修回日期: 2017-05-01
接受日期: 2017-05-08
在线出版日期: 2017-07-18
肿瘤是威胁全世界人类健康和影响社会经济的重要因素. 近年来, 随着经济的发展, 肿瘤的发病率呈明显上升趋势, 但是其病因尚未完全阐明. 越来越多的证据显示肿瘤的发生和遗传因素有关, 随着病理生理学和遗传学的发展, 许多学者认为生物标志物可以预测癌症甚至指导临床治疗. 微小RNA(microRNA, miRNA)是非编码小分子RNA, 在发育、生理、病理过程以及肿瘤发生等环节中起着重要的调节作用. miR-17-92基因簇是研究较为深入、最有特点的miRNA, 被认为是原癌基因miRNA的代表, 在多种肿瘤的发生发展中起着至关重要的作用. 本文就miR-17-92基因簇在肿瘤发生发展中的作用及功能进行综述.
核心提要: miR-17-92基因簇与肿瘤的发生密切相关, 在伯基特淋巴瘤, 弥漫性扩增大B细胞淋巴瘤, 滤泡性淋巴瘤, 套细胞淋巴瘤和肺癌, 各种造血系统恶性肿瘤, 多个实体肿瘤中高表达, 本文阐述miR-17-92基因簇作为肿瘤预防和治疗的靶点研究进展.
引文著录: 孙瑞芬, 龚建瑜, 邹海舰, 张林, 高林波. miR-17-92基因簇在肿瘤发生发展中作用的研究进展. 世界华人消化杂志 2017; 25(20): 1840-1853
Revised: May 1, 2017
Accepted: May 8, 2017
Published online: July 18, 2017
Tumors are an important factor that threatens human health and affects social economy. Although tumor incidence is increasing, their etiology is still not completely clear. Growing evidence has identified that genetic factors contribute to tumor development. Advances in the understanding of tumor pathophysiology and genetics suggest that genetic biomarkers can predict the future presence of tumors and even direct the approach to therapy. MicroRNAs are non-coding small RNA molecules that have vital effects on body development, physiological and pathological processes including tumorigenesis. MiR-17-92 gene cluster is a well-studied miRNA and considered to be oncogenic. It plays a significant role in the development of a variety of tumors. In this paper, we will review the role of miR-17-92 gene cluster in the development and progression of tumors.
- Citation: Sun RF, Gong JY, Zou HJ, Zhang L, Gao LB. Role of miR-17-92 gene cluster in tumor development and progression. Shijie Huaren Xiaohua Zazhi 2017; 25(20): 1840-1853
- URL: https://www.wjgnet.com/1009-3079/full/v25/i20/1840.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v25.i20.1840
目前, 肿瘤已经成为严重威胁全世界人类健康和社会经济发展的重大问题, 在我国, 肿瘤死亡原因占总死亡原因的1/4, 根据国家癌症中心的调查显示, 每10万人有156.83死于恶性肿瘤, 其中, 肺癌、乳腺癌、肝癌、胃癌、食管癌、结直肠癌、宫颈癌、卵巢癌以及胰腺癌是我国主要的恶性肿瘤. 而肝癌、肺癌、食管癌、胃癌、结直肠癌、胰腺癌、乳腺癌、脑瘤、宫颈癌和白血病是主要的肿瘤死因, 占全部肿瘤死亡病例的84%[1]. 鉴于肿瘤的发病率和死亡率都较高, 给社会和家庭带来了很大的负担, 因此探讨肿瘤的病因和发病机制, 深入了解其发生发展, 对肿瘤的早期发现和有效防治具有十分重要的意义.
微小RNA(microRNA, miRNA)是真核生物和病毒体内发现的一类小分子非编码微小RNA, 广泛分布在含单细胞真核藻类在内的真核生物当中, 在基因表达以及调控过程中发挥着重要的作用[2]. 成熟的miRNA是由60-110 nt的具有发卡状结构的pri-miRNA经核酸内切酶Drosha和Dicer加工切割而来, 以单链形式存在, 进化上高度保守. miRNA可特异性识别并与靶基因的3'非翻译区(3'untranslational region, 3'UTR)完全或不完全配对, 降解miRNA靶基因或者抑制蛋白质合成从而调控靶基因的转录后表达. 大多数的miRNA前体被RNA聚合酶Ⅱ转录, 随后被处理为长度为18-24 nt的成熟miRNA[3]. 成熟的miRNA双链的一条链被选择性地引入到RNA诱导的沉默复合物(RNA-induced silencing complex, RISC)中, 随后通过特异性mRNA靶基因介导转录后表达[4]. 1993年, Lee等在线虫中发现了第1个miRNA即lin-4, 目前在几十种生物中共发现超过千种miRNA, 人类有700多种[5]. 研究表明, miRNA在发育、生理、病理过程包括细胞增殖、凋亡、分化、代谢以及肿瘤发生等环节中起着重要的调节作用[6,7], 在肿瘤细胞中发挥类似癌基因或抑癌基因的作用[8]. 通过癌细胞中表达沉默的miRNA或者敲除过表达的miRNA, 可诱导肿瘤细胞死亡, 作为治疗的靶点[9]. 因此, miRNA成为目前国内外学者研究的热点.
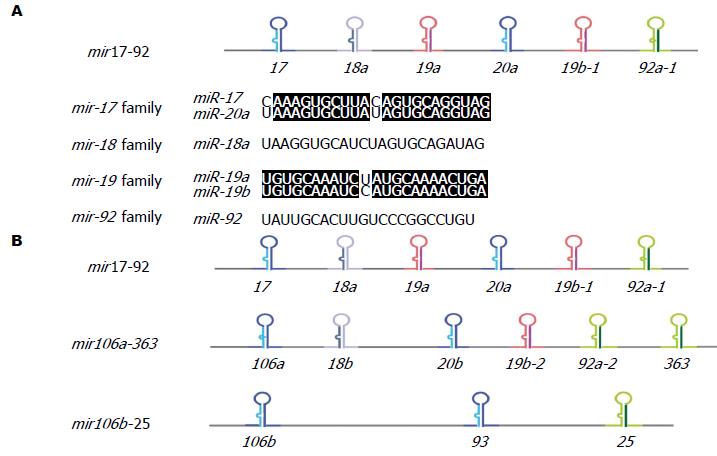
人类miRNA具有成簇聚集于染色体上的特点, 形成miRNA基因簇, 在miRNA基因簇方面, 研究较为深入、最有特点的多顺反子miRNA就是位于13q31的miR-17-92基因簇[10,11], 多数学者认为是一种强效的原癌基因, 也是原癌基因miRNA的典型代表[12,13], 该基因簇位于人类第13号染色体的MIR17HG(miR-17-92基因簇宿主基因)内含子中[14]. 初级转录因子是一个长约0.8 kb的多顺反子, 被编码为6个独立的成熟miRNAs, 即miR-17、miR-18a、miR-19a、miR-20a、miR-19b-1和miR-92a-1(图1)[15], 他们在胚胎发育、免疫系统、肾脏和心脏发育、衰老以及肿瘤发生中起着作用[16]. 在哺乳动物中, miR-17-92基因簇有两个旁系同源体: miR-106a-363和miR-106b-25基因簇, 其中, miR-106a-363基因簇位于人和小鼠的X染色体上, 包括miR-106a、miR-18b、miR-20b、miR-19b-2、miR-92-2和miR-363 6个miRNA. miR-106a-363基因簇的初级转录体称为Kis2, 在T细胞白血病中高表达, 具有致癌潜能. miR-106b-25基因簇包括miR-106b、miR-93和miR-25 3个miRNA, 基因座位于人类第7号染色体上蛋白质编码基因MCM7的第13个内含子区或小鼠的第5号染色体. miR-17-92基因簇的功能还不是很清楚, 但是他可能通过miRNA的某些成分影响保守序列的相互作用, 通过基因调控机制引起肿瘤发生. miR-17-92基因簇又称为C13orf25, 与肿瘤的发生密切相关, 在伯基特淋巴瘤, 弥漫性扩增大B细胞淋巴瘤(diffuse large B-cell lymphoma, DLBCL), 滤泡性淋巴瘤, 套细胞淋巴瘤和肺癌中发现高表达[17-20], 随后有学者在各种造血系统恶性肿瘤、肺癌[10]和多个实体肿瘤中也发现miR-17-92基因簇过表达[21]. 下面就miR-17-92基因簇在肿瘤发生发展中的作用及功能作一综述.
有研究小组[22-26]证实在体内, miR-17-92基因簇有可能在胚胎早期发育中参与并维持多潜能细胞的分化, 同时在组织器官的发育和形成当中都发挥了重要的调控作用. Koralov等[27]在2008年发现在B细胞发育过程中, Dicer基因发挥重要的作用. 在发育早期敲除Dicer基因导致B细胞的发育停留在pro-B向pre-B阶段. 而且在此阶段, miR-17-92基因簇成员miR-17和miR-92表达上调, 促凋亡蛋白Bim也高表达. 无独有偶, 同年Koralov等[27]与Ventura等[28]也证实miR-17-92基因簇在人类肿瘤中过表达, 而且在小鼠的B细胞淋巴瘤中也发挥原癌基因的作用. 当敲除小鼠的该基因簇可以导致动物出生不久便死亡, 同时伴随肺部发育不全和室间隔缺陷, 当敲除miR-17-92基因簇可引起Bim升高, B细胞发育停留在pro-B到pre-B的过程, 提示该基因簇在淋巴细胞和肺发育过程中发挥作用. Xiao等[29]研究人员通过转基因技术使小鼠miR-17-92基因簇高表达, 同样可以使得小鼠淋巴组织增生, 也进一步证实miR-17-92基因簇在淋巴细胞的发育过程中发挥功能. 以上的研究同时发现, 在淋巴细胞发育与分化过程中, miR-17-92基因簇通过靶基因PTEN和Bim发挥作用. PTEN和Bim均可促进细胞凋亡, miR-17-92基因簇可调节PTEN和Bim的表达来维持免疫细胞的正常发育[30]. 同时有学者发现在淋巴瘤细胞中, miR-17-92基因簇的靶基因还有E2F[31]、CDKN1A/p21[32]等. 但是这些靶基因除了在淋巴瘤细胞中外, 是否在正常免疫细胞中也可以调节免疫细胞分化发育还不清楚.
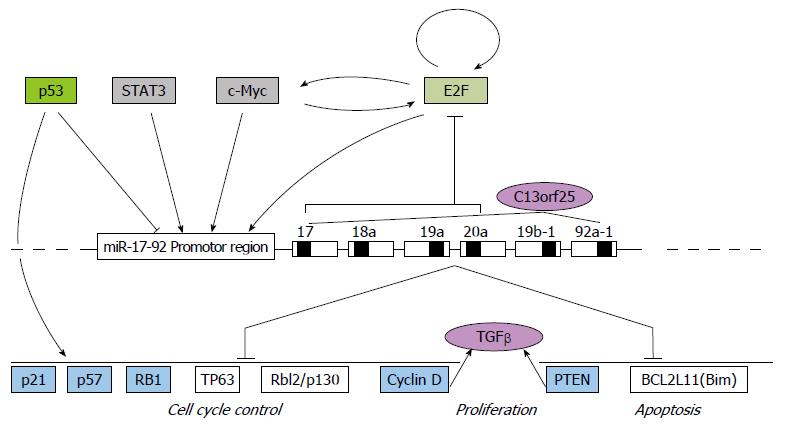
最初发现miRNA和肿瘤之间的联系是因为在各种人类肿瘤中频繁的基因改变及表达失调[33]. 在癌症的分子网络中, 多数miRNA都发挥着类癌基因或抑癌基因的作用, 通过癌症基因组学网站并未显示在不同类型的癌症中miR17HG的遗传改变[34], 同时暗示miR-17-92的转录及转录后表达在调控成熟miRNA水平中起着重要的作用. miR-17-92基因簇诱导肿瘤发生主要是通过抑制细胞周期调控基因和抑癌基因的表达实现的. E2F作为调节细胞周期和细胞凋亡的转录因子, 主要调控细胞从G1期进入S期所需基因的转录. 有证据显示, miR-17-92基因簇成员miR-17-5p和miR-20a能分别抑制E2F1、E2F2和E2F3的表达, 低表达的miR-17-5p和miR-20a可使细胞周期静止于G1期; 反过来, E2F1-3可与miR-17-92基因簇启动子区结合, 激活miR-17-92基因簇的转录, 在E2F转录因子和miR-17-92基因簇之间形成一个负反馈调节环[35]. 同时, E2F1-3和原癌基因c-myc之间可彼此相互激活转录, 形成正反馈调节环[36,37]. 此外, 抑癌基因p53和癌基因STAT3均可与miR-17-92基因簇启动子区结合, 激活miR-17-92基因簇转录, 抑制其靶基因如细胞周期负向调节因子p21等的表达, 使细胞周期调节失控, 细胞过度增殖, 引发肿瘤[29,38]. miR-17-92基因簇还由转化生长因子-β(transforming growth factor-β, TGF-β)信号通路介导, 通过抑制抑癌基因PTEN[39]和促凋亡蛋白基因Bim的表达[40]促进细胞增殖、抑制凋亡[41,42]. miR-17-92基因簇转录调控如图2.
现阶段的研究主要集中于miR-17-92基因簇在肿瘤发生发展中的调控作用, 对其自身异常表达的内在原因探讨较少. 由于E2F、p53、STAT3和c-Myc等均是通过与miR-17-92基因簇启动子区结合, 调控下游靶基因, 进而影响细胞增殖、侵袭、迁移和凋亡等生物学过程, 在肺、心脏及免疫系统的发育及肿瘤形成中起着重要作用[43-46]. miR-17-92基因簇通过攻击肿瘤抑制蛋白以及PTEN和TGFβ信号通路发挥类似癌基因的作用[47].
miR-17-92基因簇参与多种肿瘤发生发展, 在不同的肿瘤中发挥着抑癌或者促癌的作用, 该基因簇成员在同一肿瘤中的功能也不尽相同, 下面将对miR-17-92基因簇在各种肿瘤中的作用综述如下.
学者发现, 通过与正常B细胞比对, miR-92和miR-19在癌前和恶性Eμ-myc B细胞瘤中功能不同[48], 而且miR-92可以通过激活Caspase诱导细胞凋亡. miR-92a家族包括miR-25、miR-92a-1、miR-92a-2和miR-363, 从3个不同的miR-17-92旁系簇中产生, miR-106a-363和miR-106b-25在进化过程中高度保守, 与血管内皮细胞相关, 在多种癌症中均发现miR-92a家族与肿瘤发生和发展有关[49]. 甲状腺癌中, 诱导BRAF癌基因可导致miR-17-92基因簇高表达, miR-92可与miR-19a/b作用相反. 研究者[50]维持miR-92水平不变, 通过抑制miR-19增高可保护甲状腺癌高碘治疗. 这些证据均显示miR-19是一个癌基因, 而miR-92在基因簇中起负向调控作用.
在胃肠道肿瘤中, 如果骨髓中出现肿瘤细胞, 那么肿瘤的恶性程度和死亡率都会增加, 但是机制却并不清楚. 在胃癌中, miR-17-92的宿主基因MIR17HG低表达, 而且MIR17HG表达水平和胃癌的恶性度和分期呈现正相关, 但是与胃癌早期(阶段Ⅰ和Ⅱ)肿瘤的大小呈现负相关, 同时MIR17HG的低表达与转移有关. 提示在胃癌中MIR17HG发挥着抑癌基因的作用[51].
结直肠癌是一种异质性疾病, 多基因长期变化发展而来, 公认的理论认为通过启动Wnt通路来诱发腺瘤形成[52]. 在肿瘤演进过程中, 基因组不稳定是关键的因素. 这种情况在腺瘤发生率约5%, 通过失败的DNA错配修复约0.5%, 从而产生突变表型的微卫星标记不稳定性, 或者约4.5%(提高水平)染色体不稳定性. 已经发现, miR-17-92基因簇成员在结直肠癌中显著高表达[53-56], 可能成为结直肠癌诊断新的标志物, 其中miR-17-3p和miR-92诊断结直肠癌的敏感度、特异度分别为64%、70%和89%、70%[57].
腺瘤性结肠息肉病极易通过基因突变发展为散发性结直肠癌, Li等[58]发现发病机制是通过大肠癌β-连环蛋白通路调控miR-17-92基因簇诱发结直肠癌. 研究人员发现miR-92a在结肠腺瘤和结肠癌中均高表达. 而且, miR-92a在结肠癌组织中可以直接攻击抗凋亡因子BCL-2来介导细胞死亡, 抗miR-92a拮抗剂诱导结肠癌细胞来源的凋亡发生, 因此可以推测miR-92a在结直肠癌发生发展中具有举足轻重的作用[59].
在结直肠癌中, miR-17-92基因簇以及旁系同源物显著高表达[60], 同时miR-17的表达与结直肠癌患者的预后相关[61], 可以作为独立的分子标志物[56]. 在结直肠腺瘤向结直肠癌演变中, 出现并非随机的染色体频繁改变, 包括8q、13q、20q获得而8p、15q、17p以及18q缺失[62]. Diosdado等[53]也发现miR-17-92基因簇含量不同与结直肠腺瘤到腺癌的发生发展有关, 同时证实miR-17-92高表达主要是miR-18a高表达, 并且与c-Myc的转录活性相关[53]. Tsuchida等[59]通过原位杂交证实miR-92a在结直肠癌中高表达, 在正常肠黏膜中, miR-92a含量是其他几个miRNA的10到350倍, 但是机制尚不清楚. 有学者[63]发现在结直肠癌中, Bim蛋白高表达且miR-92a可以通过调控通路调控Bim的水平, 多项研究[61]表明在结直肠癌药物干预中, miR-92a是个非常有效的靶基因.
在结直肠癌中, 正常表达的miR-19通过诱导Wnt/β-连环蛋白CTNNB1介导的上皮-间充质来攻击肿瘤抑制基因PTEN, 促进肿瘤转移. 然而, 高表达的miR-17-92基因簇可以抑制肿瘤生长和转移[64]. 然而, 肿瘤细胞中CTNNB1过表达并没有导致β-连环蛋白的水平升高, 提示在抑制肿瘤生长和转移中可能还有其他因素, 并且这些因素可能参与负调控[65]. Sokolova等[66]认为在结直肠癌发生发展中, TGF-β不表达至关重要.
肿瘤依赖一些遗传机制逃避细胞凋亡, 在肿瘤发生中miRNA选择性的剪切发挥作用. Urtasun等[67]选择剪切调控因子SLU7来保证肝癌细胞活性, 发现SLU7在肝癌细胞中低表达, 同时触发与活性氧过表达相关的细胞凋亡, 但是这种情况在人原代肝细胞或者分化较好的HepaRG细胞系中并未观察到, 研究者认为SLU7使C13orf25初级转录物表达沉默, 显著降低了miR-17、miR-20和miR-92a的水平进而导致P21和BIM表达上调发挥癌基因作用. miR-17-92基因簇中, miR-18a在多种癌症中均呈现高表达, 在等离子体的癌症患者比如食管癌(AUC = 0.944)、胰腺癌(AUC = 0.936)、肝细胞癌(AUC = 0.881)、结直肠癌以及其他类型的癌症中, miR-18a的水平比健康志愿者高得多. 研究[68]表明, 在大鼠肝癌细胞中miR-17-92基因簇高表达, 同时Murakami等[69]发现miR-18和miR-92高表达则肝癌的分化程度低, 反之miR-18和miR-92低表达, 则肝癌的分化程度高, 也就是说miR-18和miR-92在分化好的肝癌组织中表达最低, 而在分化较差的肝癌组织中表达最高. Zhu等[70]通过RT-PCR和原位杂交技术证实miR-17-92基因簇在可以促进肝癌发生, 发挥癌基因作用, 同时认为miR-17-92基因簇通过下调PTEN, 上调IL-6/Stat3在胆管癌中高表达, 发挥癌基因作用[71].
Komatsu等[72]发现miR-18可能是未来的生物标志物, 并有助于通过无创液体活检筛查癌症来获得敏感性和特异性都较满意的临床结果. 在miR-17-92基因簇的抗凋亡活性中, miR-17和miR-20a的作用基本可以忽略[73].
Tao等[74]通过在膀胱癌细胞中转染miR-18a, 通过MTT测定, 集落形成测定法, 半定量RT-PCR, 荧光素酶分析和Western印迹分析发现miR-18a可以作为抑癌基因, 抑制膀胱癌T24细胞的细胞增殖. 此外, T24细胞的miR-18a可以下调在mRNA和蛋白水平Dicer的表达, 而通过反义寡核苷酸抑制miR-18a可提高T24细胞中内切酶的表达. 同时发现Dicer 3'非翻译区(3'UTR)miR-18a的两个结合位点. 荧光素酶报告分析表明在体外实验中, miR-18a可以抑制Dicer酶的表达. 此外, 可以通过siRNA的Dicer酶低表达模拟含miR-18a的T24细胞抑制现象. 这些结果表明miR-18a作为抑癌基因可以通过膀胱癌T24细胞靶向内切酶, 抑制miRNA高表达.
越来越多的证据表明miRNA表达量的改变在肺癌发生发展中有重要的作用, 研究[75]发现, miR-17-92基因簇在肺癌中高表达, 尤以进展期小细胞肺癌显著, 该基因簇能促进肺癌细胞的生长, 但是在肺癌的发生发展中的作用机制还不清楚, 推测可能通过抑制肿瘤抑制基因PTEN和RB2来发挥miR-17-92簇致癌作用. Volinia等[76]通过分析包括肺癌、胃癌、前列腺癌、直肠癌和胰腺癌等540例病例后发现, miR-17-92基因簇中miR-17-5p、miR-20a、miR-92高表达. Tai等[77]利用组织样本的表达谱和MYC诱导的细胞发现miR-17-92在肺癌中高表达, Thai等[78]发现在肺癌形成中, miR-17-92基因簇的表达水平可以通过纳米二氧化钛处理而改变, 在肺癌中miR-17-92簇被诱导, 发挥着癌基因的作用. Li等[79]发现miR-19可以触发肺癌细胞A549和HCC827中上皮和间质的转化, 下调上皮蛋白如E-钙黏蛋白、紧密连接蛋白1和α-连环蛋白以及间充质蛋白如波形蛋白、纤连蛋白1、N-钙黏蛋白和转录因子, 减少细胞黏附, 导致癌细胞迁移和侵袭能力增强. DNA微阵列结果显示miR-19表达的A549细胞中, EMT、迁移和转移相关的基因均发生显著变化. 而且, siRNA介导的miR-19靶基因PTEN沉默也可以导致A549和HCC827肺癌细胞EMT、迁移和侵袭, 提示PTEN参与了此过程.
miR-17-5p和miR-20a在人乳腺肿瘤中低表达, 可能作为抑癌基因起作用[80]. 有研究[81]将含有miR-17-5p的质粒转染到MCF-7乳腺癌细胞系, 发现细胞增殖受到抑制, 通过激素介导的信号传导通路抑制乳腺癌扩增基因-1(amplified in breast 1, AIB1)蛋白发挥抑癌基因作用. AIB1为核受体共激活因子, 作为致癌基因参与多种肿瘤的细胞增殖、转移, 在乳腺癌、卵巢癌中高表达, 可以提高雌激素受体、E2F1以及一些转录因子, 调节基因转录的活性[82,83]. 而miR-17-5p通过抑制AIB1的翻译导致雌激素受体调节基因低表达, 使乳腺癌细胞的增殖能力下降. 同时miR-17-5p抑制乳腺癌细胞胰岛素样生长因子-1介导的离体生长[84].
细胞周期蛋白D1也是乳腺癌细胞中高表达的基因, 他与CDK4等结合后激活蛋白激酶, 促进细胞从G0/G1期向S期过渡, 在乳腺组织中, 细胞周期蛋白D1高表达并在诱导癌变过程中发挥重要作用[85]. Yu等[86]发现, miR-17-5p和miR-20a都可以抑制细胞周期蛋白D1翻译mRNA, 阻止细胞进入S期, 同时抑制人乳腺癌细胞的生长和肿瘤集落因子的形成. 体外培养的乳房上皮细胞中, 细胞周期蛋白D1与miR-17-92基因簇的启动子结合诱导miR-17-92高表达, 形成负反馈调控环.
女性妇科三大恶性肿瘤为子宫内膜癌、宫颈癌和卵巢癌, 发病率在世界范围内均呈逐渐上升趋势. 卵巢癌发病率位于女性生殖系统肿瘤中的第2位, 死亡率居于首位达70%. miR-17-92基因簇可以下调肿瘤抑制因子BIM和PTEN的表达[29], 同时该基因簇和卵巢耐药性关系密切.
Umayahara等[87]研究证实在宫颈癌组织中, c-Myc和E2F1高表达, 但是miR-17-92基因簇是否在宫颈癌中通过c-Myc:miR-17-5p:E2F1调控途径发挥作用却不清楚. Wang等[88]也提出在宫颈癌组织以及宫颈癌细胞系中, 致癌性HPV可以减少抑癌基因miR-34a的表达, miR-17-92基因簇是否同时参与高危型HPV在宫颈癌发生发展中的作用机制还需要进一步探索. 研究[89]认为miR-17-92调节促分裂原活化蛋白激酶(mitogen-activated protein kinases, MAPK), 磷酸肌醇-4,5-二磷酸3-激酶, NOTCH, 细胞凋亡信号通路发挥癌基因作用.
miR-17-92基因簇在子宫内膜癌中高表达, 推测该基因簇可能参与与女性雌激素水平相关的肿瘤发生. Wang等[90]研究认为在月经周期、子宫内膜增生以及内膜癌变的过程中, 信号通路Wnt可以维持雌激素诱导的细胞增殖和孕激素诱导的细胞分化之间的平衡. 同时Boren等[91]采用61例标本(其中正常子宫内膜20例、子宫内膜癌37例、子宫内膜复杂性不典型增生4例), 通过检测发现有13个miRNA高表达, 并预测11个靶基因(KLF2、KCNMBl、IGFBP-6、ENPP2、TBLlX、RAMPl 、CNNl、MYHll、TGFBl、MYL9、SNCAIP), 证实有多种microRNA参与子宫内膜细胞转化的生物学通路. miR-17-92基因簇可能参与包括影响女性雌激素水平的Wnt信号通路在内的几条信号通路, 最终诱导子宫内膜癌的发生.
正常的前列腺和早期、进展期前列腺癌均需要雄激素受体AR维持生长和存活. 有学者利用野生型AR转录活性较高的DUCaP细胞, 通过染色质免疫沉淀实验和微阵列表达谱证实miR-17-92基因簇和靶基因可以直接调控AR[92]. miR-17-92基因簇介导前列腺癌的分子机制并未完全明确, 有学者诱导该基因簇的雄激素非依赖的DU145前列腺癌细胞证实, 可以通过破坏增殖和凋亡之间的平衡促进细胞生长. 同时有学者认为miR-17-92基因簇高表达可以显著提高DU145细胞的迁移和侵袭能力并且激活AKT信号通路, 在细胞增殖、凋亡和化疗抗性方面发挥了核心作用, 而且不断激活AKT1/2可以起放大作用[93].
O'Donnell等[94]首先发现在miR-17-92基因簇中每个miRNA含量不同, miR-18含量最多, miR-92含量最少, 但是每个生物具体含多少是有差异的. 而且miR-17-92基因簇与c-Myc联合表达可以促进小鼠造血干细胞形成肿瘤, 并且可以增加肿瘤的恶性度. 例如, 在生发中心弥漫性大B细胞淋巴瘤(GC-DLBCL)中miR-20a表达量最高[95], 在多发性骨髓瘤中, 预后差的患者miR-17、20a和92表达量最高[96]. 两个不同的研究小组[97,98]分别发现在miR-17-92基因簇中miR-19a和miR-19b是关键的癌基因. Olive等[99]发现将含有miR-19b的Eμ-myc基因的造血干细胞和祖细胞注射进小鼠时, 小鼠杀伤力明显增强. 此外, 通过特定的突变将miR-19a和miR-19b沉默, 可推迟B细胞淋巴瘤的发生同时降低动物死亡. 更为重要的是, Mu等[39]发现敲除miR-17-92基因簇可以导致Eμ-myc转基因小鼠B细胞淋巴瘤的增殖, 同时, 选择性表达miR-19a和miR-19b, 小鼠生存能力提高.
miR-17-92基因簇在多种类型的淋巴瘤中高表达, 与细胞增殖密切相关[14], 该基因簇作为致癌基因可诱发淋巴瘤等多种肿瘤发生. 同时该基因簇与胶质瘤、弥漫性B细胞淋巴瘤、髓母细胞瘤等多种肿瘤的发生发展也密切相关, 兼具癌基因与抑癌基因的双重作用[100]. 离体环境中, 将miR-17-92基因簇转染到不同的淋巴瘤细胞中, 可以使促凋亡蛋白基因BIM和抑癌基因p21低表达. Ohyashiki等[101]报道, miR-92a不仅可以用于非霍奇金淋巴瘤患者的诊断, 同时还可以作为非霍奇金淋巴瘤患者化疗后监测复发的一种指标. 富含亮氨酸重复蛋白磷酸酶家族PHLPP中的PHLPP2在实体肿瘤中可以作为肿瘤抑制剂, 有学者发现在血液系统恶性肿瘤中也参与调控, 晚期急性髓细胞白血病(acute myelocytic leukemia, AML)的亚型中, PHLPP2低表达. miR-17-92基因簇可以不依赖于c-myc和E2F转录因子抑制其表达, 而是通过全反式维甲酸(all-trans-retinoic acid, ATRA)来抑制miR-17-92水平, 增加PHLPP2的水平和磷酸酶活性. 该研究组发现ATRA通过介导miR-17-92基因簇的靶基因, 也就是C/EBPβ转录因子并相互作用抑制转录, 这些研究揭示了在终末分化骨髓细胞中, 可以通过C/EBPβ介导来发挥PHLPP2磷酸酶活性, 抑制miR-17-92表达[102]. Wu等[103]通过实验证实miR-17-92基因簇在移植物抗宿主病和白血病复发的小鼠中控制T细胞应答. Li等[104]发现在白血病细胞中通过miR-17-92基因簇成员miR17/19A白介素6(interleukin-6, IL-6)可以抑制Toll样受体, 提示在白血病中IL-6可以作为肿瘤抑制剂.
Wang等[105,106]研究表明, miR-17-92基因簇高表达可加速脂肪细胞的分化, 形成各种脂肪瘤, 机制是通过对负反馈调节抑癌基因rb2/p130加速脂肪细胞的恶性增殖. Northcott等[107]研究证实, 髓母细胞瘤患者的瘤细胞中, miR-17-92表达明显增高, 同时myc/mucn表达增高. miR-17-92基因簇在20%黑色素瘤、16.5%卵巢癌以及21.9%乳腺癌中由于13q31.3位点杂合子缺失导致miR-17-92基因簇缺失, 推测miR-17-92基因簇发挥抑癌基因的作用.
肾母细胞瘤是儿童常见的肾脏肿瘤, 预后极差. miR-17-92基因簇可以促进该肿瘤细胞增殖, 在体外实验中, 转录活性物E2F3诱导miR-17-92基因簇高表达, Kort等[108]也表明E2F3在肾母细胞瘤中高表达, 但在其他类型的肾癌中并未观察到. 同时该实验还发现该基因簇中, 成员miR-92、miR-17-5p和miR-20a在肾母细胞瘤中高表达, 发挥着癌基因的作用. 卡波氏肉瘤相关疱疹病毒KSHV可直接引发卡波氏肉瘤, Choi等[109]通过阵列miRNA表达谱发现在KSHV感染的内皮细胞中miR-17-92基因簇的所有成员均表达上调. 通过荧光素酶检测和Western blot实验发现候选基因vFLIP和vCyclin可以激活miR-17-92启动子, 下调SMAD2抑制TGF-β信号通路诱导该基因簇表达. 同时使用miR-17-92基因簇的拮抗剂转染表达vFLIP或者vCyclin的细胞中, TGF-β的活性和SMAD2表达可以完全恢复正常.
miRNA在肿瘤发生发展中起着十分重要的作用, 研究[9]表明可以通过敲除过表达的miRNA, 或者通过表达沉默的miRNA, 可以促进肿瘤细胞死亡, 故miRNA可作为肿瘤治疗的靶点. miR-17-92基因簇在人和小鼠的多种组织中广泛表达, 通过复杂的基因表达调控网络调控靶基因参与的信号通路来参与哺乳动物组织器官发育和肿瘤的发生. 目前大量的实验研究[81]证实miR-17-92基因簇在结直肠癌等肿瘤中具有原癌基因的功能, 同时在卵巢癌、黑色素瘤以及乳腺癌中却发挥抑癌基因的作用. 目前已明确的miR-17-92靶基因仅为生物信息网预测到靶基因的一小部分[40,110,111], miR-17-92还可能通过调节其他基因参与其他器官发育和肿瘤发生. 目前除了明确c-Myc、E2F和细胞周期蛋白D1等调控miR-17-92基因簇的转录以外, miR-17-92基因簇差异性表达机制尚不清楚, 对这个机制的进一步的深入探索也可以更透彻了解miR-17-92基因簇在哺乳动物器官发育和诱发肿瘤发生中的作用, 可以作为肿瘤预防和治疗的靶点[60].
近年来, 越来越多的研究[112-115]发现miR-17-92通过BIM和PTEN表达异常参与许多肿瘤的耐药性, 但是miR-17-92基因簇在哺乳动物器官发育和癌症发生中以及如何参与肿瘤耐药的作用机制还需要进一步探索, 但体外通过反义寡核苷酸抑制miR-17-92基因簇的miRNAs, 抑制多种癌细胞增殖同时促进其凋亡, 为利用miR-17-92治疗某些肿瘤提供了理论依据和新的途径.
miRNA的异常表达和多种肿瘤相关, 起着癌基因或者抑癌基因的作用. 通过癌细胞中表达沉默的miRNA或者敲除过表达miRNA, 可诱导肿瘤细胞死亡, 作为治疗的靶点. 学者发现miR-17-92基因簇遗传变异与肿瘤的发生发展相关, 通过研究筛选可能发现癌症发病机制, 发现临床诊断新的分子标志物.
miR-17-92基因簇的表达与肿瘤发生发展的关系, miR-17-92基因簇异常表达的分子机制, 鉴定出新的易感基因, 找到肿瘤诊治新的靶点, 是该领域研究的热点及需要突破的问题.
阐述了miR-17-92基因簇基因表达对肿瘤发生发展的影响的研究进展及癌症可能出现的发病机制.
miRNA在肿瘤治疗中作为新的靶点; 通过研究筛选可能发现癌症发病机制, 发现临床诊断新的分子标志物的应用前景.
陈淑珍, 研究员, 博士生导师, 中国协和医科大学•中国医学科学院医药生物技术研究所肿瘤室; 韩双印, 主任医师, 郑州大学人民医院消化内科
本文全面、系统的综述了miR-17-92基因簇在肿瘤发生发展中作用的研究进展, 内容丰富, 逻辑性强, 有关miR-17-92基因簇在消化系肿瘤的作用也给予了阐述, 对肿瘤发生发展的机制研究和临床诊断治疗有指导意义.
手稿来源: 邀请约稿
学科分类: 胃肠病学和肝病学
手稿来源地: 云南省
同行评议报告分类
A级 (优秀): 0
B级 (非常好): B, B
C级 (良好): 0
D级 (一般): 0
E级 (差): 0
编辑: 闫晋利 电编:李瑞芳
| 2. | Herranz H, Cohen SM. MicroRNAs and gene regulatory networks: managing the impact of noise in biological systems. Genes Dev. 2010;24:1339-1344. [PubMed] [DOI] |
| 3. | Kim VN, Han J, Siomi MC. Biogenesis of Small Rnas in Animals. Nat Rev Mol Cell Biol. 2009;10:126-39. [PubMed] [DOI] |
| 4. | Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102-114. [PubMed] [DOI] |
| 5. | 胡 志德, 孙 懿, 黄 元兰, 邓 安梅. Microrna的免疫调控作用及其机制的研究进展. 中国肿瘤生物治疗杂志. 2010;6:678-82. |
| 6. | Voorhoeve PM, le Sage C, Schrier M, Gillis AJ, Stoop H, Nagel R, Liu YP, van Duijse J, Drost J, Griekspoor A. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Adv Exp Med Biol. 2007;604:17-46. [PubMed] [DOI] |
| 7. | Zhang B, Chen J, Ren Z, Chen Y, Li J, Miao X, Song Y, Zhao T, Li Y, Shi Y. A specific miRNA signature promotes radioresistance of human cervical cancer cells. Cancer Cell Int. 2013;13:118. [PubMed] [DOI] |
| 8. | Khew-Goodall Y, Goodall GJ. Myc-modulated miR-9 makes more metastases. Nat Cell Biol. 2010;12:209-211. [PubMed] [DOI] |
| 9. | Yin JQ, Gao J, Shao R, Tian WN, Wang J, Wan Y. siRNA agents inhibit oncogene expression and attenuate human tumor cell growth. J Exp Ther Oncol. 2003;3:194-204. [PubMed] [DOI] |
| 10. | Ishikawa M, Sekine K, Okamura A, Zheng YW, Ueno Y, Koike N, Tanaka J, Taniguchi H. Reconstitution of hepatic tissue architectures from fetal liver cells obtained from a three-dimensional culture with a rotating wall vessel bioreactor. J Biosci Bioeng. 2011;111:711-718. [PubMed] [DOI] |
| 11. | Nelson LJ, Walker SW, Hayes PC, Plevris JN. Low-shear modelled microgravity environment maintains morphology and differentiated functionality of primary porcine hepatocyte cultures. Cells Tissues Organs. 2010;192:125-140. [PubMed] [DOI] |
| 12. | Aigner A. MicroRNAs (miRNAs) in cancer invasion and metastasis: therapeutic approaches based on metastasis-related miRNAs. J Mol Med (Berl). 2011;89:445-457. [PubMed] [DOI] |
| 13. | Blenkiron C, Miska EA. miRNAs in cancer: approaches, aetiology, diagnostics and therapy. Hum Mol Genet. 2007;16 Spec No 1:R106-R113. [PubMed] [DOI] |
| 14. | He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828-833. [PubMed] [DOI] |
| 15. | Mendell JT. miRiad roles for the miR-17-92 cluster in development and disease. Cell. 2008;133:217-222. [PubMed] [DOI] |
| 16. | Xie R, Lin X, Du T, Xu K, Shen H, Wei F, Hao W, Lin T, Lin X, Qin Y. Targeted Disruption of miR-17-92 Impairs Mouse Spermatogenesis by Activating mTOR Signaling Pathway. Medicine (Baltimore). 2016;95:e2713. [PubMed] [DOI] |
| 17. | Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y, Takahashi T. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628-9632. [PubMed] [DOI] |
| 18. | Ota A, Tagawa H, Karnan S, Tsuzuki S, Karpas A, Kira S, Yoshida Y, Seto M. Identification and characterization of a novel gene, C13orf25, as a target for 13q31-q32 amplification in malignant lymphoma. Cancer Res. 2004;64:3087-3095. [PubMed] [DOI] |
| 19. | Tagawa H, Seto M. A microRNA cluster as a target of genomic amplification in malignant lymphoma. Leukemia. 2005;19:2013-2016. [PubMed] [DOI] |
| 20. | Takakura S, Mitsutake N, Nakashima M, Namba H, Saenko VA, Rogounovitch TI, Nakazawa Y, Hayashi T, Ohtsuru A, Yamashita S. Oncogenic role of miR-17-92 cluster in anaplastic thyroid cancer cells. Cancer Sci. 2008;99:1147-1154. [PubMed] [DOI] |
| 21. | Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834-838. [PubMed] [DOI] |
| 22. | Gruber AJ, Grandy WA, Balwierz PJ, Dimitrova YA, Pachkov M, Ciaudo C, Nimwegen Ev, Zavolan M. Embryonic stem cell-specific microRNAs contribute to pluripotency by inhibiting regulators of multiple differentiation pathways. Nucleic Acids Res. 2014;42:9313-9326. [PubMed] [DOI] |
| 23. | Tsai ZY, Chou CH, Lu CY, Singh S, Yu SL, Li SS. Proteomic comparison of human embryonic stem cells with their differentiated fibroblasts: Identification of 206 genes targeted by hES cell-specific microRNAs. Kaohsiung J Med Sci. 2011;27:299-306. [PubMed] [DOI] |
| 24. | Judson RL, Babiarz JE, Venere M, Blelloch R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol. 2009;27:459-461. [PubMed] [DOI] |
| 25. | Wang Y, Baskerville S, Shenoy A, Babiarz JE, Baehner L, Blelloch R. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat Genet. 2008;40:1478-1483. [PubMed] [DOI] |
| 26. | Houbaviy HB, Murray MF, Sharp PA. Embryonic stem cell-specific MicroRNAs. Dev Cell. 2003;5:351-358. [PubMed] [DOI] |
| 27. | Koralov SB, Muljo SA, Galler GR, Krek A, Chakraborty T, Kanellopoulou C, Jensen K, Cobb BS, Merkenschlager M, Rajewsky N. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell. 2008;132:860-874. [PubMed] [DOI] |
| 28. | Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875-886. [PubMed] [DOI] |
| 29. | Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, Henderson JM, Kutok JL, Rajewsky K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9:405-414. [PubMed] [DOI] |
| 30. | Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136:26-36. [PubMed] [DOI] |
| 31. | Inomata M, Tagawa H, Guo YM, Kameoka Y, Takahashi N, Sawada K. MicroRNA-17-92 down-regulates expression of distinct targets in different B-cell lymphoma subtypes. Blood. 2009;113:396-402. [PubMed] [DOI] |
| 32. | Zhou B, Wang S, Mayr C, Bartel DP, Lodish HF. miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc Natl Acad Sci USA. 2007;104:7080-7085. [PubMed] [DOI] |
| 33. | Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004;101:2999-3004. [PubMed] [DOI] |
| 34. | Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401-404. [PubMed] [DOI] |
| 35. | Li Y, Li Y, Zhang H, Chen Y. MicroRNA-mediated positive feedback loop and optimized bistable switch in a cancer network Involving miR-17-92. PLoS One. 2011;6:e26302. [PubMed] [DOI] |
| 36. | Aguda BD, Kim Y, Piper-Hunter MG, Friedman A, Marsh CB. MicroRNA regulation of a cancer network: consequences of the feedback loops involving miR-17-92, E2F, and Myc. Proc Natl Acad Sci USA. 2008;105:19678-19683. [PubMed] [DOI] |
| 37. | Li Y, Vecchiarelli-Federico LM, Li YJ, Egan SE, Spaner D, Hough MR, Ben-David Y. The miR-17-92 cluster expands multipotent hematopoietic progenitors whereas imbalanced expression of its individual oncogenic miRNAs promotes leukemia in mice. Blood. 2012;119:4486-4498. [PubMed] [DOI] |
| 38. | Wu W, Takanashi M, Borjigin N, Ohno SI, Fujita K, Hoshino S, Osaka Y, Tsuchida A, Kuroda M. MicroRNA-18a modulates STAT3 activity through negative regulation of PIAS3 during gastric adenocarcinogenesis. Br J Cancer. 2013;108:653-661. [PubMed] [DOI] |
| 39. | Fuziwara CS, Kimura ET. Insights into Regulation of the miR-17-92 Cluster of miRNAs in Cancer. Front Med (Lausanne). 2015;2:64. [PubMed] [DOI] |
| 40. | Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787-798. [PubMed] [DOI] |
| 41. | Gao LB, Li LJ, Pan XM, Li ZH, Liang WB, Bai P, Zhu YH, Zhang L. A genetic variant in the promoter region of miR-34b/c is associated with a reduced risk of colorectal cancer. Biol Chem. 2013;394:415-420. [PubMed] [DOI] |
| 42. | Mestdagh P, Boström AK, Impens F, Fredlund E, Van Peer G, De Antonellis P, von Stedingk K, Ghesquière B, Schulte S, Dews M. The miR-17-92 microRNA cluster regulates multiple components of the TGF-β pathway in neuroblastoma. Mol Cell. 2010;40:762-773. [PubMed] [DOI] |
| 43. | Fonsato V, Herrera MB, Buttiglieri S, Gatti S, Camussi G, Tetta C. Use of a rotary bioartificial liver in the differentiation of human liver stem cells. Tissue Eng Part C Methods. 2010;16:123-132. [PubMed] [DOI] |
| 44. | Mygind T, Stiehler M, Baatrup A, Li H, Zou X, Flyvbjerg A, Kassem M, Bünger C. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials. 2007;28:1036-1047. [PubMed] [DOI] |
| 45. | Yeatts AB, Choquette DT, Fisher JP. Bioreactors to influence stem cell fate: augmentation of mesenchymal stem cell signaling pathways via dynamic culture systems. Biochim Biophys Acta. 2013;1830:2470-2480. [PubMed] [DOI] |
| 46. | Yu B, Yu D, Cao L, Zhao X, Long T, Liu G, Tang T, Zhu Z. Simulated microgravity using a rotary cell culture system promotes chondrogenesis of human adipose-derived mesenchymal stem cells via the p38 MAPK pathway. Biochem Biophys Res Commun. 2011;414:412-418. [PubMed] [DOI] |
| 47. | Aguda BD, del Rosario RC, Chan MW. Oncogene-tumor suppressor gene feedback interactions and their control. Math Biosci Eng. 2015;12:1277-1288. [PubMed] [DOI] |
| 48. | Han YC, Vidigal JA, Mu P, Yao E, Singh I, González AJ, Concepcion CP, Bonetti C, Ogrodowski P, Carver B. An allelic series of miR-17-92-mutant mice uncovers functional specialization and cooperation among members of a microRNA polycistron. Nat Genet. 2015;47:766-775. [PubMed] [DOI] |
| 49. | Li M, Guan X, Sun Y, Mi J, Shu X, Liu F, Li C. miR-92a family and their target genes in tumorigenesis and metastasis. Exp Cell Res. 2014;323:1-6. [PubMed] [DOI] |
| 50. | Fuziwara CS, Kimura ET. High iodine blocks a Notch/miR-19 loop activated by the BRAF(V600E) oncoprotein and restores the response to TGFβ in thyroid follicular cells. Thyroid. 2014;24:453-462. [PubMed] [DOI] |
| 51. | Bahari F, Emadi-Baygi M, Nikpour P. miR-17-92 host gene, uderexpressed in gastric cancer and its expression was negatively correlated with the metastasis. Indian J Cancer. 2015;52:22-25. [PubMed] [DOI] |
| 52. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [PubMed] [DOI] |
| 53. | Diosdado B, van de Wiel MA, Terhaar Sive Droste JS, Mongera S, Postma C, Meijerink WJ, Carvalho B, Meijer GA. MiR-17-92 cluster is associated with 13q gain and c-myc expression during colorectal adenoma to adenocarcinoma progression. Br J Cancer. 2009;101:707-714. [PubMed] [DOI] |
| 54. | Koga Y, Yasunaga M, Takahashi A, Kuroda J, Moriya Y, Akasu T, Fujita S, Yamamoto S, Baba H, Matsumura Y. MicroRNA expression profiling of exfoliated colonocytes isolated from feces for colorectal cancer screening. Cancer Prev Res (Phila). 2010;3:1435-1442. [PubMed] [DOI] |
| 55. | Ng EK, Chong WW, Jin H, Lam EK, Shin VY, Yu J, Poon TC, Ng SS, Sung JJ. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375-1381. [PubMed] [DOI] |
| 56. | Yu G, Tang JQ, Tian ML, Li H, Wang X, Wu T, Zhu J, Huang SJ, Wan YL. Prognostic values of the miR-17-92 cluster and its paralogs in colon cancer. J Surg Oncol. 2012;106:232-237. [PubMed] [DOI] |
| 57. | Almeida MI, Nicoloso MS, Zeng L, Ivan C, Spizzo R, Gafà R, Xiao L, Zhang X, Vannini I, Fanini F. Strand-specific miR-28-5p and miR-28-3p have distinct effects in colorectal cancer cells. Gastroenterology. 2012;142:886-896.e9. [PubMed] [DOI] |
| 58. | Li Y, Lauriola M, Kim D, Francesconi M, D'Uva G, Shibata D, Malafa MP, Yeatman TJ, Coppola D, Solmi R. Adenomatous polyposis coli (APC) regulates miR17-92 cluster through β-catenin pathway in colorectal cancer. Oncogene. 2016;35:4558-4568. [PubMed] [DOI] |
| 59. | Tsuchida A, Ohno S, Wu W, Borjigin N, Fujita K, Aoki T, Ueda S, Takanashi M, Kuroda M. miR-92 is a key oncogenic component of the miR-17-92 cluster in colon cancer. Cancer Sci. 2011;102:2264-2271. [PubMed] [DOI] |
| 60. | Meng WJ, Yang L, Ma Q, Zhang H, Adell G, Arbman G, Wang ZQ, Li Y, Zhou ZG, Sun XF. MicroRNA Expression Profile Reveals miR-17-92 and miR-143-145 Cluster in Synchronous Colorectal Cancer. Medicine (Baltimore). 2015;94:e1297. [PubMed] [DOI] |
| 61. | Knudsen KN, Nielsen BS, Lindebjerg J, Hansen TF, Holst R, Sørensen FB. microRNA-17 Is the Most Up-Regulated Member of the miR-17-92 Cluster during Early Colon Cancer Evolution. PLoS One. 2015;10:e0140503. [PubMed] [DOI] |
| 62. | Hermsen M, Postma C, Baak J, Weiss M, Rapallo A, Sciutto A, Roemen G, Arends JW, Williams R, Giaretti W. Colorectal adenoma to carcinoma progression follows multiple pathways of chromosomal instability. Gastroenterology. 2002;123:1109-1119. [PubMed] [DOI] |
| 63. | Sinicrope FA, Rego RL, Okumura K, Foster NR, O'Connell MJ, Sargent DJ, Windschitl HE. Prognostic impact of bim, puma, and noxa expression in human colon carcinomas. Clin Cancer Res. 2008;14:5810-5818. [PubMed] [DOI] |
| 64. | Humphreys KJ, Cobiac L, Le Leu RK, Van der Hoek MB, Michael MZ. Histone deacetylase inhibition in colorectal cancer cells reveals competing roles for members of the oncogenic miR-17-92 cluster. Mol Carcinog. 2013;52:459-474. [PubMed] [DOI] |
| 65. | Cui YM, Jiang D, Zhang SH, Wu P, Ye YP, Chen CM, Tang N, Liang L, Li TT, Qi L. FOXC2 promotes colorectal cancer proliferation through inhibition of FOXO3a and activation of MAPK and AKT signaling pathways. Cancer Lett. 2014;353:87-94. [PubMed] [DOI] |
| 66. | Sokolova V, Fiorino A, Zoni E, Crippa E, Reid JF, Gariboldi M, Pierotti MA. The Effects of miR-20a on p21: Two Mechanisms Blocking Growth Arrest in TGF-β-Responsive Colon Carcinoma. J Cell Physiol. 2015;230:3105-3114. [PubMed] [DOI] |
| 67. | Urtasun R, Elizalde M, Azkona M, Latasa MU, García-Irigoyen O, Uriarte I, Fernández-Barrena MG, Vicent S, Alonso MM, Muntané J. Splicing regulator SLU7 preserves survival of hepatocellular carcinoma cells and other solid tumors via oncogenic miR-17-92 cluster expression. Oncogene. 2016;35:4719-4729. [PubMed] [DOI] |
| 68. | Kutay H, Bai S, Datta J, Motiwala T, Pogribny I, Frankel W, Jacob ST, Ghoshal K. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem. 2006;99:671-678. [PubMed] [DOI] |
| 69. | Murakami Y, Yasuda T, Saigo K, Urashima T, Toyoda H, Okanoue T, Shimotohno K. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene. 2006;25:2537-2545. [PubMed] [DOI] |
| 70. | Zhu H, Han C, Wu T. MiR-17-92 cluster promotes hepatocarcinogenesis. Carcinogenesis. 2015;36:1213-1222. [PubMed] [DOI] |
| 71. | Zhu H, Han C, Lu D, Wu T. miR-17-92 cluster promotes cholangiocarcinoma growth: evidence for PTEN as downstream target and IL-6/Stat3 as upstream activator. Am J Pathol. 2014;184:2828-2839. [PubMed] [DOI] |
| 72. | Komatsu S, Ichikawa D, Takeshita H, Morimura R, Hirajima S, Tsujiura M, Kawaguchi T, Miyamae M, Nagata H, Konishi H. Circulating miR-18a: a sensitive cancer screening biomarker in human cancer. In Vivo. 2014;28:293-297. [PubMed] |
| 73. | Hong L, Lai M, Chen M, Xie C, Liao R, Kang YJ, Xiao C, Hu WY, Han J, Sun P. The miR-17-92 cluster of microRNAs confers tumorigenicity by inhibiting oncogene-induced senescence. Cancer Res. 2010;70:8547-8557. [PubMed] [DOI] |
| 74. | Tao J, Wu D, Li P, Xu B, Lu Q, Zhang W. microRNA-18a, a member of the oncogenic miR-17-92 cluster, targets Dicer and suppresses cell proliferation in bladder cancer T24 cells. Mol Med Rep. 2012;5:167-172. [PubMed] [DOI] |
| 75. | Kim HJ, Kim YH, Lee DS, Chung JK, Kim S. In vivo imaging of functional targeting of miR-221 in papillary thyroid carcinoma. J Nucl Med. 2008;49:1686-1693. [PubMed] [DOI] |
| 76. | Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257-2261. [PubMed] [DOI] |
| 77. | Tai MC, Kajino T, Nakatochi M, Arima C, Shimada Y, Suzuki M, Miyoshi H, Yatabe Y, Yanagisawa K, Takahashi T. miR-342-3p regulates MYC transcriptional activity via direct repression of E2F1 in human lung cancer. Carcinogenesis. 2015;36:1464-1473. [PubMed] [DOI] |
| 78. | Thai SF, Wallace KA, Jones CP, Ren H, Prasad RY, Ward WO, Kohan MJ, Blackman CF. Signaling Pathways and MicroRNA Changes in Nano-TiO2 Treated Human Lung Epithelial (BEAS-2B) Cells. J Nanosci Nanotechnol. 2015;15:492-503. [PubMed] [DOI] |
| 79. | Li J, Yang S, Yan W, Yang J, Qin YJ, Lin XL, Xie RY, Wang SC, Jin W, Gao F. MicroRNA-19 triggers epithelial-mesenchymal transition of lung cancer cells accompanied by growth inhibition. Lab Invest. 2015;95:1056-1070. [PubMed] [DOI] |
| 80. | Smith L, Baxter EW, Chambers PA, Green CA, Hanby AM, Hughes TA, Nash CE, Millican-Slater RA, Stead LF, Verghese ET. Down-Regulation of miR-92 in Breast Epithelial Cells and in Normal but Not Tumour Fibroblasts Contributes to Breast Carcinogenesis. PLoS One. 2015;10:e0139698. [PubMed] [DOI] |
| 81. | Zhang L, Huang J, Yang N, Greshock J, Megraw MS, Giannakakis A, Liang S, Naylor TL, Barchetti A, Ward MR. microRNAs exhibit high frequency genomic alterations in human cancer. Proc Natl Acad Sci USA. 2006;103:9136-9141. [PubMed] [DOI] |
| 82. | Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965-968. [PubMed] [DOI] |
| 83. | Wang Y, Wu MC, Sham JS, Zhang W, Wu WQ, Guan XY. Prognostic significance of c-myc and AIB1 amplification in hepatocellular carcinoma. A broad survey using high-throughput tissue microarray. Cancer. 2002;95:2346-2352. [PubMed] [DOI] |
| 84. | Hossain A, Kuo MT, Saunders GF. Mir-17-5p regulates breast cancer cell proliferation by inhibiting translation of AIB1 mRNA. Mol Cell Biol. 2006;26:8191-8201. [PubMed] [DOI] |
| 85. | Hunter T, Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994;79:573-582. [PubMed] [DOI] |
| 86. | Yu Z, Wang C, Wang M, Li Z, Casimiro MC, Liu M, Wu K, Whittle J, Ju X, Hyslop T. A cyclin D1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. J Cell Biol. 2008;182:509-517. [PubMed] [DOI] |
| 87. | Umayahara K, Numa F, Suehiro Y, Sakata A, Nawata S, Ogata H, Suminami Y, Sakamoto M, Sasaki K, Kato H. Comparative genomic hybridization detects genetic alterations during early stages of cervical cancer progression. Genes Chromosomes Cancer. 2002;33:98-102. [PubMed] [DOI] |
| 88. | Wang X, Wang HK, McCoy JP, Banerjee NS, Rader JS, Broker TR, Meyers C, Chow LT, Zheng ZM. Oncogenic HPV infection interrupts the expression of tumor-suppressive miR-34a through viral oncoprotein E6. RNA. 2009;15:637-647. [PubMed] [DOI] |
| 89. | Servín-González LS, Granados-López AJ, López JA. Families of microRNAs Expressed in Clusters Regulate Cell Signaling in Cervical Cancer. Int J Mol Sci. 2015;16:12773-12790. [PubMed] [DOI] |
| 90. | Wang Y, Hanifi-Moghaddam P, Hanekamp EE, Kloosterboer HJ, Franken P, Veldscholte J, van Doorn HC, Ewing PC, Kim JJ, Grootegoed JA. Progesterone inhibition of Wnt/beta-catenin signaling in normal endometrium and endometrial cancer. Clin Cancer Res. 2009;15:5784-5793. [PubMed] [DOI] |
| 91. | Boren T, Xiong Y, Hakam A, Wenham R, Apte S, Wei Z, Kamath S, Chen DT, Dressman H, Lancaster JM. MicroRNAs and their target messenger RNAs associated with endometrial carcinogenesis. Gynecol Oncol. 2008;110:206-215. [PubMed] [DOI] |
| 92. | Pasqualini L, Bu H, Puhr M, Narisu N, Rainer J, Schlick B, Schäfer G, Angelova M, Trajanoski Z, Börno ST. miR-22 and miR-29a Are Members of the Androgen Receptor Cistrome Modulating LAMC1 and Mcl-1 in Prostate Cancer. Mol Endocrinol. 2015;29:1037-1054. [PubMed] [DOI] |
| 93. | Zhou P, Ma L, Zhou J, Jiang M, Rao E, Zhao Y, Guo F. miR-17-92 plays an oncogenic role and conveys chemo-resistance to cisplatin in human prostate cancer cells. Int J Oncol. 2016;48:1737-1748. [PubMed] [DOI] |
| 94. | O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839-843. [PubMed] [DOI] |
| 95. | Fassina A, Marino F, Siri M, Zambello R, Ventura L, Fassan M, Simonato F, Cappellesso R. The miR-17-92 microRNA cluster: a novel diagnostic tool in large B-cell malignancies. Lab Invest. 2012;92:1574-1582. [PubMed] [DOI] |
| 96. | Gao X, Zhang R, Qu X, Zhao M, Zhang S, Wu H, Jianyong L, Chen L. MiR-15a, miR-16-1 and miR-17-92 cluster expression are linked to poor prognosis in multiple myeloma. Leuk Res. 2012;36:1505-1509. [PubMed] [DOI] |
| 97. | Mu P, Han YC, Betel D, Yao E, Squatrito M, Ogrodowski P, de Stanchina E, D'Andrea A, Sander C, Ventura A. Genetic dissection of the miR-17~92 cluster of microRNAs in Myc-induced B-cell lymphomas. Genes Dev. 2009;23:2806-2811. [PubMed] [DOI] |
| 98. | Olive V, Bennett MJ, Walker JC, Ma C, Jiang I, Cordon-Cardo C, Li QJ, Lowe SW, Hannon GJ, He L. miR-19 is a key oncogenic component of mir-17-92. Genes Dev. 2009;23:2839-2849. [PubMed] [DOI] |
| 99. | Mihailovich M, Bremang M, Spadotto V, Musiani D, Vitale E, Varano G, Zambelli F, Mancuso FM, Cairns DA, Pavesi G. miR-17-92 fine-tunes MYC expression and function to ensure optimal B cell lymphoma growth. Nat Commun. 2015;6:8725. [PubMed] [DOI] |
| 100. | Wong P, Iwasaki M, Somervaille TC, Ficara F, Carico C, Arnold C, Chen CZ, Cleary ML. The miR-17-92 microRNA polycistron regulates MLL leukemia stem cell potential by modulating p21 expression. Cancer Res. 2010;70:3833-3842. [PubMed] [DOI] |
| 101. | Ohyashiki K, Umezu T, Yoshizawa S, Ito Y, Ohyashiki M, Kawashima H, Tanaka M, Kuroda M, Ohyashiki JH. Clinical impact of down-regulated plasma miR-92a levels in non-Hodgkin's lymphoma. PLoS One. 2011;6:e16408. [PubMed] [DOI] |
| 102. | Wang CY, Guo ST, Wang JY, Liu F, Zhang YY, Yari H, Yan XG, Jin L, Zhang XD, Jiang CC. Inhibition of HSP90 by AUY922 Preferentially Kills Mutant KRAS Colon Cancer Cells by Activating Bim through ER Stress. Mol Cancer Ther. 2016;15:448-459. [PubMed] [DOI] |
| 103. | Wu Y, Heinrichs J, Bastian D, Fu J, Nguyen H, Schutt S, Liu Y, Jin J, Liu C, Li QJ. MicroRNA-17-92 controls T-cell responses in graft-versus-host disease and leukemia relapse in mice. Blood. 2015;126:1314-1323. [PubMed] [DOI] |
| 104. | Li Y, Shi Y, McCaw L, Li YJ, Zhu F, Gorczynski R, Duncan GS, Yang B, Ben-David Y, Spaner DE. Microenvironmental interleukin-6 suppresses toll-like receptor signaling in human leukemia cells through miR-17/19A. Blood. 2015;126:766-778. [PubMed] [DOI] |
| 105. | Wang Q, Li YC, Wang J, Kong J, Qi Y, Quigg RJ, Li X. miR-17-92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc Natl Acad Sci USA. 2008;105:2889-2894. [PubMed] [DOI] |
| 106. | Brändstedt J, Wangefjord S, Nodin B, Eberhard J, Jirström K, Manjer J. Associations of hormone replacement therapy and oral contraceptives with risk of colorectal cancer defined by clinicopathological factors, beta-catenin alterations, expression of cyclin D1, p53, and microsatellite-instability. BMC Cancer. 2014;14:371. [PubMed] [DOI] |
| 107. | Northcott PA, Fernandez-L A, Hagan JP, Ellison DW, Grajkowska W, Gillespie Y, Grundy R, Van Meter T, Rutka JT, Croce CM. The miR-17/92 polycistron is up-regulated in sonic hedgehog-driven medulloblastomas and induced by N-myc in sonic hedgehog-treated cerebellar neural precursors. Cancer Res. 2009;69:3249-3255. [PubMed] [DOI] |
| 108. | Yu X, Li Z, Chan MT, Wu WK. The roles of microRNAs in Wilms' tumors. Tumour Biol. 2016;37:1445-1450. [PubMed] [DOI] |
| 109. | Choi HS, Jain V, Krueger B, Marshall V, Kim CH, Shisler JL, Whitby D, Renne R. Kaposi's Sarcoma-Associated Herpesvirus (KSHV) Induces the Oncogenic miR-17-92 Cluster and Down-Regulates TGF-β Signaling. PLoS Pathog. 2015;11:e1005255. [PubMed] [DOI] |
| 110. | Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495-500. [PubMed] [DOI] |
| 111. | Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15-20. [PubMed] [DOI] |
| 112. | Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856-859. [PubMed] [DOI] |
| 113. | Wu H, Cao Y, Weng D, Xing H, Song X, Zhou J, Xu G, Lu Y, Wang S, Ma D. Effect of tumor suppressor gene PTEN on the resistance to cisplatin in human ovarian cancer cell lines and related mechanisms. Cancer Lett. 2008;271:260-271. [PubMed] [DOI] |
| 114. | Olive V, Sabio E, Bennett MJ, De Jong CS, Biton A, McGann JC, Greaney SK, Sodir NM, Zhou AY, Balakrishnan A. A component of the mir-17-92 polycistronic oncomir promotes oncogene-dependent apoptosis. Elife. 2013;2:e00822. [PubMed] [DOI] |
| 115. | Grillari J, Hackl M, Grillari-Voglauer R. miR-17-92 cluster: ups and downs in cancer and aging. Biogerontology. 2010;11:501-506. [PubMed] [DOI] |