1-9 |
| 1983-1993 | 23-93 |
| 1994-2012 | 126-952 |
| 2013-2016 | 1081-1585 |
对肿瘤对化疗药耐药的认识已经有数十年的历史, 并对耐药机制提出了数种假说, 但耐药的机制尚未完全明了.
长链非编RNA和microRNA调节是近年来肿瘤多药耐药机制研究的热点.
本文对胃癌多药耐药最重要的两种机制(ABC转运蛋白和细胞凋亡)以及长链非编RNA和microRNA的调节作用近年来研究进展作了总结论述.
临床医生在选择化学治疗药物之前若能对患者癌细胞对相应化疗药的敏感性做一测试, 应该非常有助于合理选择药物和优化治疗效果.
范辉, 副教授, 主任医师, 江苏省南通市第二人民医院消化科; 李琦, 主任医师, 上海中医药大学附属普陀医院肿瘤科; 杨秋蒙, 副主任医师, 上海交通大学医学院附属瑞金医院普外科; 朱永良, 副研究员, 浙江大学医学院附属第二医院消化病学
该综述试图从P-糖蛋白, 细胞凋亡以及长链非编码RNA三个角度探讨在胃癌多药耐药的发生机制. 文章具有一定的新颖. 此外, 论文的逻辑性强, 有较多作者的见解和评论. 对胃癌耐药研究的相关学者、研究人员的研究工作具有参考价值.
手稿来源: 邀请约稿
学科分类: 胃肠病学和肝病学
手稿来源地: 陕西省
同行评议报告分类
A级 (优秀): A, A
B级 (非常好): 0
C级 (良好): C, C
D级 (一般): 0
E级 (差): 0
编辑: 马亚娟 电编:杜冉冉
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [PubMed] [DOI] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [PubMed] [DOI] |
| 3. | Zhang D, Fan D. Multidrug resistance in gastric cancer: recent research advances and ongoing therapeutic challenges. Expert Rev Anticancer Ther. 2007;7:1369-1378. [PubMed] [DOI] |
| 4. | Zhang D, Fan D. New insights into the mechanisms of gastric cancer multidrug resistance and future perspectives. Future Oncol. 2010;6:527-537. [PubMed] [DOI] |
| 5. | Long S, Sousa E, Kijjoa A, Pinto MM. Marine Natural Products as Models to Circumvent Multidrug Resistance. Molecules. 2016;21:pii: E892. [PubMed] [DOI] |
| 6. | Yan LH, Wang XT, Yang J, Lian C, Kong FB, Wei WY, Luo W, Xiao Q, Xie YB. Reversal of multidrug resistance in gastric cancer cells by CDX2 downregulation. World J Gastroenterol. 2013;19:4155-4165. [PubMed] [DOI] |
| 7. | Huang GL, Shen DY, Cai CF, Zhang QY, Ren HY, Chen QX. β-escin reverses multidrug resistance through inhibition of the GSK3β/β-catenin pathway in cholangiocarcinoma. World J Gastroenterol. 2015;21:1148-1157. [PubMed] [DOI] |
| 8. | Andersen V, Svenningsen K, Knudsen LA, Hansen AK, Holmskov U, Stensballe A, Vogel U. Novel understanding of ABC transporters ABCB1/MDR/P-glycoprotein, ABCC2/MRP2, and ABCG2/BCRP in colorectal pathophysiology. World J Gastroenterol. 2015;21:11862-11876. [PubMed] [DOI] |
| 9. | Stolarczyk EI, Reiling CJ, Paumi CM. Regulation of ABC transporter function via phosphorylation by protein kinases. Curr Pharm Biotechnol. 2011;12:621-635. [PubMed] [DOI] |
| 10. | Srinivasan S, Bingham JL, Johnson D. The ABCs of human alternative splicing: a review of ATP-binding cassette transporter splicing. Curr Opin Drug Discov Devel. 2009;12:149-158. [PubMed] |
| 11. | Hollenstein K, Dawson RJ, Locher KP. Structure and mechanism of ABC transporter proteins. Curr Opin Struct Biol. 2007;17:412-418. [PubMed] [DOI] |
| 12. | Jones PM, George AM. The ABC transporter structure and mechanism: perspectives on recent research. Cell Mol Life Sci. 2004;61:682-699. [PubMed] [DOI] |
| 13. | Frelet A, Klein M. Insight in eukaryotic ABC transporter function by mutation analysis. FEBS Lett. 2006;580:1064-1084. [PubMed] [DOI] |
| 14. | El-Awady R, Saleh E, Hashim A, Soliman N, Dallah A, Elrasheed A, Elakraa G. The Role of Eukaryotic and Prokaryotic ABC Transporter Family in Failure of Chemotherapy. Front Pharmacol. 2017;7:535. [PubMed] [DOI] |
| 15. | Li W, Zhang H, Assaraf YG, Zhao K, Xu X, Xie J, Yang DH, Chen ZS. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist Updat. 2016;27:14-29. [PubMed] [DOI] |
| 16. | Cui H, Zhang AJ, Chen M, Liu JJ. ABC Transporter Inhibitors in Reversing Multidrug Resistance to Chemotherapy. Curr Drug Targets. 2015;16:1356-1371. [PubMed] [DOI] |
| 17. | Kathawala RJ, Gupta P, Ashby CR Jr, Chen ZS. The modulation of ABC transporter-mediated multidrug resistance in cancer: a review of the past decade. Drug Resist Updat. 2015;18:1-17. [PubMed] [DOI] |
| 18. | Shukla S, Chen ZS, Ambudkar SV. Tyrosine kinase inhibitors as modulators of ABC transporter-mediated drug resistance. Drug Resist Updat. 2012;15:70-80. [PubMed] [DOI] |
| 19. | Chung FS, Santiago JS, Jesus MF, Trinidad CV, See MF. Disrupting P-glycoprotein function in clinical settings: what can we learn from the fundamental aspects of this transporter? Am J Cancer Res. 2016;6:1583-1598. [PubMed] |
| 20. | Higgins CF, Callaghan R, Linton KJ, Rosenberg MF, Ford RC. Structure of the multidrug resistance P-glycoprotein. Semin Cancer Biol. 1997;8:135-142. [PubMed] [DOI] |
| 21. | Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718-1722. [PubMed] [DOI] |
| 22. | Ferreira RJ, dos Santos DJ, Ferreira MJ. P-glycoprotein and membrane roles in multidrug resistance. Future Med Chem. 2015;7:929-946. [PubMed] [DOI] |
| 23. | Chufan EE, Sim HM, Ambudkar SV. Molecular basis of the polyspecificity of P-glycoprotein (ABCB1): recent biochemical and structural studies. Adv Cancer Res. 2015;125:71-96. [PubMed] [DOI] |
| 24. | Xu HW, Xu L, Hao JH, Qin CY, Liu H. Expression of P-glycoprotein and multidrug resistance-associated protein is associated with multidrug resistance in gastric cancer. J Int Med Res. 2010;38:34-42. [PubMed] [DOI] |
| 25. | Hu WQ, Peng CW, Li Y. The expression and significance of P-glycoprotein, lung resistance protein and multidrug resistance-associated protein in gastric cancer. J Exp Clin Cancer Res. 2009;28:144. [PubMed] [DOI] |
| 26. | Shi H, Lu D, Shu Y, Shi W, Lu S, Wang K. Expression of multidrug-resistance-related proteins P-glycoprotein, glutathione-S-transferases, topoisomerase-II and lung resistance protein in primary gastric cardiac adenocarcinoma. Cancer Invest. 2008;26:344-351. [PubMed] [DOI] |
| 27. | Lu C, Shan Z, Li C, Yang L. MiR-129 regulates cisplatin-resistance in human gastric cancer cells by targeting P-gp. Biomed Pharmacother. 2017;86:450-456. [PubMed] [DOI] |
| 28. | Zhao X, Yang L, Hu J. Down-regulation of miR-27a might inhibit proliferation and drug resistance of gastric cancer cells. J Exp Clin Cancer Res. 2011;30:55. [PubMed] [DOI] |
| 29. | 刘 东晓. JAK2基因多态性与胃癌风险相关性研究及miR-27a对胃癌细胞生长的影响. 江苏: 南京医科大学 2012; pp33-55. |
| 30. | Wang F, Li T, Zhang B, Li H, Wu Q, Yang L, Nie Y, Wu K, Shi Y, Fan D. MicroRNA-19a/b regulates multidrug resistance in human gastric cancer cells by targeting PTEN. Biochem Biophys Res Commun. 2013;434:688-694. [PubMed] [DOI] |
| 31. | Shang Y, Zhang Z, Liu Z, Feng B, Ren G, Li K, Zhou L, Sun Y, Li M, Zhou J. miR-508-5p regulates multidrug resistance of gastric cancer by targeting ABCB1 and ZNRD1. Oncogene. 2014;33:3267-3276. [PubMed] [DOI] |
| 32. | Shang Y, Feng B, Zhou L, Ren G, Zhang Z, Fan X, Sun Y, Luo G, Liang J, Wu K. The miR27b-CCNG1-P53-miR-508-5p axis regulates multidrug resistance of gastric cancer. Oncotarget. 2016;7:538-549. [PubMed] [DOI] |
| 33. | Chen M, Li D, Gong N, Wu H, Su C, Xie C, Xiang H, Lin C, Li X. miR-133b down-regulates ABCC1 and enhances the sensitivity of CRC to anti-tumor drugs. Oncotarget. 2017; May 8. [Epub ahead of print]. [PubMed] [DOI] |
| 34. | Ocelotl J, Sánchez J, Gómez I, Tabashnik BE, Bravo A, Soberón M. ABCC2 is associated with Bacillus thuringiensis Cry1Ac toxin oligomerization and membrane insertion in diamondback moth. Sci Rep. 2017;7:2386. [PubMed] [DOI] |
| 35. | Ideozu JE, Zhang X, Pan A, Ashrafi Z, Woods KJ, Hessner MJ, Simpson P, Levy H. Increased Expression of Plasma-Induced ABCC1 mRNA in Cystic Fibrosis. Int J Mol Sci. 2017;18:pii: E1752. [PubMed] [DOI] |
| 36. | Cao Z, Liang N, Yang H, Li S. Visfatin mediates doxorubicin resistance in human non-small-cell lung cancer via Akt-mediated up-regulation of ABCC1. Cell Prolif. 2017;50. [PubMed] [DOI] |
| 37. | Cao D, Qin S, Mu Y, Zhong M. The role of MRP1 in the multidrug resistance of colorectal cancer. Oncol Lett. 2017;13:2471-2476. [PubMed] [DOI] |
| 38. | Ling S, Li J, Shan Q, Dai H, Lu D, Wen X, Song P, Xie H, Zhou L, Liu J. USP22 mediates the multidrug resistance of hepatocellular carcinoma via the SIRT1/AKT/MRP1 signaling pathway. Mol Oncol. 2017;11:682-695. [PubMed] [DOI] |
| 39. | Ding J, Zhou XT, Zou HY, Wu J. Hedgehog signaling pathway affects the sensitivity of hepatoma cells to drug therapy through the ABCC1 transporter. Lab Invest. 2017;97:819-832. [PubMed] [DOI] |
| 40. | Au A, Baba AA, Azlan H, Norsa'adah B, Ankathil R. Clinical impact of ABCC1 and ABCC2 genotypes and haplotypes in mediating imatinib resistance among chronic myeloid leukaemia patients. J Clin Pharm Ther. 2014;39:685-690. [PubMed] [DOI] |
| 41. | Herraez E, Sanchez-Vicente L, Macias RIR, Briz O, Marin JJG. Usefulness of the MRP2 promoter to overcome the chemoresistance of gastrointestinal and liver tumors by enhancing the expression of the drug transporter OATP1B1. Oncotarget. 2017;8:34617-34629. [PubMed] [DOI] |
| 42. | Wang Z, Sun X, Feng Y, Liu X, Zhou L, Sui H, Ji Q, E Q, Chen J, Wu L. Dihydromyricetin reverses MRP2-mediated MDR and enhances anticancer activity induced by oxaliplatin in colorectal cancer cells. Anticancer Drugs. 2017;28:281-288. [PubMed] [DOI] |
| 43. | Tian J, Xu YY, Li L, Hao Q. MiR-490-3p sensitizes ovarian cancer cells to cisplatin by directly targeting ABCC2. Am J Transl Res. 2017;9:1127-1138. [PubMed] |
| 44. | Chen X, Zhang M, Liu LX. The overexpression of multidrug resistance-associated proteins and gankyrin contribute to arsenic trioxide resistance in liver and gastric cancer cells. Oncol Rep. 2009;22:73-80. [PubMed] |
| 45. | Yu P, Du Y, Yang L, Fan S, Wu J, Zheng S. Significance of multidrug resistance gene-related proteins in the postoperative chemotherapy of gastric cancer. Int J Clin Exp Pathol. 2014;7:7945-7950. [PubMed] |
| 47. | Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan Y, Wang M, Zhu W, Qian H, Xu W. Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell Cycle. 2015;14:2473-2483. [PubMed] [DOI] |
| 48. | Takegawa N, Nonagase Y, Yonesaka K, Sakai K, Maenishi O, Ogitani Y, Tamura T, Nishio K, Nakagawa K, Tsurutani J. DS-8201a, a new HER2-targeting antibody-drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int J Cancer. 2017;141:1682-1689. [PubMed] [DOI] |
| 49. | Taylor NMI, Manolaridis I, Jackson SM, Kowal J, Stahlberg H, Locher KP. Structure of the human multidrug transporter ABCG2. Nature. 2017;546:504-509. [PubMed] [DOI] |
| 50. | Kim JB, Hwang SE, Yoon SP. Dexamethasone reduces side population fraction through downregulation of ABCG2 transporter in MCF-7 breast cancer cells. Mol Med Rep. 2017;16:453-458. [PubMed] [DOI] |
| 51. | Ishikawa T, Kajimoto Y, Sun W, Nakagawa H, Inoue Y, Ikegami Y, Miyatake S, Kuroiwa T. Role of Nrf2 in cancer photodynamic therapy: regulation of human ABC transporter ABCG2. J Pharm Sci. 2013;102:3058-3069. [PubMed] [DOI] |
| 52. | Ishikawa T, Nakagawa H. Human ABC transporter ABCG2 in cancer chemotherapy and pharmacogenomics. J Exp Ther Oncol. 2009;8:5-24. [PubMed] |
| 53. | Horsey AJ, Cox MH, Sarwat S, Kerr ID. The multidrug transporter ABCG2: still more questions than answers. Biochem Soc Trans. 2016;44:824-830. [PubMed] [DOI] |
| 54. | Hasanabady MH, Kalalinia F. ABCG2 inhibition as a therapeutic approach for overcoming multidrug resistance in cancer. J Biosci. 2016;41:313-324. [PubMed] [DOI] |
| 55. | Noguchi K, Katayama K, Sugimoto Y. Human ABC transporter ABCG2/BCRP expression in chemoresistance: basic and clinical perspectives for molecular cancer therapeutics. Pharmgenomics Pers Med. 2014;7:53-64. [PubMed] [DOI] |
| 56. | Schnepf R, Zolk O. Effect of the ATP-binding cassette transporter ABCG2 on pharmacokinetics: experimental findings and clinical implications. Expert Opin Drug Metab Toxicol. 2013;9:287-306. [PubMed] [DOI] |
| 57. | Kawabata KC, Hayashi Y, Inoue D, Meguro H, Sakurai H, Fukuyama T, Tanaka Y, Asada S, Fukushima T, Nagase R. High expression of ABCG2 induced by EZH2 disruption has pivotal roles in MDS pathogenesis. Leukemia. 2017; Jul. [Epub ahead of print]. [PubMed] [DOI] |
| 58. | Gantner ME, Peroni RN, Morales JF, Villalba ML, Ruiz ME, Talevi A. Development and Validation of a Computational Model Ensemble for the Early Detection of BCRP/ABCG2 Substrates during the Drug Design Stage. J Chem Inf Model. 2017;57:1868-1880. [PubMed] [DOI] |
| 59. | Wang J, Yunyun Z, Wang L, Chen X, Zhu Z. ABCG2 confers promotion in gastric cancer through modulating downstream CRKL in vitro combining with biostatistics mining. Oncotarget. 2017;8:5256-5267. [PubMed] [DOI] |
| 60. | Zhang L, Guo X, Zhang D, Fan Y, Qin L, Dong S, Zhang L. Upregulated miR-132 in Lgr5<sup>+</sup> gastric cancer stem cell-like cells contributes to cisplatin-resistance via SIRT1/CREB/ABCG2 signaling pathway. Mol Carcinog. 2017;56:2022-2034. [PubMed] [DOI] |
| 61. | Zhao L, Pan Y, Gang Y, Wang H, Jin H, Tie J, Xia L, Zhang Y, He L, Yao L. Identification of GAS1 as an epirubicin resistance-related gene in human gastric cancer cells with a partially randomized small interfering RNA library. J Biol Chem. 2009;284:26273-26285. [PubMed] [DOI] |
| 62. | Chang CH, Wang Y, Zalath M, Liu D, Cardillo TM, Goldenberg DM. Combining ABCG2 Inhibitors with IMMU-132, an Anti-Trop-2 Antibody Conjugate of SN-38, Overcomes Resistance to SN-38 in Breast and Gastric Cancers. Mol Cancer Ther. 2016;15:1910-1919. [PubMed] [DOI] |
| 63. | Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153-164. [PubMed] [DOI] |
| 64. | Ahmad A, Sakr WA, Rahman KM. Anticancer properties of indole compounds: mechanism of apoptosis induction and role in chemotherapy. Curr Drug Targets. 2010;11:652-666. [PubMed] [DOI] |
| 65. | Hickman JA. Apoptosis and chemotherapy resistance. Eur J Cancer. 1996;32A:921-926. [PubMed] [DOI] |
| 66. | Pommier Y, Sordet O, Antony S, Hayward RL, Kohn KW. Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene. 2004;23:2934-2949. [PubMed] [DOI] |
| 67. | Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798-4811. [PubMed] [DOI] |
| 68. | Fu ZY, Han XD, Wang AH, Liu XB. Apoptosis of human gastric carcinoma cells induced by Euphorbia esula latex. World J Gastroenterol. 2016;22:3564-3572. [PubMed] [DOI] |
| 69. | Gao F, Fu Z, Tian H, He Z. The Euphorbia lunulata Bge extract inhibits proliferation of human hepatoma HepG2 cells and induces apoptosis. J BUON. 2013;18:491-495. [PubMed] |
| 70. | Dasgupta A, Nomura M, Shuck R, Yustein J. Cancer's Achilles' Heel: Apoptosis and Necroptosis to the Rescue. Int J Mol Sci. 2016;18:pii: E23. [PubMed] [DOI] |
| 71. | Aung LHH, Li R, Prabhakar BS, Maker AV, Li P. Mitochondrial protein 18 (MTP18) plays a pro-apoptotic role in chemotherapy-induced gastric cancer cell apoptosis. Oncotarget. 2017; Apr 28. [Epub ahead of print]. [DOI] |
| 72. | Liang W, Cai A, Chen G, Xi H, Wu X, Cui J, Zhang K, Zhao X, Yu J, Wei B. Shikonin induces mitochondria-mediated apoptosis and enhances chemotherapeutic sensitivity of gastric cancer through reactive oxygen species. Sci Rep. 2016;6:38267. [PubMed] [DOI] |
| 73. | Tang QF, Sun J, Yu H, Shi XJ, Lv R, Wei HC, Yin PH. The Zuo Jin Wan Formula Induces Mitochondrial Apoptosis of Cisplatin-Resistant Gastric Cancer Cells via Cofilin-1. Evid Based Complement Alternat Med. 2016;2016:8203789. [PubMed] |
| 74. | Yin F, Shi YQ, Zhao WP, Xiao B, Miao JY, Fan DM. Suppression of P-gp induced multiple drug resistance in a drug resistant gastric cancer cell line by overexpression of Fas. World J Gastroenterol. 2000;6:664-670. [PubMed] [DOI] |
| 75. | Zhang X, Peng X, Yu W, Hou S, Zhao Y, Zhang Z, Huang X, Wu K. Alpha-tocopheryl succinate enhances doxorubicin-induced apoptosis in human gastric cancer cells via promotion of doxorubicin influx and suppression of doxorubicin efflux. Cancer Lett. 2011;307:174-181. [PubMed] [DOI] |
| 76. | Lim SC, Han SI. Ursodeoxycholic acid effectively kills drug-resistant gastric cancer cells through induction of autophagic death. Oncol Rep. 2015;34:1261-1268. [PubMed] [DOI] |
| 77. | Li BP, Liu JL, Chen JQ, Wang Z, Mao YT, Chen YY. Effects of siRNA-mediated silencing of myeloid cell leukelia-1 on the biological behaviors and drug resistance of gastric cancer cells. Am J Transl Res. 2015;7:2397-2411. [PubMed] |
| 78. | Na YJ, Lee DH, Kim JL, Kim BR, Park SH, Jo MJ, Jeong S, Kim HJ, Lee SY, Jeong YA. Cyclopamine sensitizes TRAIL-resistant gastric cancer cells to TRAIL-induced apoptosis via endoplasmic reticulum stress-mediated increase of death receptor 5 and survivin degradation. Int J Biochem Cell Biol. 2017;89:147-156. [PubMed] [DOI] |
| 79. | Fan L, Tan B, Li Y, Zhao Q, Liu Y, Wang D, Zhang Z. Silencing of ZNF139-siRNA induces apoptosis in human gastric cancer cell line BGC823. Int J Clin Exp Pathol. 2015;8:12428-12436. [PubMed] |
| 80. | 周 露婷, 王 翠翠, 景 洪标, 李 培峰, 耿 明. Survivin和Bcl-2蛋白在胃癌细胞中的表达及其与化疗药物耐药性关系的研究. 现代肿瘤医学. 2014;22:1627-1629. |
| 81. | Ji N, Yu JW, Ni XC, Wu JG, Wang SL, Jiang BJ. Bone marrow-derived mesenchymal stem cells increase drug resistance in CD133-expressing gastric cancer cells by regulating the PI3K/AKT pathway. Tumour Biol. 2016;37:14637-14651. [PubMed] [DOI] |
| 82. | Zhao Q, Tan BB, Li Y, Fan LQ, Yang PG, Tian Y. Enhancement of Drug Sensitivity by Knockdown of HIF-1α in Gastric Carcinoma Cells. Oncol Res. 2016;23:129-136. [PubMed] [DOI] |
| 83. | Xu GY, Tang XJ. Troxerutin (TXN) potentiated 5-Fluorouracil (5-Fu) treatment of human gastric cancer through suppressing STAT3/NF-κB and Bcl-2 signaling pathways. Biomed Pharmacother. 2017;92:95-107. [PubMed] [DOI] |
| 84. | Chen Y, Zuo J, Liu Y, Gao H, Liu W. Inhibitory effects of miRNA-200c on chemotherapy-resistance and cell proliferation of gastric cancer SGC7901/DDP cells. Chin J Cancer. 2010;29:1006-1011. [PubMed] [DOI] |
| 85. | 智 慧, 朱 伟, 王 同杉, 王 建, 束 永前, 刘 平. miR-125b靶向抑制BCL2、MCL1表达对胃癌SGC7901/VCR细胞多药耐药性的影响. 南京医科大学学报(自然科学版). 2011;31:777-782. |
| 86. | Wang T, Ge G, Ding Y, Zhou X, Huang Z, Zhu W, Shu Y, Liu P. MiR-503 regulates cisplatin resistance of human gastric cancer cell lines by targeting IGF1R and BCL2. Chin Med J (Engl). 2014;127:2357-2362. [PubMed] |
| 87. | Zhuang M, Shi Q, Zhang X, Ding Y, Shan L, Shan X, Qian J, Zhou X, Huang Z, Zhu W. Involvement of miR-143 in cisplatin resistance of gastric cancer cells via targeting IGF1R and BCL2. Tumour Biol. 2015;36:2737-2745. [PubMed] [DOI] |
| 88. | Wang Y, Liu L, Liu X, Zhang H, Liu J, Feng B, Shang Y, Zhou L, Wu K, Nie Y. Shugoshin1 enhances multidrug resistance of gastric cancer cells by regulating MRP1, Bcl-2, and Bax genes. Tumour Biol. 2013;34:2205-2214. [PubMed] [DOI] |
| 89. | Zhao Q, Li Y, Tan BB, Fan LQ, Yang PG, Tian Y. HIF-1α Induces Multidrug Resistance in Gastric Cancer Cells by Inducing MiR-27a. PLoS One. 2015;10:e0132746. [PubMed] [DOI] |
| 90. | Fang Q, Chen X, Zhi X. Long Non-Coding RNA (LncRNA) Urothelial Carcinoma Associated 1 (UCA1) Increases Multi-Drug Resistance of Gastric Cancer via Downregulating miR-27b. Med Sci Monit. 2016;22:3506-3513. [PubMed] [DOI] |
| 91. | You HY, Xie XM, Zhang WJ, Zhu HL, Jiang FZ. Berberine modulates cisplatin sensitivity of human gastric cancer cells by upregulation of miR-203. In Vitro Cell Dev Biol Anim. 2016;52:857-863. [PubMed] [DOI] |
| 92. | 赵 梦雅, 陈 虹羽, 刘 月, 汪 凯, 张 晓丹, 张 亚飞. miR-29通过调节抗凋亡蛋白Mcl-1表达参与胃癌细胞多药耐药的发生. 世界华人消化杂志. 2016;24:4781-4787. [DOI] |
| 93. | Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155-159. [PubMed] [DOI] |
| 94. | Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15:7-21. [PubMed] [DOI] |
| 95. | Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391-407. [PubMed] [DOI] |
| 97. | Yang ZG, Gao L, Guo XB, Shi YL. Roles of long non-coding RNAs in gastric cancer metastasis. World J Gastroenterol. 2015;21:5220-5230. [PubMed] [DOI] |
| 98. | Zhao J, Liu Y, Huang G, Cui P, Zhang W, Zhang Y. Long non-coding RNAs in gastric cancer: versatile mechanisms and potential for clinical translation. Am J Cancer Res. 2015;5:907-927. [PubMed] |
| 99. | Huang YK, Yu JC. Circulating microRNAs and long non-coding RNAs in gastric cancer diagnosis: An update and review. World J Gastroenterol. 2015;21:9863-9886. [PubMed] [DOI] |
| 100. | Chen QN, Wei CC, Wang ZX, Sun M. Long non-coding RNAs in anti-cancer drug resistance. Oncotarget. 2017;8:1925-1936. [PubMed] |
| 101. | 王 颖. 长链非编码RNA DMTF1v4(NR_024549)在胃癌多药耐药中的作用及机制研究. 陕西省: 第四军医大学 2012; 1-121. |
| 102. | Wang Y, Wu K, Yang Z, Zhao Q, Fan D, Xu P, Nie Y, Fan D. Multidrug-Resistance Related Long Non-Coding RNA Expression Profile Analysis of Gastric Cancer. PLoS One. 2015;10:e0135461. [PubMed] [DOI] |
| 103. | 张 哲. 长链非编码RNA分子UCA1介导胃癌多药耐药的机制研究. 陕西省: 第四军医大学 2015; 1-72. |
| 104. | Shang C, Sun L, Zhang J, Zhao B, Chen X, Xu H, Huang B. Silence of cancer susceptibility candidate 9 inhibits gastric cancer and reverses chemoresistance. Oncotarget. 2017;8:15393-15398. [PubMed] [DOI] |
| 105. | Lan WG, Xu DH, Xu C, Ding CL, Ning FL, Zhou YL, Ma LB, Liu CM, Han X. Silencing of long non-coding RNA ANRIL inhibits the development of multidrug resistance in gastric cancer cells. Oncol Rep. 2016;36:263-270. [PubMed] [DOI] |
| 106. | Wang Y, Zhang D, Wu K, Zhao Q, Nie Y, Fan D. Long noncoding RNA MRUL promotes ABCB1 expression in multidrug-resistant gastric cancer cell sublines. Mol Cell Biol. 2014;34:3182-3193. [PubMed] [DOI] |
| 107. | Hang Q, Sun R, Jiang C, Li Y. Notch 1 promotes cisplatin-resistant gastric cancer formation by upregulating lncRNA AK022798 expression. Anticancer Drugs. 2015;26:632-640. [PubMed] [DOI] |
| 108. | Shang C, Guo Y, Zhang J, Huang B. Silence of long noncoding RNA UCA1 inhibits malignant proliferation and chemotherapy resistance to adriamycin in gastric cancer. Cancer Chemother Pharmacol. 2016;77:1061-1067. [PubMed] [DOI] |
| 109. | Zhang J, Zhao B, Chen X, Wang Z, Xu H, Huang B. Silence of Long Noncoding RNA NEAT1 Inhibits Malignant Biological Behaviors and Chemotherapy Resistance in Gastric Cancer. Pathol Oncol Res. 2017; Apr 11. [Epub ahead of print]. [PubMed] |
| 110. | Yan J, Dang Y, Liu S, Zhang Y, Zhang G. LncRNA HOTAIR promotes cisplatin resistance in gastric cancer by targeting miR-126 to activate the PI3K/AKT/MRP1 genes. Tumour Biol. 2016; Nov 30. [Epub ahead of print]. [PubMed] [DOI] |
| 111. | Zhang X, Bo P, Liu L, Zhang X, Li J. Overexpression of long non-coding RNA GHET1 promotes the development of multidrug resistance in gastric cancer cells. Biomed Pharmacother. 2017;92:580-585. [PubMed] [DOI] |
| 112. | Zhang XW, Bu P, Liu L, Zhang XZ, Li J. Overexpression of long non-coding RNA PVT1 in gastric cancer cells promotes the development of multidrug resistance. Biochem Biophys Res Commun. 2015;462:227-232. [PubMed] [DOI] |
| 113. | LAW LW. Differences between cancers in terms of evolution of drug resistance. Cancer Res. 1956;16:698-716. [PubMed] |
| 114. | WELCH AD. The problem of drug resistance in cancer chemotherapy. Cancer Res. 1959;19:359-371. [PubMed] |
| 115. | HUTCHISON DJ. CROSS RESISTANCE AND COLLATERAL SENSITIVITY STUDIES IN CANCER CHEMOTHERAPY. Adv Cancer Res. 1963;7:235-250. [PubMed] [DOI] |
| 116. | Baskin F, Rosenberg RN, Dev V. Correlation of double-minute chromosomes with unstable multidrug cross-resistance in uptake mutants of neuroblastoma cells. Proc Natl Acad Sci USA. 1981;78:3654-3658. [PubMed] [DOI] |
| 117. | Kartner N, Riordan JR, Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983;221:1285-1288. [PubMed] [DOI] |
| 118. | Roninson IB, Chin JE, Choi KG, Gros P, Housman DE, Fojo A, Shen DW, Gottesman MM, Pastan I. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci USA. 1986;83:4538-4542. [PubMed] [DOI] |
| 119. | Leyland-Jones B, Dalton W, Fisher GA, Sikic BI. Reversal of multidrug resistance to cancer chemotherapy. Cancer. 1993;72:3484-3488. [PubMed] [DOI] |
| 120. | Xie Q, Wu MY, Zhang DX, Yang YM, Wang BS, Zhang J, Xu J, Zhong WD, Hu JN. Synergistic anticancer effect of exogenous wild-type p53 gene combined with 5-FU in human colon cancer resistant to 5-FU in vivo. World J Gastroenterol. 2016;22:7342-7352. [PubMed] [DOI] |