修回日期: 2015-10-30
接受日期: 2015-11-09
在线出版日期: 2016-01-18
肠易激综合征(irritable bowel syndrome, IBS)通常由肠道功能异常引发, 是一种常见的功能性肠胃道紊乱疾病, 其发病机制目前尚不清楚. IBS一般不会威胁患者的生命安全, 但会严重干扰患者的日常生活. 目前, 随着高通量测序技术的发展, 大量研究发现肠道菌失衡, 特别是双歧杆菌失衡, 在IBS的发生发展中具有重要作用. 双歧杆菌能够抵抗肠道病原菌的定植和入侵、增强肠道上皮的屏障作用, 其代谢产物还能够增强肠道的防御作用. 在IBS患者肠道内, 双歧杆菌通常明显减少, 这提示增加肠道双歧杆菌数量可能在治疗IBS中具有积极作用. 本文综述了双歧杆菌与IBS的关系, 探讨了双歧杆菌在辅助治疗IBS的作用, 以期为后续研究奠定基础.
核心提示: 双歧杆菌是人体肠道益生菌, 在治疗肠易激综合征(irritable bowel syndrome, IBS)中具有积极作用. 本文综述了双歧杆菌在治疗IBS过程中可能的机制, 阐述了双歧杆菌类益生菌制剂的价值.
引文著录: 吴彬彬, 杨莉丽, 梁岩. 双歧杆菌与肠易激综合征的研究进展. 世界华人消化杂志 2016; 24(2): 229-235
Revised: October 30, 2015
Accepted: November 9, 2015
Published online: January 18, 2016
Irritable bowel syndrome (IBS) is a common functional gastrointestinal (GI) disorder usually originated from gut dysfunction, and the mechanisms underlying IBS are not clear. IBS can seriously disrupt patient's normal routine, even though it is not life-threatening. With the development of high-throughput sequencing technology, a large number of studies have showed that intestinal flora imbalance does play an important role in the pathogenesis of IBS, especially Bifidobacterium. Bifidobacterium can resist the colonization and invasion of intestinal pathogenic bacteria, and enhance the intestinal epithelial barrier function. Besides, its metabolites also can improve the defense function of the intestinal tract. In the gut of patients with IBS, the number of Bifidobacteria is usually significantly reduced, suggesting that increasing the number of intestinal Bifidobacteria may play a positive role in the treatment of IBS. This paper summarizes the relationship between Bifidobacterium and IBS, and discusses the effect of Bifidobacterium in the adjuvant treatment of IBS.
- Citation: Wu BB, Yang LL, Liang Y. Bifidobacterium and irritable bowel syndrome. Shijie Huaren Xiaohua Zazhi 2016; 24(2): 229-235
- URL: https://www.wjgnet.com/1009-3079/full/v24/i2/229.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v24.i2.229
肠易激综合征(irritable bowel syndrome, IBS)是一种常见的消化系统功能性疾病[1,2]. 据统计, IBS在普通人群中的患病率最高达20%[3], 其主要特征为肠道紊乱、腹痛及腹胀等[4]. 近年来, 随着高通量测序、基因芯片等技术的发展, 研究[5]发现IBS不仅与肠道紊乱有关, 还与肠道菌群紊乱有关. 目前, 已有的研究认为双歧杆菌(Bifidobacterium)在IBS的发生发展中具有重要作用. 本文综述了双歧杆菌与IBS的关系, 阐述了双歧杆菌对IBS的作用机制及在治疗IBS中的潜在价值.
目前, IBS疾病的发病机制尚不清楚, 但大多数学者认为IBS疾病是由多种因素综合作用的结果, 这些因素包括心理变化、肠道能动性和免疫功能的改变、肠道敏感性的增加、神经递质失衡等[6]. IBS诊断的主要依据是罗马委员会修订的现行罗马Ⅲ标准, 包括: (1)腹部不适或疼痛, 至少25%的时间符合排便后改善、发病伴排便频率改变、发病伴粪便性状改变中两项或两项以上; (2)无炎症、解剖学、代谢或肿瘤性疾病的证据可以解释患者的症状. 诊断前至少2 mo症状符合以上标准, 每周至少发作1次. IBS通常分为四类: (1)便秘型IBS(constipation-predominant IBS, IBS-C); (2)腹泻型IBS(diarrhoea-predominant IBS, IBS-D); (3)混合型IBS(mixed-type IBS, IBS-M); (4)未定型IBS(unsubtyped IBS)[7,8]. IBS的分类没有严格的标准, 主要是根据各类病症的严重程度, 因此经常出现交替情况. IBS疾病的治疗有饮食干涉、药物治疗等[9-13], 其中饮食干涉主要针对轻症患者, 而药物治疗主要针对严重患者, 常用的治疗药物有止泻剂、离子通道阻滞剂和微生态制剂等, 其中止泻剂、离子通道阻滞剂具有较大的不良反应[14], 而微生态制剂(如益生菌、益生元)不良反应低, 效果显著[15-17], 越来越受到患者的喜爱.
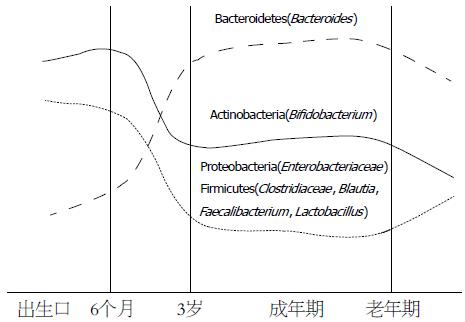
双歧杆菌是由法国学者首次分离出的一种厌氧革兰氏阳性杆菌, 末端常常分叉, 因此称为双歧杆菌. 目前已经发现并测序几十种双歧杆菌, 包括长双歧杆菌(B. longum)、短双歧杆菌(B. breve)和青春双歧杆菌等. 双歧杆菌是肠道内的重要细菌, 在肠道功能和营养等方面发挥着重要作用. 双歧杆菌在人体肠道内是时刻变化的, 不同年龄段, 人体肠道内双歧杆菌具有较大差异(图1). 婴幼儿时期, 肠道内双歧杆菌较多, 但随着年龄的增加, 双歧杆菌逐渐减少. 在整个生命历程中, 肠道内的双歧杆菌维持着动态平衡. 但在某一特定时期, 肠道内双歧杆菌则相对稳定, 这对于肠道正常功能的发挥及人体健康是至关重要的. 一旦双歧杆菌紊乱, 可能会引发危害人体健康的疾病[18]. 科学研究[19,20]发现双歧杆菌失衡与IBS疾病间具有密切关系. 最近, Si等[21]也采用培养基培养的方法研究了IBS患者肠道菌的变化, 发现与正常组相比, IBS患者粪便中双歧杆菌的数量显著减少(P<0.05). Kerckhoffs等[22]采用FISH方法研究了IBS患者和健康人的十二指肠及粪便样本微生物组成, 同样发现IBS患者的双歧杆菌, 特别是长双歧杆菌水平显著降低, Shukla等[23]则通过16S rRNA方法测定了IBS患者的粪便肠道菌群, 发现与正常组相比, IBS亚类水平(IBS-C、IBS-D和IBS-U)的双歧杆菌没有显著变化, 但是在IBS整体水平上, 双歧杆菌显著减少(P<0.05). 大量学者通过不同实验方法发现双歧杆菌与IBS具有密切关系, 这表明双歧杆菌具有潜在的辅助治疗IBS疾病的作用.
双歧杆菌是人体肠道内的益生菌, 研究表明双歧杆菌在IBS的治疗中具有积极意义, 但其治疗的机制目前尚不完全清楚, 可能是其自身及代谢产物等共同作用的结果.
双歧杆菌具有强大的定植和黏附能力, 能够稳定定植在肠道系统内[24]. 在体外实验中, 已有学者证实双歧杆菌能黏附于人类细胞或黏蛋白. 在体内, 双歧杆菌也具有强烈的黏附和定植作用, 能够阻止病原菌及条件致病菌的定植和入侵, 还能够避免其随粪便排出. 双歧杆菌也能够分泌多种与黏附相关的蛋白, 使自己的黏附性强于肠产毒性大肠杆菌、致病性大肠杆菌等大肠埃希氏菌以及痢疾杆菌、沙门氏菌、金黄色葡萄球菌等多种致病菌, 通过竞争性占位黏附于肠上皮细胞, 达到拮抗致病菌和抗感染的作用. 此外, 双歧杆菌还通过与周围环境的其他厌氧菌联合, 占据肠黏膜表面形成特异性微生态圈, 产生生物学屏障, 抑制病原菌及条件致病菌的定植和入侵; 最后, 双歧杆菌无论是否灭活, 均可黏附于肠黏膜上皮细胞, 加强肠道黏膜机械屏障作用.
双歧杆菌对人体的作用目前还不完全清楚, 但随着科技的发展和研究的深入, 有学者发现细菌胞外多糖(surface exopolysaccharide, sEPS)与细菌的定植和持续有关. 细菌sEPS通常是一个复杂的、由单糖或寡糖通过糖苷键连接的结构, 其相关作用是在病毒中首次研究发现的. 最近, Salazar等[25]发现, 长双歧杆菌IPLA E44和B. animalis subsp. lactis IPLA R1的EPS能够耐受胃肠道环境且结构稳定, 而Alp等[26]发现双歧杆菌EPS产量与耐酸耐胆盐之间存在线性关系, 提示EPS能够保护双歧杆菌耐受酸和胆盐, 便于在肠道内存活. Wu等[27]发现长双歧杆菌BCRC 14634胞外多糖在体外具有抑制病原菌的作用, 推测EPS有助于宿主抵抗肠道病原菌感染. Fanning等[28]研究发现产EPS的短双歧杆菌比不产EPS的突变短双歧杆菌能更好地耐受酸和胆盐, 他们还发现短双歧杆菌UCC2003细菌的EPS能够在小鼠肠道内调节B细胞介导的适应性免疫反应的逃避, 预防促炎症因子[如干扰素(interferon, IFN)-g、肿瘤坏死因子-α(tumor necrosis factor α, TNF-α)和白介素(interleukin, IL)-12]的产生, 在与宿主间相互作用和病原体防护方面具有重要作用.
细菌菌毛常常参与肠道菌与肠黏膜的相互作用, 在肠道菌的定植过程、蛋白的分泌和结合等方面中发挥重要作用, 与这些作用有关的菌毛有两种, 一种是Ⅳb, 也称为严谨性菌毛(tight adherence pili, Tad pili), 另一种是分选酶依赖性菌毛(sortase-dependent pili). 最近研究发现在两歧双歧杆菌(B. bifidum)PRL2010中, 分选酶依赖性菌毛不仅能特异性结合到胞外基质成分, 发挥细菌集群作用, 还在调节宿主免疫方面发挥作用. 一方面他能刺激TNF-α的高表达; 另一方面能作为其他促炎症因子(如IL-12)的低水平诱导物. 对于免疫系统尚不成熟的婴儿肠道, 两歧双歧杆菌PRL2010菌毛激发的促炎症因子的存在可能是婴儿发展适当的免疫程序中所必须的[29].
双歧杆菌是人体内重要的益生菌, 对IBS的治疗具有积极作用. 目前, 已有基于双歧杆菌开发益生菌制剂的研究报道, 他们在治疗IBS、缓解腹胀和疼痛等方面具有良好的效果. 最近, Wang等[32]通过使用旋毛虫感染的小鼠PI-IBS(post-infectious irritable bowel syndrome)模型, 研究了双歧杆菌、乳杆菌和链球菌对PI-IBS的治疗效果, 结果发现三种益生菌混合物能够减缓内脏高敏感性, 恢复肠屏蔽作用, 并且效果优于单一益生菌. Guglielmetti等[33]研究了两歧双歧杆菌MIMBb75干涉4 wk后的IBS患者的症状, 发现两歧双歧杆菌MIMBb75能够有效缓解IBS患者的疼痛, 改善IBS症状. Agrawal等[34]则进一步将双歧杆菌用于牛奶发酵, 并研究了发酵牛奶产品对IBS患者的作用, 他们首先使用乳双歧杆菌(B. lactis)DN-173 010和典型发酵细菌S. thermophilus及L. bulgaricus发酵牛奶, 然后让IBS患者饮用, 对患者的腹胀, 结肠运动和IBS症状进行评估, 发现三种细菌发酵的牛奶能够降低最大腹胀百分比, 改善肠道运动和整体症状严重程度. 目前, 研究双歧杆菌或其混菌治疗IBS的报道[32-42]逐渐增多(表1), 且大多数研究结果表明双歧杆菌或其相关产品具有良好的功效, 这为双歧杆菌类益生菌或制剂的研发奠定了基础.
| 双歧杆菌菌株 | 其他菌株 | 治疗效果 | 参考文献 |
| B. longum HB55020 | L. acidophilus HB56003 | 减轻内脏过敏、恢复肠屏障 | [32] |
| S. faecalis HB62001 | |||
| B. bifidum MIMBb75 | - | 改善IBS症状 | [33] |
| B. lactis DN-173 010 | S. thermophilus; L. bulgaricus | 改善肠道转运 | [34] |
| B. infantis 35624 | 减缓腹痛、气胀等 | [35] | |
| B. infantis 35624 | L. salivarius UCC4331 | 疼痛、排便困难减轻 | [36] |
| B. animalis ssp. lactis Bb12 | LGG, DSM7061, DSM7067 | 稳定肠道菌群 | [37] |
| B. lactis DN-173 010 | S. thermophilus; L. bulgaricus | 改善HRQoL不适得分, 减轻腹胀 | [38] |
| B. lactis CNCM I-2494 | L. lactis CNCM I-1632/ 1519, L. lactis CNCM I-1631, S. thermophiles CNCM I-1630 | 改善患者状态 | [39] |
| B. bifidum/lactis/longum | L. rhamnosus/acidophilus, S. thermophilus | 改善腹部疼痛、不适和腹胀 | [40] |
| B. bifidum BGN4/lactis AD011 | L. casei IBS041, L. acidophilus AD031 | 缓解腹痛 | [41] |
| B. lactis CUL34/B. bifidum CUL20 | L. acidophilus CUL60, L. acidophilus CUL21 | 降低疼痛、改善IBS症状得分 | [42] |
益生菌是指对宿主有益的一类活性微生物[43], 他们通常定植在人体肠道或生殖系统内, 能改善宿主微生态平衡并产生确切健康功效. 双歧杆菌是常见的益生菌, 卫生部《益生菌类保健食品评审规定》中指出双歧杆菌中的两歧双歧杆菌、长双歧杆菌、短双歧杆菌、青春双歧杆菌和婴儿双歧杆菌均属于益生菌菌株. 双歧杆菌类益生菌及制剂在IBS等疾病治疗中具有积极作用[44], 其发挥作用的机制可能是[45-47]: (1)双歧杆菌类益生菌及制剂通过调节宿主肠道内的细菌, 改善肠道菌群组成和稳定性; (2)通过结合到肠道上皮细胞, 抑制病原菌的结合; (3)通过增加黏蛋白的产生增强肠道上皮的屏障作用, 预防病原菌对上皮组织的损害, 降低细胞通透性; (4)通过肠道内容物的发酵, 产生短链脂肪酸, 降低肠道pH值, 酸化肠道, 抑制病原菌的生长; (5)IBS患者常常具有免疫紊乱现象, 而双歧杆菌类益生菌能够调节IBS的免疫系统, 治疗IBS; (6)双歧杆菌类益生菌及制剂还能改善IBS患者的肠道蠕动性, 增强患者的肠道运动.
IBS是一种反复发作的肠道疾病, 虽然不会威胁患者的生命安全, 但严重困扰着患者的健康和生活[8,48]. 目前, IBS的发病机制尚不十分清楚, 这给临床上的治疗带来了极大的困难. 双歧杆菌是一类作用效果显著的益生菌[49,50], 近年来, 大量科研成果发现双歧杆菌及其相关制剂能够缓解IBS患者疼痛, 改善IBS症状, 在治疗IBS中具有良好的功效. 因此, 利用双歧杆菌及其制剂治疗IBS具有较强的可行性, 将给IBS患者的康复带来新的希望.
肠易激综合征(irritable bowel syndrome, IBS)是常见的消化系统功能性疾病, 其病因尚不清楚. 目前, 越来越多的研究发现双歧杆菌与IBS密切关系, 补充双歧杆菌能有效缓解IBS症状, 针对IBS疾病的双歧杆菌相关研究越来越受到重视.
姚登福, 教授, 南通大学附属医院
双歧杆菌在治疗IBS中效果显著, 研发已成为本领域的研究热点和重点. 但其作用机制不清一直制约着双歧杆菌的应用.
Wu等发现双歧杆菌胞外多糖具有体外抑制病原菌作用, 提示双歧杆菌有助于宿主抵抗病原菌的感染.
利用双歧杆菌治疗IBS的研究已有报道, 但其作用机制十分复杂, 本文根据已有报道进行了总结.
本文较系统的阐述了双歧杆菌作用于IBS的机制, 为IBS的治疗及相关益生菌制剂的研发起到一定的铺垫作用.
细菌胞外多糖(sEPS): 通常由单糖或寡糖通过糖苷键连接而来, 具有复杂的结构. 研究发现其与细菌的定植和持续有关.
本文综述了双歧杆菌与IBS的关系, 阐述了双歧杆菌对IBS的作用机制及在治疗IBS中的潜在价值, 选题新颖, 对于IBS的治疗具有一定的参考价值.
编辑: 郭鹏 电编:闫晋利
| 2. | Chey WD, Eswaran S, Kurlander J. JAMA patient page. Irritable bowel syndrome. JAMA. 2015;313:982. [PubMed] [DOI] |
| 3. | Torpy JM, Golub RM. JAMA patient page. Irritable bowel syndrome. JAMA. 2011;306:1501. [PubMed] [DOI] |
| 4. | Rao S, Lembo AJ, Shiff SJ, Lavins BJ, Currie MG, Jia XD, Shi K, MacDougall JE, Shao JZ, Eng P. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012;107:1714-1724; quiz 1725. [PubMed] [DOI] |
| 6. | Ringel Y, Maharshak N. Intestinal microbiota and immune function in the pathogenesis of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2013;305:G529-G541. [PubMed] [DOI] |
| 7. | Krogius-Kurikka L, Lyra A, Malinen E, Aarnikunnas J, Tuimala J, Paulin L, Mäkivuokko H, Kajander K, Palva A. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009;9:95. [PubMed] [DOI] |
| 8. | Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G775-G785. [PubMed] [DOI] |
| 11. | de Roest RH, Dobbs BR, Chapman BA, Batman B, O'Brien LA, Leeper JA, Hebblethwaite CR, Gearry RB. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: a prospective study. Int J Clin Pract. 2013;67:895-903. [PubMed] [DOI] |
| 12. | Rahimi R, Abdollahi M. Herbal medicines for the management of irritable bowel syndrome: a comprehensive review. World J Gastroenterol. 2012;18:589-600. [PubMed] [DOI] |
| 13. | Chey WD, Lembo AJ, Lavins BJ, Shiff SJ, Kurtz CB, Currie MG, MacDougall JE, Jia XD, Shao JZ, Fitch DA. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702-1712. [PubMed] [DOI] |
| 14. | Markland AD, Burgio KL, Whitehead WE, Richter HE, Wilcox CM, Redden DT, Beasley TM, Goode PS. Loperamide Versus Psyllium Fiber for Treatment of Fecal Incontinence: The Fecal Incontinence Prescription (Rx) Management (FIRM) Randomized Clinical Trial. Dis Colon Rectum. 2015;58:983-993. [PubMed] [DOI] |
| 15. | 兰 英, 罗 和生. 益生菌制剂治疗肠易激综合征的荟萃分析. 胃肠病学和肝病学杂志. 2011;20:809-813. |
| 17. | Clarke G, Cryan JF, Dinan TG, Quigley EM. Review article: probiotics for the treatment of irritable bowel syndrome--focus on lactic acid bacteria. Aliment Pharmacol Ther. 2012;35:403-413. [PubMed] [DOI] |
| 19. | Jalanka-Tuovinen J, Salojärvi J, Salonen A, Immonen O, Garsed K, Kelly FM, Zaitoun A, Palva A, Spiller RC, de Vos WM. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut. 2014;63:1737-1745. [PubMed] [DOI] |
| 20. | Saulnier DM, Riehle K, Mistretta TA, Diaz MA, Mandal D, Raza S, Weidler EM, Qin X, Coarfa C, Milosavljevic A. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology. 2011;141:1782-1791. [PubMed] [DOI] |
| 21. | Si JM, Yu YC, Fan YJ, Chen SJ. Intestinal microecology and quality of life in irritable bowel syndrome patients. World J Gastroenterol. 2004;10:1802-1805. [PubMed] [DOI] |
| 22. | Kerckhoffs AP, Samsom M, van der Rest ME, de Vogel J, Knol J, Ben-Amor K, Akkermans LM. Lower Bifidobacteria counts in both duodenal mucosa-associated and fecal microbiota in irritable bowel syndrome patients. World J Gastroenterol. 2009;15:2887-2892. [PubMed] [DOI] |
| 23. | Shukla R, Ghoshal U, Dhole TN, Ghoshal UC. Fecal Microbiota in Patients with Irritable Bowel Syndrome Compared with Healthy Controls Using Real-Time Polymerase Chain Reaction: An Evidence of Dysbiosis. Dig Dis Sci. 2015;60:2953-2962. [PubMed] [DOI] |
| 25. | Salazar N, Ruas-Madiedo P, Kolida S, Collins M, Rastall R, Gibson G, de Los Reyes-Gavilán CG. Exopolysaccharides produced by Bifidobacterium longum IPLA E44 and Bifidobacterium animalis subsp. lactis IPLA R1 modify the composition and metabolic activity of human faecal microbiota in pH-controlled batch cultures. Int J Food Microbiol. 2009;135:260-267. [PubMed] [DOI] |
| 26. | Alp G, Aslim B. Relationship between the resistance to bile salts and low pH with exopolysaccharide (EPS) production of Bifidobacterium spp. isolated from infants feces and breast milk. Anaerobe. 2010;16:101-105. [PubMed] [DOI] |
| 27. | Wu MH, Pan TM, Wu YJ, Chang SJ, Chang MS, Hu CY. Exopolysaccharide activities from probiotic bifidobacterium: Immunomodulatory effects (on J774A.1 macrophages) and antimicrobial properties. Int J Food Microbiol. 2010;144:104-110. [PubMed] [DOI] |
| 28. | Fanning S, Hall LJ, van Sinderen D. Bifidobacterium breve UCC2003 surface exopolysaccharide production is a beneficial trait mediating commensal-host interaction through immune modulation and pathogen protection. Gut Microbes. 2012;3:420-425. [PubMed] [DOI] |
| 29. | Turroni F, Serafini F, Foroni E, Duranti S, O'Connell Motherway M, Taverniti V, Mangifesta M, Milani C, Viappiani A, Roversi T. Role of sortase-dependent pili of Bifidobacterium bifidum PRL2010 in modulating bacterium-host interactions. Proc Natl Acad Sci USA. 2013;110:11151-11156. [PubMed] [DOI] |
| 30. | Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543-547. [PubMed] [DOI] |
| 31. | De Vuyst L, Leroy F. Cross-feeding between bifidobacteria and butyrate-producing colon bacteria explains bifdobacterial competitiveness, butyrate production, and gas production. Int J Food Microbiol. 2011;149:73-80. [PubMed] [DOI] |
| 32. | Wang H, Gong J, Wang W, Long Y, Fu X, Fu Y, Qian W, Hou X. Are there any different effects of Bifidobacterium, Lactobacillus and Streptococcus on intestinal sensation, barrier function and intestinal immunity in PI-IBS mouse model? PLoS One. 2014;9:e90153. [PubMed] [DOI] |
| 33. | Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life--a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132. [PubMed] [DOI] |
| 34. | Agrawal A, Houghton LA, Morris J, Reilly B, Guyonnet D, Goupil Feuillerat N, Schlumberger A, Jakob S, Whorwell PJ. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2009;29:104-114. [PubMed] [DOI] |
| 35. | Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O'Mahony L, Kiely B, Shanahan F, Quigley EM. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581-1590. [PubMed] [DOI] |
| 36. | O'Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O'Sullivan GC, Kiely B, Collins JK, Shanahan F. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541-551. [PubMed] [DOI] |
| 37. | Kajander K, Myllyluoma E, Rajilić-Stojanović M, Kyrönpalo S, Rasmussen M, Järvenpää S, Zoetendal EG, de Vos WM, Vapaatalo H, Korpela R. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27:48-57. [PubMed] [DOI] |
| 38. | Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier CH, Matuchansky C. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. 2007;26:475-486. [PubMed] [DOI] |
| 39. | Veiga P, Pons N, Agrawal A, Oozeer R, Guyonnet D, Brazeilles R, Faurie JM, van Hylckama Vlieg JE, Houghton LA, Whorwell PJ. Changes of the human gut microbiome induced by a fermented milk product. Sci Rep. 2014;4:6328. [PubMed] [DOI] |
| 40. | Yoon JS, Sohn W, Lee OY, Lee SP, Lee KN, Jun DW, Lee HL, Yoon BC, Choi HS, Chung WS. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52-59. [PubMed] [DOI] |
| 41. | Hong KS, Kang HW, Im JP, Ji GE, Kim SG, Jung HC, Song IS, Kim JS. Effect of probiotics on symptoms in korean adults with irritable bowel syndrome. Gut Liver. 2009;3:101-107. [PubMed] [DOI] |
| 42. | Williams EA, Stimpson J, Wang D, Plummer S, Garaiova I, Barker ME, Corfe BM. Clinical trial: a multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2009;29:97-103. [PubMed] [DOI] |
| 43. | 雷 芸. 益生菌及其制品研究和应用开发的最新进展. 甘肃联合大学学报(自然科学版). 2013;27:114-116. |
| 44. | Whelan K, Quigley EM. Probiotics in the management of irritable bowel syndrome and inflammatory bowel disease. Curr Opin Gastroenterol. 2013;29:184-189. [PubMed] [DOI] |
| 45. | Hosseini A, Nikfar S, Abdollahi M. Probiotics use to treat irritable bowel syndrome. Expert Opin Biol Ther. 2012;12:1323-1334. [PubMed] [DOI] |
| 46. | Dupont HL. Review article: evidence for the role of gut microbiota in irritable bowel syndrome and its potential influence on therapeutic targets. Aliment Pharmacol Ther. 2014;39:1033-1042. [PubMed] [DOI] |
| 47. | Korpela R, Niittynen L. Probiotics and irritable bowel syndrome. Microb Ecol Health Dis. 2012;23:18573. [PubMed] [DOI] |
| 48. | Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71-80. [PubMed] [DOI] |
| 49. | Ringel-Kulka T, Palsson OS, Maier D, Carroll I, Galanko JA, Leyer G, Ringel Y. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol. 2011;45:518-525. [PubMed] [DOI] |
| 50. | Agostini S, Goubern M, Tondereau V, Salvador-Cartier C, Bezirard V, Lévèque M, Keränen H, Theodorou V, Bourdu-Naturel S, Goupil-Feuillerat N. A marketed fermented dairy product containing Bifidobacterium lactis CNCM I-2494 suppresses gut hypersensitivity and colonic barrier disruption induced by acute stress in rats. Neurogastroenterol Motil. 2012;24:376-e172. [PubMed] [DOI] |