修回日期: 2017-04-19
接受日期: 2017-05-02
在线出版日期: 2017-07-08
结直肠癌(colorectal cancer, CRC)是消化系最常见的恶性肿瘤之一, 在我国, 其发病率及死亡率处于逐年上升趋势, 且总体预后相对较差. 近年来, 免疫治疗的基础和临床研究都获得了快速发展, 已成为肿瘤研究的热点. 其中, 免疫检查点抑制剂已经被批准用于包括CRC在内的多种实体肿瘤的临床治疗. 本文将重点阐述免疫检查点的作用、机制和免疫检查点抑制剂在CRC中应用的最新进展, 以及影响其抗肿瘤疗效的因素. 已经完成和正在进行的临床试验肯定了免疫检查点抑制剂在CRC的治疗中的潜力, 尽管部分患者仍对免疫检查点治疗无应答. 因此, 探究免疫检查点抑制剂治疗CRC患者的敏感因素, 对实现个体化精准治疗至关重要. 未来, 免疫检查点抑制剂有望和其他多种治疗方法相联合, 提高患者反应率, 延长患者的生存期.
核心提要: 免疫检查点抑制剂已被批准用于包括结直肠癌(colorectal cancer, CRC)在内的多种实体瘤的治疗, 具有较大的潜力. 探究免疫检查点抑制剂治疗CRC患者的因素, 有利于实现个体化精准治疗. 联合治疗将是未来CRC治疗的发展方向.
引文著录: 王维嘉, 王丹, 秦国慧, 陈新峰, 张毅. 免疫检查点抑制剂在结直肠癌中的应用以及未来发展方向. 世界华人消化杂志 2017; 25(19): 1714-1727
Revised: April 19, 2017
Accepted: May 2, 2017
Published online: July 8, 2017
Colorectal cancer is one of the most common malignancies in the digestive system. The incidence and mortality of colorectal cancer in China have been gradually increasing in recent years, but its overall prognosis remains poor. Nowadays both the basic and clinical research of immunotherapy has advanced rapidly. Immune-checkpoint blockade as an immunotherapy has recently been approved for the clinical treatment of multiple solid cancers including colorectal cancer. This review will focus on the latest advances on the role and mechanism of immune-checkpoint blockade as well as the factors that affect the prognosis of colorectal cancer patients under therapy with immune-checkpoint blockade. Recent clinical investigations and ongoing studies indicate that immune-checkpoint blockade might have potential in the treatment of patients with colorectal cancer, although some patients have no response to this therapy. Therefore, it is important to explore the factors that affect the response to immune-checkpoint blockade in colorectal cancer patients and to select appropriate treatments for individual patients. Immune-checkpoint blockade is expected to be combined with a variety of other treatments to improve patient response and survival in the future.
- Citation: Wang WJ, Wang D, Qin GH, Chen XF, Zhang Y. Immune-checkpoint blockade in colorectal cancer: Current research and future perspectives. Shijie Huaren Xiaohua Zazhi 2017; 25(19): 1714-1727
- URL: https://www.wjgnet.com/1009-3079/full/v25/i19/1714.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v25.i19.1714
结直肠癌(colorectal cancer, CRC)是严重危害人类健康的常见消化系恶性肿瘤之一. 在我国, 其发病率和致死率分别位列第5位和第3位, 且自2000年至2011年, 发病率和致死率呈逐年上升趋势[1]. 研究[2,3]表明, 65岁以上的女性具有更高的死亡率和更低的5年生存率. 目前, CRC的治疗手段主要是手术治疗, T3期中/低位直肠癌(位于距离肛门边缘12 cm)或淋巴结阳性CRC需要结合术前放化疗, 以及额外的辅助治疗[4-8]. 然而, 手术治疗辅以术后化疗, CRC患者的生存期并没有得到明显的改善[9]. 因此, 探究新的治疗手段, 帮助CRC患者改善预后十分必要.
近年来, 免疫治疗的基础和临床研究都获得了快速发展, 已成为国际上肿瘤治疗的研究热点, 《科学》杂志将肿瘤免疫治疗评选为2013年度最重要的科学突破[10]. 2016-02-04美国临床肿瘤学会癌症研究进展年报[11]指出, 肿瘤的免疫治疗已获得突破性进展, 他不仅能够改善患者的预后, 同时也为未来的研究指明方向. 在肿瘤的免疫治疗中, 过继性细胞治疗和免疫检查点抑制剂(immune checkpoint blockade, ICB)在临床试验中取得了巨大的成功[12,13]. 尤其是PD-1/PD-L1阻断剂在黑色素瘤、肺癌、膀胱癌、肾细胞癌、霍奇金淋巴瘤和CRC的治疗中取得了革命性突破[14-19], 被食品和药品管理局(Food and Drug Administration, FDA)批准用于临床治疗, 使得ICB成为肿瘤治疗的热点. 近年来, 越来越多的证据表明, ICB在CRC的治疗方面具有广阔的应用前景[20].
本文旨在探讨免疫检查点的作用和ICB在CRC中应用的最新进展, 以及影响免疫检查点抑制剂效果的因素, 为更全面深入地了解ICB在CRC治疗中的研究现状提供指导意义.
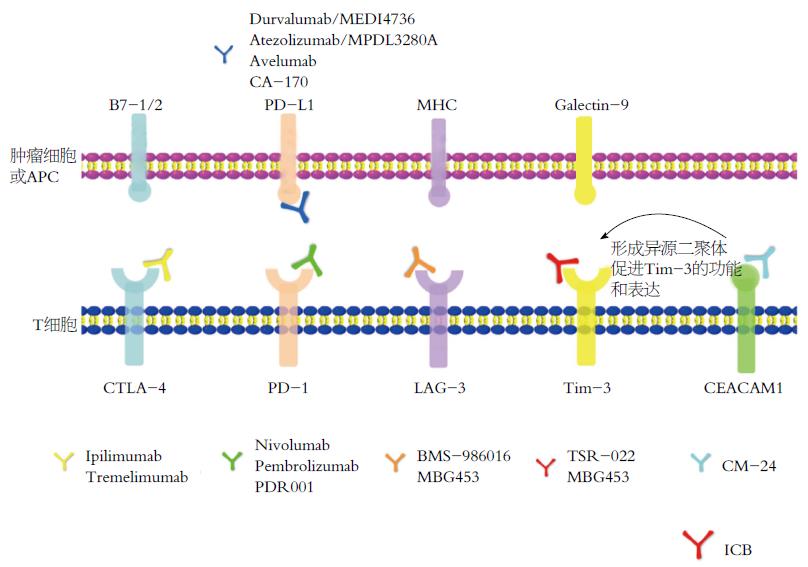
T淋巴细胞作为抗肿瘤免疫应答中的主要效应细胞, 通过识别肿瘤特异性抗原(包括致癌病毒、分化抗原, 表观遗传调控分子, 以及致癌过程中产生的新抗原等)产生细胞毒性反应[21], 其数量与CRC患者复发率和生存率高度相关[22,23]. 正常状态下, T淋巴细胞通过表达一系列激活性(促进T细胞分化增殖)和抑制性(抑制T细胞分化增殖)受体来调控免疫平衡, 既可以调控生理性免疫应答, 又不会过度激活免疫系统而造成机体自我损伤[24,25]. 但在CRC肿瘤微环境中, 随着肿瘤抗原对T细胞的持续刺激, T细胞表面的一系列抑制性受体表达水平升高; 同时, 其配体在CRC细胞或抗原呈递细胞(antigen-presenting cells, APC)表面表达水平增高, 抑制性受体与其配体结合后, 将抑制T细胞活化增殖并诱导T细胞凋亡, 从而导致免疫抑制性肿瘤微环境形成, 使CRC细胞逃避机体免疫系统的监控和杀伤, 造成免疫逃逸[26,27]. T细胞表面表达的抑制性受体主要包括细胞毒T淋巴细胞相关抗原-4(cytotoxic T lymphocyte antigen-4, CTLA-4)[28]、程序性死亡受体-1(programmed death receptor 1, PD-1)[29]、T细胞免疫球蛋白及黏蛋白域3(T cell immune globulin and mucindomain-containing protein 3, Tim-3)[30]、淋巴细胞活化基因-3分子(lymphocyte activation gene-3, LAG-3)[31]、杀伤性免疫球蛋白样受体(killer-cell immunoglobulin-like receptors, KIR)、CD47、TIGIT(T cell immuno receptor with immunoglobulin and ITIM domain)、癌胚抗原相关细胞黏附分子1(carcino-embryonic antigen related cellular adhesion molecule 1, CEACAM1)、腺苷A2A受体(adenosine A2A receptor, A2AR)[32]、吲哚胺2,3-双加氧酶(2,3-dioxygenase 1, IDO1)、B和T淋巴细胞衰减器(B and T lymphocyte attenuator, BTLA)、T细胞活化的V结构域免疫球蛋白抑制剂(V-domain Ig suppressor of T cell activation, VISTA)[33]、CD276[34]、VTCN1[35]等. 这些抑制性受体所对应的抑制性信号通路称为免疫检查点(图1).
CTLA-4, 也称为CD152, 内段含有一个免疫受体酪氨酸抑制基序(immuno receptor tyrosine-based inhibitory motif, ITIM), 主要表达在活化的T细胞或自然杀伤细胞(natural killer cell, NK)表面[36], 可以同CD28受体竞争结合APC上的B7-1/B7-2配体, 且其亲和力强于CD28, 故能抑制免疫刺激[37], 调节T细胞活化的早期步骤. CTLA-4高表达的CD4+调节性T细胞(regulatory T cells, Treg)则通过减少白介素-2(interleukin-2, IL-2)的分泌和下调IL-2受体的表达将T细胞阻滞在G1期[37,38].
PD-1为CD28超家族成员, 是一种重要的免疫抑制分子, 于1992年被Tasuku Honjo教授首先发现. PD-1在T细胞、B细胞、NK细胞、单核细胞和树突状细胞中都有表达[39], 其胞内段含有ITIM、免疫受体酪氨酸转换基序(immuno receptor tyrosine-based switch motif, ITSM), 其中, ITSM的激活与效应性T细胞应答活性密切相关. PD-L1为PD-1的主要配体, 在肿瘤免疫微环境中高度表达. PD-1与PD-L1结合后, 通过mTOR以及PI3K/AKT通路抑制效应T细胞的活性、增强Treg的功能、诱导无反应性和抗原特异性T淋巴细胞的细胞凋亡, 从而抑制干扰素-γ(interferon-γ, IFN-γ)、IL-2和肿瘤坏死因子-α的合成, 增加IL-10的生成[40,41].
LAG-3是在活化的T细胞、NK细胞、B细胞、APC等细胞表面表达的另一种分子, 与其配体MHCⅡ类分子结合后, 既可以抑制CD4+ T淋巴细胞的抗原依赖性刺激[42], 又能够调节Treg细胞的功能[43-46], 从而维持机体免疫耐受. LAG-3既与CTLA-4功能相似, 可以促进T细胞周期延长并诱导T细胞凋亡; 又能够和PD-1/PD-L1发挥协同作用, 诱导T细胞凋亡, 降低自体免疫功能[47,48]. 在慢性感染模型中和肿瘤患者体内均发现LAG-3和PD-1的共同表达[49,50].
Tim-3于2002年被发现, 表达在活化的T细胞、NK细胞和单核细胞表面, 通过与其配体半乳凝素-9(galectin-9)相结合, 抑制IFN-γ的分泌并诱导Th1细胞的凋亡[51,52], 维持免疫耐受; 同时, 也能通过调控T细胞的平衡, 维持肠道免疫稳态[52]. Tim-3通过其胞内段的第256位和第263位两个关键酪氨酸酶位点, 构成SH2结构域结合位点, 进而招募STAT1, 抑制STAT1-miR-155-SOCS1信号轴, 继而上调IL-2、Arg-1等分子的转录与表达, 最终极化巨噬细胞[53].
CEACAM1是癌胚抗原(CEA)家族的成员, 有两个亚型, 分别是CEACAM1-L和CEACAM1-S, CEACAM1-L胞内段含两个ITIM, T细胞活化时引起CEACAM1-L丝氨酸残基磷酸化, 并促使其转移到T细胞膜表面, CEACAM1-L单体通过氨基末端的免疫球蛋白区域与肿瘤或免疫细胞膜表面CEACAM1单体相连, 从而参与抑制性信号的转导, 主要表现为抑制T细胞活化, 减少IFN-γ、IL-4和IL-2等细胞因子的分泌[54]. 其可通过自身的相互作用或者与Tim-3分子远端N-黏性末端顺式及反式结合形成异二聚体, 促进Tim-3的成熟和表达, 协助其介导T细胞衰竭, 在抗肿瘤免疫中具有关键作用[55].
近几年, 研究人员开发出多种ICB, 以阻断免疫抑制信号的传递, 逆转肿瘤免疫微环境, 恢复T细胞抗肿瘤活性, 增强内源性抗肿瘤免疫效应(表1).
| 免疫检查点 | ICB | 肿瘤 | 联合治疗方案 | 研究期别 | NCT编号 |
| CTLA-4 | ipilimumab | 复发或转移的MSI-H和MSI-L的结肠癌 | +ivolumab | Ⅱ期 | NCT02060188 |
| 包括CRC在内的多种转移瘤 | +放疗 | Ⅰ/Ⅱ期 | NCT01769222 | ||
| tremelimumab | 可切除的CRC肝转移 | +MEDI4736(in resectable mets) | Ⅰ期 | NCT02754856 | |
| (ticilimumab,CP675, | 转移性CRC或非小细胞肺癌 | +MEDI4736& XR | Ⅱ期 | NCT02888743 | |
| 206) | CRC | +MEDI4736 | Ⅱ期 | NCT02870920 | |
| 包括CRC在内的多种晚期肿瘤 | +MEDI4736 | Ⅱ期 | NCT01975831 | ||
| PD-1 | nivolumab | MSI-HCRC | +TAS-102 | Ⅱ期 | NCT02860546 |
| 包括CRC在内的多种肿瘤 | + Epacadostat | Ⅰ/Ⅱ期 | NCT02327078 | ||
| 包括CRC在内的多种转移瘤 | +化疗 | Ⅰ/Ⅱ期 | NCT02423954 | ||
| 包括CRC在内的多种肿瘤 | +Enadenotucirev | Ⅰ期 | NCT02636036 | ||
| 包括CRC在内的多种实体瘤 | +BMS-986016 | Ⅰ/Ⅱ期 | NCT01968109 | ||
| pembrolizumab | MSI-H和MSI-L的CRC以及MSI-H的其他肿瘤 | Alone | Ⅱ期 | NCT01876511 | |
| CRC | Alone | Ⅱ期 | NCT02460198 | ||
| CRC | +化疗 | Ⅲ期 | NCT02563002 | ||
| 复发IV期CRC | +cetuximab | Ⅰ/Ⅱ期 | NCT02713373 | ||
| IV期CRC或转移性肝癌 | +放疗 | Ⅰ期 | NCT02837263 | ||
| 转移性CRC | +放疗或射频消融术 | Ⅱ期 | NCT02437071 | ||
| CRC | +mFOLFOX6 | Ⅱ期 | NCT02375672 | ||
| CRC | +romidepsin or +romidepsin | Ⅰ期 | NCT02512172 | ||
| 转移性CRC | +Azacitidine | Ⅱ期 | NCT02260440 | ||
| 转移性CRC | +Napabucasin | Ⅰ/Ⅱ期 | NCT02851004 | ||
| 包括CRC在内的多种肿瘤 | +AMG820 | Ⅰ/Ⅱ期 | NCT02713529 | ||
| 包括IV期CRC在内的多种肿瘤 | +Ziv-aflibercept | Ⅰ期 | NCT02298959 | ||
| 胃肠道肿瘤(包括CRC) | +mFOLFOX6 | Ⅰ/Ⅱ期 | NCT02268825 | ||
| 包括CRC在内的多种肿瘤 | +cetuximab | Ⅰ/Ⅱ期 | NCT02318901 | ||
| 包括MSI-H的CRC在内的多种肿瘤 | +itacitinib; +INCB050465 | Ⅰ期 | NCT02646748 | ||
| 包括MSI-HCRC在内的多种肿瘤 | +INCB024360 | Ⅰ/Ⅱ期 | NCT02178722 | ||
| 包括CRC在内的多种实体肿瘤 | +Nintedanib | Ⅰ期 | NCT02856425 | ||
| CRC | +Poly-ICLC | Ⅰ/Ⅱ期 | NCT02834052 | ||
| PDR001 | 包括CRC在内的多种恶性肿瘤 | Alone | Ⅰ期 | NCT02678260 | |
| 包括CRC在内的多种肿瘤 | +LCL161, Everolimus or Panobinostat | Ⅰ期 | NCT02890069 | ||
| 包括CRC在内的多种肿瘤 | +ACZ885+CJM112+TMT212+EGF816 | Ⅰ期 | NCT02900664 | ||
| 包括CRC在内的多种实体肿瘤 | +BLZ945 | Ⅰ/Ⅱ期 | NCT02829723 | ||
| 包括CRC在内的多种高级实体瘤 | +LAG525 | Ⅰ/Ⅱ期 | NCT02460224 | ||
| PD-L1 | durvaluma | CRC | Alone | Ⅱ期 | NCT02227667 |
| b/MEDI4736 | 包括CRC在内的多种肿瘤 | +Pexidartinib | Ⅰ期 | NCT02777710 | |
| 包括CRC在内的多种肿瘤 | +Olaparib+Olaparib&Cediranib | Ⅰ/Ⅱ期 | NCT02484404 | ||
| 包括MSI-H的CRC在内的多种肿瘤 | +Azacitidine | Ⅱ期 | NCT02811497 | ||
| 包括CRC在内的多种肿瘤 | +Selumetinib | Ⅰ期 | NCT02586987 | ||
| atezolizumab/MPD | 包括CRC在内的多种晚期或转移瘤 | Alone | Ⅰ期 | NCT01375842 | |
| L3280A | CRC | +Cobimetinib&Regorafenib | Ⅲ期 | NCT02788279 | |
| CRC | +Bevacizumab&Cobimetinib | Ⅰ期 | NCT02876224 | ||
| 包括CRC在内的多种肿瘤 | +CPI-444 | Ⅰ期 | NCT02655822 | ||
| 包括CRC在内的多种转移瘤 | +Bevacizumab+化疗 | Ⅰ期 | NCT01633970 | ||
| 复发或晚期CRC | +Capecitabine&Bevacizumab | Ⅱ期 | NCT02873195 | ||
| MSI-H的Ⅲ期CRC | +化疗 | Ⅲ期 | NCT02912559 | ||
| avelumab | 包括CRC在内的转移或高级别实体瘤 | Alone | Ⅰ期 | NCT01772004 | |
| 包括CRC在内的高级恶性实体瘤 | +PF05082566+PF-04518600+PD 0360324 | Ⅰ期 | NCT02554812 | ||
| CA-170 | 包括CRC在内的多种肿瘤 | Alone | Ⅰ期 | NCT02812875 | |
| LAG-3 | BMS-986016 | 包括CRC在内的多种高级或转移实体瘤 | +nivolumab | Ⅰ期 | NCT02817633 |
| MBG453 | 包括CRC在内的多种高级恶性肿瘤 | +PDR001 | Ⅰ/Ⅱ期 | NCT02608268 | |
| TIM3 | TSR-022 | 包括CRC在内的多种肿瘤 | ±nivolumab | Ⅰ期 | NCT02817633 |
| MBG453 | 包括CRC在内的多种高级恶性肿瘤 | ±PDR001 | Ⅰ/Ⅱ期 | NCT02608268 | |
| CEACAM1 | CM-24 | 包括CRC在内的多种肿瘤 | ±pembrolizumab | Ⅰ期 | NCT02346955 |
3.1.1 ipilimumab: ipilimumab是最早获得FDA批准并用于临床的ICB[59], 但在CRC中治疗效果却并不理想. 3例CRC患者对ipilimumab无应答, 其中一名患者因疾病进展在30 d内死亡[60]. 目前有一项采用Ipilimumab与化疗联用的方法, 针对包括CRC在内的多种转移瘤患者的Ⅱ期临床试验正在进行(NCT01769222).
3.1.2 tremelimumab: tremelimumab是完全人源化的抗CTLA-4单克隆抗体, 已获得FDA批准用于临床治疗[61]. 在一项由Chung[62]主持的Ⅱ期临床试验中, 采用tremelimumab治疗标准化疗失败的结直肠转移癌患者, 其中仅有1例患者接受了第2次治疗, 其余44例患者均在3 mo之内出现了疾病进展或死亡. 同时, 治疗伴随显著的不良反应, 包括腹泻、溃疡性结肠炎等. 有研究[63]表明, 相比于微卫星不稳定性(microsatellite instability, MSI)低的CRC, CTLA-4在MSI高的CRC中的TIL细胞以及肿瘤周围基质均有较高水平的表达. 故MSI-H和MSI-L的CRC患者分别采用tremelimumab治疗的临床试验正在进行(NCT02060188).
3.2.1 nivolumab: 虽然在黑色素瘤和肺癌的治疗中取得了鼓舞人心的临床效果[14,64], 但抗PD-1抗体nivolumab在CRC中却未能取得同样的效果[65]. Topalian等[66]提出, nivolumab在接受治疗的19例CRC患者中均没有产生临床反应, 且有2例患者死于药物相关性肺炎. 推测可能与CRC肿瘤细胞表面PD-L1表达为阴性有关. 进一步对CRC患者基因测序显示: MSI-H的CRC患者比MSI-L的CRC患者的肿瘤细胞表面表达更多的PD-L1, 提示MSI的高低与抗PD-1/PD-L1单克隆抗体治疗效果有关. 最新研究[67]结果表明, 对74例MSI-H的CRC患者应用Nivolumab治疗, 效果良好.
3.2.2 pembrolizumab: 2015年, Le等[68]报道: 在一项使用pembrolizumab治疗41例CRC患者的Ⅱ期临床试验中, 1次/2 wk, 每次10 mg/kg, 20 wk后, 观察到MSI-H的CRC患者的客观缓解率为40%(4/10), 无进展生存率(progression-free-survival, PFS)为78%(7/9); 相反, 在MSI-L的CRC患者中, 并未观察到客观缓解率(0/18), PFS仅为11%(2/18). 最常见不良反应为皮疹/瘙痒(17%)、胰腺炎(15%)和甲状腺炎/甲状腺功能减退(10%)进一步经过全基因组外显子测序后, 研究人员[69]发现体细胞突变高载荷的肿瘤患者无进展生存期相对较长(P = 0.02). 这一观点随后得到了证实. 基于此, pembrolizumab已经获得FDA的批准, 主要用于MSI-H的转移性CRC患者的治疗.
3.2.3 PD-L1抗体: 采用抗PD-L1抗体BMS936559对18例CRC患者进行治疗的Ⅰ期临床试验中没有观察到客观反应[70]. 然而, 使用另一种抗PD-L1抗体MPDL3280A治疗CRC患者, 4例患者中有1例获得客观临床反应[71]. 有研究者[72]认为, 细胞表面的PD-L1表达水平提示CRC患者是否适宜接受ICB治疗和化疗.
类似于CTLA-4和PD-L1, 相比微卫星稳定的肿瘤, LAG-3在MSI-H的肿瘤中的表达水平高得多. 通过对108对CRC肿瘤组织与癌旁组织进行对比分析, 发现CRC组织中, LAG-3的表达水平与预后呈负相关[73]. 体内实验证实PD-1和LAG-3联用对CD8+ T细胞的功能抑制更强, 且减弱了抗肿瘤免疫应答[49]. 在卵巢癌模型中, 应用抗LAG-3/PD-1抗体联合治疗小鼠可以促进肿瘤特异性应答, 比单抗治疗具有更好的预后, 且相较于CTLA-4阻断剂毒性更低[47]. 基于此, 一项中单独和联合使用的抗LAG-3单克隆抗体(BMS-986016)治疗CRC患者临床试验已经展开(NCT01968109).
在CRC患者体内, Tim-3+PD-1+CD8+T细胞水平在肿瘤内显著升高, 但分泌的IFN-γ却显著低于Tim-3-PD-1+CD8+T细胞[74], 且其表达水平与结肠癌淋巴转移、TNM分期以及患者生存期高度相关(P<0.0001)[75]. 基于PD-1和Tim-3的同时高表达, 一项将抗Tim-3抗体TSR-022与抗PD-1抗体联用的临床试验正在进行.
CEACAM1是一种调节结肠细胞凋亡的肿瘤抑制因子, 其表达水平降低是CRC早期肿瘤发生中最常见的事件之一[76]. 在黑色素瘤小鼠模型中使用鼠抗体MRG1, 能显著增强T细胞抗肿瘤效应, 其主要在肿瘤细胞和迟发型效应淋巴细胞间相互作用, 因此被认为是一种安全有效的治疗手段[77]. 同时, 有研究者[78]证明, 在非小细胞肺癌中, 抗CEACAM1抗体可以增强CIK的疗效. 抗CEACAM1抗体CM-24单用或与抗PD-1单抗联用以治疗CRC患者的临床实验正在进行.
除此之外, IDO抗体NLG-919、NLG-9189、INCB024360, KIR抗体lirimulab等抗体也已经进入临床试验阶段, 为ICB治疗临床CRC患者提供了前期基础.
4.1.1 MSI: MSI-H来自缺陷型错配修复蛋白(defcient mismatch repair, dMMR), 在遗传性Lynch综合征和约15%的散发性CRC病例中可见, 在年轻CRC患者和疾病进展早期常见[79]. MSI-H的CRC的特征在于具有异常高的突变负荷、肿瘤-增殖淋巴细胞(tumor infiltuating lymphocyte, TIL)和多个免疫检查点的表达, 包括PD-1、PDL1、CTLA-4、LAG-3和IDO, 其原因主要为对细胞因子如IFN-γ等的应答[80,81]. 与MSI-L的患者相比, MSI-H的CRC患者通常具有更好的预后与更低的复发率[82,83], 且用ICB治疗效果更好[84]. 但是, 据报道[85], 有一例属于微卫星稳定(microsatellite stable , MSS)的polymerase ε(POLE)突变的81岁CRC患者, 其90%以上CD8+TIL细胞表达PD-1, 肿瘤微环境中聚集在TIL细胞周围的非肿瘤细胞有99%表达PD-L1.
4.1.2 KRAS: 与MSI相反, KRAS和NRAS突变与相对少的免疫细胞浸润和相对低的抑制分子表达相关. KRAS突变的CRC中, CD4+ T细胞水平显著降低[86]. 因此, KRAS突变CRC处于免疫相对静止的肿瘤微环境状态, ICB的治疗效果可能不佳, 相反, 化疗会相对适合. 此外, 在小鼠模型中, 提示致癌性KRAS驱动并维持了CRC的侵袭和转移[87].
此外, CRC中常见的BRAF突变通常提示预后不良, 将BRAF抑制剂与ICB联合使用, 可进一步增强免疫激活. 这提示我们基因测序有助于帮助CRC患者选择更合适的治疗方案.
自Stephen Paget于1889年针对肿瘤转移的器官特异性提出肿瘤转移的"种子-土壤"学说以来, 免疫微环境的概念逐渐清晰[88]. 肿瘤微环境由内皮细胞和他的前体细胞、周皮细胞、骨髓来源的抑制细胞(myeloid-derived suppressor cells, MDSC)、肿瘤相关成纤维细胞(cancer-associated fibroblast, CAF)、肿瘤相关巨噬细胞(tumor-associated macrophage, TAM)、T细胞和B细胞、NK细胞、DC细胞等组成. 肿瘤微环境中存在影响T细胞的活化和功能的因素[89]. 肿瘤微环境通过多种机制影响T细胞的活化、代谢, 促进T细胞表面抑制性分子的上调及诱导T细胞向终末状态分化, 使T细胞耗竭、无能[90].
4.2.1 MDSC: 既可以直接作用于T细胞抑制其活化[91], 也可以通过产生活性氮诱导CCL2转变为N-CCL2, 从而抑制T细胞浸润, 帮助肿瘤细胞实现免疫逃逸[92].
4.2.2 Treg: Treg细胞能够促进肿瘤细胞和CAF中血管内皮生长因子的产生, 降低T细胞产生的IFN-γ和颗粒酶来抑制免疫杀伤作用[93,94]. 在CRC肝转移患者体内具有大量激活的Treg细胞, 能够抑制特异性T细胞反应, 并表达高水平的糖皮质激素诱导的肿瘤坏死因子受体相关蛋白(glucocorticoid-induced TNFR-related protein, GITR )和CTLA-4[95].
4.2.3 PSGL-1: CD8+T细胞表面黏附分子P-选择素糖蛋白配体-1(P-selectin glycoprotein ligand-1, PSGL-1)的分子能够增加免疫检查点水平, 并抑制TCR和IL-2的信号转导进而抑制T细胞活性. 当PSGL-1缺失, T细胞的制动系统解除, T细胞就能够保持活跃状态[96]. 在黑色素瘤小鼠模型中, PSGL-1缺乏导致PD-1下调, 改善的T细胞应答, 肿瘤生长速度相较于PSGL-1未缺失小鼠明显减缓[96]. 而肿瘤浸润的T淋巴细胞可以产生IFN-γ, 增加PD-1/PD-L1的表达水平[97].
4.2.4 共刺激受体: 此外, 肿瘤浸润性T细胞常表现为低反应性, 同肿瘤患者的生存期呈正相关[98,99]. 研究[100-102]表明, 肿瘤浸润性T细胞可以通过共刺激受体(例如肿瘤坏死因子受体超家族成员OX40、CD40、41BB, B7-CD28免疫球蛋白超家族成员ICOS等)活化, 共刺激受体与其配体相结合, 通过增强Th1细胞的功能或者抑制Treg细胞的功能, 杀伤肿瘤细胞. 共刺激受体激动剂能够增强T细胞的活化, 与ICB联用能够增强抗肿瘤效率.
4.2.5 肿瘤浸润的T淋巴细胞密度: 肿瘤浸润的T淋巴细胞具有免疫监视的作用, 能够抑制与PD-1与PD-L1的结合, 对抗肿瘤的发生和发展, 其密度与抵抗效果正相关, 并能够用于预测肿瘤的发生[103].
除了肿瘤微环境以及癌细胞内源信号的特征以外, 患者的其他一些生理特征也造成了免疫疗法效应不佳的结果. 其中包括年龄、HLA类型、遗传背景、饮食与代谢差异以及慢性感染病史等[104]. 另外, CRC患者的肠道的微生物能够调控机体免疫状态. 有证据表明CTLA-4的阻断疗法能够通过影响上皮间淋巴细胞与肠道表皮细胞的稳态而促进多种拟杆菌属的菌类的增殖, 这些细菌能够通过影响黏膜处的DC促进抗肿瘤Th1细胞的激活[105]. 临床试验结果表明, 在接受了ipilimumab治疗后, 部分黑色素瘤患者表现出肠道拟杆菌数量的上升.
总之, CRC患者经过基因筛选后, 进一步通过靶向抑制性细胞或激活T细胞表面的共刺激受体等多种重塑免疫微环境的手段进行治疗, 能够有效提高CRC患者ICB的治疗效果; 另外, 如果能够找到促进肿瘤免疫反应的微生物抗原结构, 并将其作为肿瘤疫苗的组成部分进行研发, 与ICB联用可能会有意想不到的效果.
以PD-1为代表的ICB在CRC患者的临床治疗中取得了显著疗效, 但由于肿瘤中存在多种免疫抵抗机制, 仍有部分患者无应答. 临床实践证明采用单一的治疗方法难以取得最佳的效果, 只有联合治疗才能更好地改善患者的预后, 是未来的发展方向. 除了多种ICB联合应用以外, ICB既可以和放化疗[106,107]、手术治疗等传统治疗方法联合应用, 也可以和疫苗、细胞因子[108]、VEGF抗体[109]、共刺激受体激动剂[110]、PI3K激酶抑制剂[111]、过继性细胞免疫疗法以及其他ICB等免疫疗法共同使用. 总之, ICB和其他各种治疗方案的联合将给癌症患者提供了长期控制、甚至于治愈疾病的新型疗法, 其疗效和具体方案等仍有待探索.
随着对免疫检查点探索的不断深入和临床试验的不断开展, 若干种ICB已经或正在被FDA批准, 预计下一步将以单药的形式或者与其他治疗模式相结合应用于临床[112,113]. 然而, 机遇与挑战并存, 仍有部分关键问题并未得到解决. 首先, 在CRC的ICB的治疗过程中, 目前仅有pembrolizumab已被FDA批准用于临床转移性MSI-H的CRC治疗. 然而, MSI-H的患者仅占CRC患者的15%, 对于MSI-L的患者仍无很好的治疗方法. 其次, PD-1/PD-L1抑制剂用于POLEε突变的CRC患者的良好预后提示我们有必要对CRC患者进行更全面的基因测序和更精确严谨的分类. 再者, 由于患者个体化差异较大, 相同的治疗方案用于不同的患者可能预后会有较大差异, 因此, 除了需要全面检查患者基因突变的状态外, 寻找预测免疫治疗效果的生物标记势在必行. 而且, 大量证据表明辐射和化疗药物的暴露可能影响肿瘤细胞DNA突变率, 促使一些新抗原的形成或者MSI的改变. 当ICB与放化疗联合应用时, 确定放疗的剂量、强度及持续时间, 或者定时放化疗是联合治疗取得最大效益的先决条件.
最后, 临床研究[114]表明, ICB可能会产生耐药性. 以PD-L1为例, 接受PD-L1单克隆抗体治疗后的小鼠, 其体内原本的耗竭的T细胞的表观遗传学模式只发生了微小变化, 无法变成效应T细胞或记忆T细胞. 研究表明JAK抑制剂[115]和PI3K抑制剂[111]和能够帮助检查点抑制剂无反应的CRC患者克服这种耐药性. 未来关于如何克服ICB的耐药性, 延长CRC患者生存时间尚需进一步探索.
通过阻断T细胞表面表达的抑制性受体, 提高机体免疫应答水平, 进而杀伤肿瘤已被证明是肿瘤免疫疗法临床发展的一个十分有前景的领域. 明确CRC患者影响免疫检查点治疗效果的因素可以帮助我们筛选出对ICB疗法敏感的患者. 采取ICB疗法与现有的或者新的治疗模式相结合的治疗策略将是今后CRC治疗的发展方向.
《科学》杂志将肿瘤免疫治疗评选为2013年度最重要的科学突破. 2016-02-04, 美国临床肿瘤学会癌症研究进展年报指出, 肿瘤的免疫治疗已获得突破性进展, 他不仅能够改善患者的预后, 同时也为未来的研究指明方向. 在肿瘤的免疫治疗中, 过继性细胞治疗和免疫检查点抑制剂在临床试验中取得了巨大的成功, 尤其是PD-1/PD-L1阻断剂在黑色素瘤、肺癌、膀胱癌、肾细胞癌、霍奇金淋巴瘤和结直肠癌(colorectal cancer, CRC)的治疗中都取得了革命性突破, 被食品和药品管理局批准用于临床治疗.
免疫检查点抑制剂治疗通过抑制免疫检查点活性, 重新激活T细胞对肿瘤的免疫应答效应, 从而达到抗肿瘤的作用. 在已经完成和正在进行的临床试验中, 针对CRC的免疫检查点抑制剂已经展示了潜力, 并且已被批准用于部分CRC的临床治疗.
随着CRC诊断和治疗水平的不断进步, 不同角度不同层面对CRC诊疗的报道逐渐增多, 为大家更深入的了解CRC提供了有利条件. 如, Bhattacharya等报道, 在CRC模型小鼠中, 视黄酸通过激活CD8+ T细胞来阻断或延缓癌症产生. 在小鼠体内, 低水平的视黄酸能够导致肠道组织中CD8+ T细胞的数量下降, 从而增加小鼠体内的肿瘤负荷. 这提示大家微生物群可能驱动结肠癌发生以及视黄酸代谢可能作为CRC治疗靶标的机制.
本文针对及直肠癌免疫检查点抑制剂治疗的现状进行了系统的介绍, 通过现有的或正在进行的具体临床试验结果来说明免疫检查点抑制剂在CRC治疗中的应用情况, 并针对影响CRC免疫检查点抑制剂治疗的因素进行了评价. 较之前类似的文章更系统、具体、清晰, 具有针对性, 对临床应用和研究有较好的借鉴作用.
在CRC免疫检查点抑制剂治疗的临床应用分析的基础上提出与其他靶点、其他免疫治疗方法或者现有治疗模式的结合是今后CRC治疗方向的观点, 并辅以具体试验的证据, 为CRC免疫治疗在临床具体应用方案的制定提供了有力证据.
免疫检查点: 在肿瘤微环境中, 随着肿瘤抗原对T细胞的持续刺激, T细胞表面会高表达一系列抑制性受体, 同时, 其配体在肿瘤细胞或抗原呈递细胞表面表达水平增高, 抑制性受体与其配体结合后, 将抑制T细胞活化增殖并诱导T细胞凋亡, 从而导致免疫抑制性肿瘤微环境形成, 使肿瘤细胞逃避机体免疫系统的监控和杀伤, 造成免疫逃逸;
肿瘤微环境: 指癌细胞周围的各种免疫细胞、成纤维细胞、内皮细胞、血管旁细胞、神经细胞、脂肪细胞、细胞外基质成分及微环境中存在的细胞因子等构成的调控肿瘤细胞发生发展的微环境.
范辉, 副教授, 副主任医师, 江苏省南通市第二人民医院消化科; 赵春玲, 副教授, 潍坊医学院细胞生物学教研室
本文重点阐述免疫检查点的作用、机制和免疫检查点抑制剂在CRC中应用的最新进展, 以及影响免疫检查点抑制剂抗肿瘤疗效的因素. 内容新颖充实, 具有较强的科学性和可读性, 有一定的参考价值.
手稿来源: 邀请约稿
学科分类: 胃肠病学和肝病学
手稿来源地: 河南省
同行评议报告分类
A级 (优秀): 0
B级 (非常好): B, B
C级 (良好): 0
D级 (一般): 0
E级 (差): 0
编辑: 闫晋利 电编:李瑞芳
| 1. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [PubMed] [DOI] |
| 2. | Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, Lee DH, Lee KH. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2013. Cancer Res Treat. 2016;48:436-450. [PubMed] [DOI] |
| 3. | Matsuda A, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H. Cancer incidence and incidence rates in Japan in 2008: a study of 25 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2014;44:388-396. [PubMed] [DOI] |
| 4. | Popek S, Tsikitis VL. Neoadjuvant vs adjuvant pelvic radiotherapy for locally advanced rectal cancer: Which is superior? World J Gastroenterol. 2011;17:848-854. [PubMed] [DOI] |
| 5. | Cellini F, Valentini V. Current perspectives on preoperative integrated treatments for locally advanced rectal cancer: a review of agreement and controversies. Oncology (Williston Park). 2012;26:730-735, 741. [PubMed] |
| 6. | Du CZ, Chen YC, Cai Y, Xue WC, Gu J. Oncologic outcomes of primary and post-irradiated early stage rectal cancer: a retrospective cohort study. World J Gastroenterol. 2011;17:3229-3234. [PubMed] [DOI] |
| 7. | Petersen SH, Harling H, Kirkeby LT, Wille-Jørgensen P, Mocellin S. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev. 2012;CD004078. [PubMed] [DOI] |
| 8. | Benson AB, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, Engstrom PF, Enzinger PC, Fakih MG, Fenton MJ. Metastatic colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:141-152; quiz 152. [PubMed] [DOI] |
| 9. | Grothey A, Sargent DJ. Adjuvant Therapy for Colon Cancer: Small Steps Toward Precision Medicine. JAMA Oncol. 2016;2:1133-1134. [PubMed] [DOI] |
| 10. | Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432-1433. [PubMed] [DOI] |
| 11. | Burstein HJ, Krilov L, Aragon-Ching JB, Baxter NN, Chiorean EG, Chow WA, De Groot JF, Devine SM, DuBois SG, El-Deiry WS. Clinical Cancer Advances 2017: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology. J Clin Oncol. 2017;35:1341-1367. [PubMed] [DOI] |
| 12. | Verdegaal EM. Adoptive cell therapy: a highly successful individualized therapy for melanoma with great potential for other malignancies. Curr Opin Immunol. 2016;39:90-95. [PubMed] [DOI] |
| 13. | Tran E, Robbins PF, Rosenberg SA. 'Final common pathway' of human cancer immunotherapy: targeting random somatic mutations. Nat Immunol. 2017;18:255-262. [PubMed] [DOI] |
| 14. | Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627-1639. [PubMed] [DOI] |
| 15. | Nivolumab Gets FDA Nod for Bladder Cancer. Cancer Discov. 2017;7:OF7. [PubMed] [DOI] |
| 16. | Abdelaziz A, Vaishampayan U. Cabozantinib for Renal Cell Carcinoma: Current and Future Paradigms. Curr Treat Options Oncol. 2017;18:18. [PubMed] [DOI] |
| 17. | Ansell SM. Nivolumab in the Treatment of Hodgkin Lymphoma. Clin Cancer Res. 2017;23:1623-1626. [PubMed] [DOI] |
| 18. | Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:1270-1271. [PubMed] [DOI] |
| 19. | Bever KM, Le DT. An Expanding Role for Immunotherapy in Colorectal Cancer. J Natl Compr Canc Netw. 2017;15:401-410. [PubMed] [DOI] |
| 20. | Oh DY, Venook AP, Fong L. On the Verge: Immunotherapy for Colorectal Carcinoma. J Natl Compr Canc Netw. 2015;13:970-978. [PubMed] [DOI] |
| 21. | Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56-61. [PubMed] [DOI] |
| 22. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. [PubMed] [DOI] |
| 23. | Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944-5951. [PubMed] [DOI] |
| 24. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [PubMed] [DOI] |
| 25. | Yao S, Zhu Y, Chen L. Advances in targeting cell surface signalling molecules for immune modulation. Nat Rev Drug Discov. 2013;12:130-146. [PubMed] [DOI] |
| 26. | Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565-1570. [PubMed] [DOI] |
| 27. | Bryan LJ, Gordon LI. Blocking tumor escape in hematologic malignancies: the anti-PD-1 strategy. Blood Rev. 2015;29:25-32. [PubMed] [DOI] |
| 28. | Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405-413. [PubMed] [DOI] |
| 29. | Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027-1034. [PubMed] [DOI] |
| 30. | Severson JJ, Serracino HS, Mateescu V, Raeburn CD, McIntyre RC, Sams SB, Haugen BR, French JD. PD-1+Tim-3+ CD8+ T Lymphocytes Display Varied Degrees of Functional Exhaustion in Patients with Regionally Metastatic Differentiated Thyroid Cancer. Cancer Immunol Res. 2015;3:620-630. [PubMed] [DOI] |
| 31. | Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492-499. [PubMed] [DOI] |
| 32. | Leone RD, Lo YC, Powell JD. A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy. Comput Struct Biotechnol J. 2015;13:265-272. [PubMed] [DOI] |
| 33. | Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, Lu LF, Gondek D, Wang Y, Fava RA. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208:577-592. [PubMed] [DOI] |
| 34. | Leitner J, Klauser C, Pickl WF, Stöckl J, Majdic O, Bardet AF, Kreil DP, Dong C, Yamazaki T, Zlabinger G. B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur J Immunol. 2009;39:1754-1764. [PubMed] [DOI] |
| 35. | Dangaj D, Lanitis E, Zhao A, Joshi S, Cheng Y, Sandaltzopoulos R, Ra HJ, Danet-Desnoyers G, Powell DJ, Scholler N. Novel recombinant human b7-h4 antibodies overcome tumoral immune escape to potentiate T-cell antitumor responses. Cancer Res. 2013;73:4820-4829. [PubMed] [DOI] |
| 36. | Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235-271. [PubMed] [DOI] |
| 37. | Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res. 2013;19:4917-4924. [PubMed] [DOI] |
| 38. | Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985;161:72-87. [PubMed] [DOI] |
| 39. | Shi L, Chen S, Yang L, Li Y. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol. 2013;6:74. [PubMed] [DOI] |
| 40. | Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704. [PubMed] [DOI] |
| 41. | Liu C, Jiang J, Gao L, Hu X, Wang F, Shen Y, Yu G, Zhao Z, Zhang X. A Promoter Region Polymorphism in PDCD-1 Gene Is Associated with Risk of Rheumatoid Arthritis in the Han Chinese Population of Southeastern China. Int J Genomics. 2014;2014:247637. [PubMed] [DOI] |
| 42. | Huard B, Tournier M, Hercend T, Triebel F, Faure F. Lymphocyte-activation gene 3/major histocompatibility complex class II interaction modulates the antigenic response of CD4+ T lymphocytes. Eur J Immunol. 1994;24:3216-3221. [PubMed] [DOI] |
| 43. | Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503-513. [PubMed] [DOI] |
| 44. | Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004;172:5450-5455. [PubMed] [DOI] |
| 45. | Workman CJ, Vignali DA. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur J Immunol. 2003;33:970-979. [PubMed] [DOI] |
| 46. | Goldberg MV, Drake CG. LAG-3 in Cancer Immunotherapy. Curr Top Microbiol Immunol. 2011;344:269-278. [PubMed] [DOI] |
| 47. | Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917-927. [PubMed] [DOI] |
| 48. | Lucas CL, Workman CJ, Beyaz S, LoCascio S, Zhao G, Vignali DA, Sykes M. LAG-3, TGF-β, and cell-intrinsic PD-1 inhibitory pathways contribute to CD8 but not CD4 T-cell tolerance induced by allogeneic BMT with anti-CD40L. Blood. 2011;117:5532-5540. [PubMed] [DOI] |
| 49. | Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci USA. 2010;107:7875-7880. [PubMed] [DOI] |
| 50. | Baitsch L, Legat A, Barba L, Fuertes Marraco SA, Rivals JP, Baumgaertner P, Christiansen-Jucht C, Bouzourene H, Rimoldi D, Pircher H. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PLoS One. 2012;7:e30852. [PubMed] [DOI] |
| 51. | Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276:97-111. [PubMed] [DOI] |
| 52. | Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141-1143. [PubMed] [DOI] |
| 53. | Jiang X, Zhou T, Xiao Y, Yu J, Dou S, Chen G, Wang R, Xiao H, Hou C, Wang W. Tim-3 promotes tumor-promoting M2 macrophage polarization by binding to STAT1 and suppressing the STAT1-miR-155 signaling axis. Oncoimmunology. 2016;5:e1211219. [PubMed] [DOI] |
| 54. | Chen D, Iijima H, Nagaishi T, Nakajima A, Russell S, Raychowdhury R, Morales V, Rudd CE, Utku N, Blumberg RS. Carcinoembryonic antigen-related cellular adhesion molecule 1 isoforms alternatively inhibit and costimulate human T cell function. J Immunol. 2004;172:3535-3543. [PubMed] [DOI] |
| 55. | Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517:386-390. [PubMed] [DOI] |
| 56. | Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R, Muller AJ. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014;63:721-735. [PubMed] [DOI] |
| 57. | Iwayama Y, Tsuruma T, Mizuguchi T, Furuhata T, Toyota N, Matsumura M, Torigoe T, Sato N, Hirata K. Prognostic value of HLA class I expression in patients with colorectal cancer. World J Surg Oncol. 2015;13:36. [PubMed] [DOI] |
| 58. | Pittari G, Filippini P, Gentilcore G, Grivel JC, Rutella S. Revving up Natural Killer Cells and Cytokine-Induced Killer Cells Against Hematological Malignancies. Front Immunol. 2015;6:230. [PubMed] [DOI] |
| 59. | Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [PubMed] [DOI] |
| 60. | O'Mahony D, Morris JC, Quinn C, Gao W, Wilson WH, Gause B, Pittaluga S, Neelapu S, Brown M, Fleisher TA. A pilot study of CTLA-4 blockade after cancer vaccine failure in patients with advanced malignancy. Clin Cancer Res. 2007;13:958-964. [PubMed] [DOI] |
| 61. | Calabrò L, Morra A, Fonsatti E, Cutaia O, Amato G, Giannarelli D, Di Giacomo AM, Danielli R, Altomonte M, Mutti L. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2013;14:1104-1111. [PubMed] [DOI] |
| 62. | Chung KY, Gore I, Fong L, Venook A, Beck SB, Dorazio P, Criscitiello PJ, Healey DI, Huang B, Gomez-Navarro J. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2010;28:3485-3490. [PubMed] [DOI] |
| 63. | Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43-51. [PubMed] [DOI] |
| 64. | Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23-34. [PubMed] [DOI] |
| 65. | Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 2015;27:39-46. [PubMed] [DOI] |
| 66. | Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454. [PubMed] [DOI] |
| 67. | Toh JW, de Souza P, Lim SH, Singh P, Chua W, Ng W, Spring KJ. The Potential Value of Immunotherapy in Colorectal Cancers: Review of the Evidence for Programmed Death-1 Inhibitor Therapy. Clin Colorectal Cancer. 2016;15:285-291. [PubMed] [DOI] |
| 68. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [PubMed] [DOI] |
| 69. | Lee LH, Cavalcanti MS, Segal NH, Hechtman JF, Weiser MR, Smith JJ, Garcia-Aguilar J, Sadot E, Ntiamoah P, Markowitz AJ. Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod Pathol. 2016;29:1433-1442. [PubMed] [DOI] |
| 70. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [PubMed] [DOI] |
| 71. | Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1-10. [PubMed] [DOI] |
| 72. | Dunne PD, McArt DG, O'Reilly PG, Coleman HG, Allen WL, Loughrey M, Van Schaeybroeck S, McDade S, Salto-Tellez M, Longley DB. Immune-Derived PD-L1 Gene Expression Defines a Subgroup of Stage II/III Colorectal Cancer Patients with Favorable Prognosis Who May Be Harmed by Adjuvant Chemotherapy. Cancer Immunol Res. 2016;4:582-591. [PubMed] [DOI] |
| 73. | Chen J, Chen Z. The effect of immune microenvironment on the progression and prognosis of colorectal cancer. Med Oncol. 2014;31:82. [PubMed] [DOI] |
| 74. | Xu B, Yuan L, Gao Q, Yuan P, Zhao P, Yuan H, Fan H, Li T, Qin P, Han L. Circulating and tumor-infiltrating Tim-3 in patients with colorectal cancer. Oncotarget. 2015;6:20592-20603. [PubMed] [DOI] |
| 75. | Zhou E, Huang Q, Wang J, Fang C, Yang L, Zhu M, Chen J, Chen L, Dong M. Up-regulation of Tim-3 is associated with poor prognosis of patients with colon cancer. Int J Clin Exp Pathol. 2015;8:8018-8027. [PubMed] |
| 76. | Neumaier C, Nittka S, Neumaier M. Loss of expression of the tumor suppressor CEACAM1 links different hereditary colorectal carcinoma subtypes to the genesis of sporadic colorectal carcinoma. Onkologie. 2012;35:563-568. [PubMed] [DOI] |
| 77. | Sapoznik S, Hammer O, Ortenberg R, Besser MJ, Ben-Moshe T, Schachter J, Markel G. Novel anti-melanoma immunotherapies: disarming tumor escape mechanisms. Clin Dev Immunol. 2012;2012:818214. [PubMed] [DOI] |
| 78. | Zhang L, Wang J, Wei F, Wang K, Sun Q, Yang F, Jin H, Zheng Y, Zhao H, Wang L. Profiling the dynamic expression of checkpoint molecules on cytokine-induced killer cells from non-small-cell lung cancer patients. Oncotarget. 2016;7:43604-43615. [PubMed] [DOI] |
| 79. | Raut CP, Pawlik TM, Rodriguez-Bigas MA. Clinicopathologic features in colorectal cancer patients with microsatellite instability. Mutat Res. 2004;568:275-282. [PubMed] [DOI] |
| 80. | Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, Huynh TG, Mino-Kenudson M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod Pathol. 2016;29:1104-1112. [PubMed] [DOI] |
| 81. | Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91:2417-2422. [PubMed] [DOI] |
| 82. | Sinicrope FA, Foster NR, Thibodeau SN, Marsoni S, Monges G, Labianca R, Kim GP, Yothers G, Allegra C, Moore MJ. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 2011;103:863-875. [PubMed] [DOI] |
| 83. | Hutchins G, Southward K, Handley K, Magill L, Beaumont C, Stahlschmidt J, Richman S, Chambers P, Seymour M, Kerr D. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011;29:1261-1270. [PubMed] [DOI] |
| 84. | Gelsomino F, Barbolini M, Spallanzani A, Pugliese G, Cascinu S. The evolving role of microsatellite instability in colorectal cancer: A review. Cancer Treat Rev. 2016;51:19-26. [PubMed] [DOI] |
| 85. | Gong J, Wang C, Lee PP, Chu P, Fakih M. Response to PD-1 Blockade in Microsatellite Stable Metastatic Colorectal Cancer Harboring a POLE Mutation. J Natl Compr Canc Netw. 2017;15:142-147. [PubMed] [DOI] |
| 86. | Lal N, Beggs AD, Willcox BE, Middleton GW. An immunogenomic stratification of colorectal cancer: Implications for development of targeted immunotherapy. Oncoimmunology. 2015;4:e976052. [PubMed] [DOI] |
| 87. | Boutin AT, Liao WT, Wang M, Hwang SS, Karpinets TV, Cheung H, Chu GC, Jiang S, Hu J, Chang K. Oncogenic Kras drives invasion and maintains metastases in colorectal cancer. Genes Dev. 2017;31:370-382. [PubMed] [DOI] |
| 88. | Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98-101. [PubMed] |
| 89. | Liu X, Ranganathan R, Jiang S, Fang C, Sun J, Kim S, Newick K, Lo A, June CH, Zhao Y. A Chimeric Switch-Receptor Targeting PD1 Augments the Efficacy of Second-Generation CAR T Cells in Advanced Solid Tumors. Cancer Res. 2016;76:1578-1590. [PubMed] [DOI] |
| 90. | Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74-80. [PubMed] [DOI] |
| 91. | Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689-695. [PubMed] [DOI] |
| 92. | Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, De Palma A, Mauri P, Monegal A, Rescigno M. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949-1962. [PubMed] [DOI] |
| 93. | Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407:348-354. [PubMed] [DOI] |
| 94. | Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620-2627. [PubMed] [DOI] |
| 95. | Pedroza-Gonzalez A, Zhou G, Singh SP, Boor PP, Pan Q, Grunhagen D, de Jonge J, Tran TK, Verhoef C, IJzermans JN. GITR engagement in combination with CTLA-4 blockade completely abrogates immunosuppression mediated by human liver tumor-derived regulatory T cells ex vivo. Oncoimmunology. 2015;4:e1051297. [PubMed] [DOI] |
| 96. | Tinoco R, Carrette F, Barraza ML, Otero DC, Magaña J, Bosenberg MW, Swain SL, Bradley LM. PSGL-1 Is an Immune Checkpoint Regulator that Promotes T Cell Exhaustion. Immunity. 2016;44:1190-1203. [PubMed] [DOI] |
| 97. | Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I, Mandai M. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112:1501-1509. [PubMed] [DOI] |
| 98. | Reissfelder C, Stamova S, Gossmann C, Braun M, Bonertz A, Walliczek U, Grimm M, Rahbari NN, Koch M, Saadati M. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. J Clin Invest. 2015;125:739-751. [PubMed] [DOI] |
| 99. | Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, Schemmer P, Bruns H, Eiermann T, Price DA. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59:1415-1426. [PubMed] [DOI] |
| 100. | Kumari A, Garnett-Benson C. Effector function of CTLs is increased by irradiated colorectal tumor cells that modulate OX-40L and 4-1BBL and is reversed following dual blockade. BMC Res Notes. 2016;9:92. [PubMed] [DOI] |
| 101. | Zhang Y, Luo Y, Qin SL, Mu YF, Qi Y, Yu MH, Zhong M. The clinical impact of ICOS signal in colorectal cancer patients. Oncoimmunology. 2016;5:e1141857. [PubMed] [DOI] |
| 102. | Schaer DA, Hirschhorn-Cymerman D, Wolchok JD. Targeting tumor-necrosis factor receptor pathways for tumor immunotherapy. J Immunother Cancer. 2014;2:7. [PubMed] [DOI] |
| 103. | Maccalli C, Parmiani G, Ferrone S. Immunomodulating and Immunoresistance Properties of Cancer-Initiating Cells: Implications for the Clinical Success of Immunotherapy. Immunol Invest. 2017;46:221-238. [PubMed] [DOI] |
| 104. | Pitt JM, Vétizou M, Daillère R, Roberti MP, Yamazaki T, Routy B, Lepage P, Boneca IG, Chamaillard M, Kroemer G. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity. 2016;44:1255-1269. [PubMed] [DOI] |
| 105. | Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079-1084. [PubMed] [DOI] |
| 106. | Rodriguez-Ruiz ME, Rodriguez I, Garasa S, Barbes B, Solorzano JL, Perez-Gracia JL, Labiano S, Sanmamed MF, Azpilikueta A, Bolaños E. Abscopal Effects of Radiotherapy Are Enhanced by Combined Immunostimulatory mAbs and Are Dependent on CD8 T Cells and Crosspriming. Cancer Res. 2016;76:5994-6005. [PubMed] [DOI] |
| 107. | He C, Duan X, Guo N, Chan C, Poon C, Weichselbaum RR, Lin W. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat Commun. 2016;7:12499. [PubMed] [DOI] |
| 108. | Chen X, Xu J, Guo Q, Wang L, Yang Y, Guo H, Gu N, Zhang D, Qian W, Hou S. Therapeutic efficacy of an anti-PD-L1 antibody based immunocytokine in a metastatic mouse model of colorectal cancer. Biochem Biophys Res Commun. 2016;480:160-165. [PubMed] [DOI] |
| 109. | Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362. [PubMed] [DOI] |
| 110. | Linch SN, McNamara MJ, Redmond WL. OX40 Agonists and Combination Immunotherapy: Putting the Pedal to the Metal. Front Oncol. 2015;5:34. [PubMed] [DOI] |
| 111. | De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, Budhu S, Ghosh A, Pink M, Tchaicha J. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature. 2016;539:443-447. [PubMed] [DOI] |
| 112. | Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482-491. [PubMed] [DOI] |
| 113. | Kroemer G, Zitvogel L. Abscopal but desirable: The contribution of immune responses to the efficacy of radiotherapy. Oncoimmunology. 2012;1:407-408. [PubMed] [DOI] |