修回日期: 2016-03-27
接受日期: 2016-04-06
在线出版日期: 2016-05-18
林奇综合征曾被称为遗传性非息肉病性结直肠癌, 是一种显性遗传性癌症综合征, 由细胞错配修复基因的可遗传突变引起, 易导致消化系统及女性生殖系统等肿瘤. 目前, 林奇综合征的误诊漏诊率极高, 本文就林奇综合征监测、手术、药物、生活方式、筛查等风险管理的最新进展做一综述.
核心提示: 本文就林奇综合征监测、手术、药物、生活方式、筛查等风险管理方面进行了详实的分析和阐述, 内容较丰富, 学术价值良好, 对临床也有一定的借鉴作用.
引文著录: 沈燕如, 冶生芳, 索朗央金, 陈晓红. 林奇综合征的风险管理. 世界华人消化杂志 2016; 24(14): 2191-2197
Revised: March 27, 2016
Accepted: April 6, 2016
Published online: May 18, 2016
Lynch syndrome has been known as hereditary non-polyposis colorectal cancer (HNPCC), and it is a dominantly inherited cancer syndrome caused by genetic mutations in cell mismatch repair genes, often leading to digestive system and female reproductive system tumors. At present, there is a high misdiagnosis rate for Lynch syndrome. This paper reviews the latest progress in Lynch syndrome risk management with regards to its monitoring, surgical treatment, pharmaceutical treatment, life style improvement and screening.
- Citation: Shen YR, Ye SF, Suo-Lang YJ, Chen XH. Lynch syndrome risk management. Shijie Huaren Xiaohua Zazhi 2016; 24(14): 2191-2197
- URL: https://www.wjgnet.com/1009-3079/full/v24/i14/2191.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v24.i14.2191
林奇综合征(Lynch syndrome, LS)曾被称为遗传性非息肉病性结直肠癌(hereditary non-polyposis colorectal cancer, HNPCC), 是一种显性遗传性癌症综合征, 由细胞错配修复(mismatch repair, MMR)基因的可遗传突变引起, 表现为易患结直肠癌、子宫内膜癌、卵巢癌、胃癌、小肠癌等肿瘤. MMR系统组成复杂, 作用是检测和纠正DNA复制错误, 而MLH1、MSH2、MSH6、PMS2是翻译MMR蛋白质的基因[1-3]. 目前LS的诊断标准主要有AmsterdamⅠ标准、AmsterdamⅡ标准及Bethesda指南, 敏感性和特异性越为60%和70%[4,5]. LS是最常见的遗传性结直肠癌综合征, 发病率约为1/440, 占结直肠癌总数的2%-3%, 美国每35例结直肠癌就有1例源于LS[6], 但目前LS误诊漏诊率极高. 2015年7月美国胃肠病学会(American Gastroenterological Association, AGA)发布了《林奇综合征诊断和管理指南》[6](以下简称AGA指南), 结合2014年美国国立综合癌症网络(National Comprehensive Cancer Network, NCCN)发布的《遗传/家族高风险评估-结直肠癌》[7](以下简称NCCN指南)和2013年欧洲专家组发布的《林奇综合征临床管理指南》[8](以下简称欧洲指南)里的建议和指导方针, 本文就LS的监测、手术、药物、生活方式和筛查等风险管理方面的进展做一阐述.
LS具有肿瘤倾向特征, 使某些特定癌症在没有典型危险因素参与的情况下患病风险明显增高. LS患者患结直肠癌风险高达75%, 中位年龄为44-61岁[9-12]. 普通人群中的结直肠癌约10%发生在50岁之前, 而LS的结直肠癌患者约50%在50岁之前就出现结直肠癌病灶, 并且10年内出现第二个原发病灶的风险约为15%-20%, 20年内为40%-50%风险, 而30年内>60%[13,14]. LS患者从出现结直肠息肉到发展为结直肠癌的平均时间比普通人群短得多, 可能在2-3年内息肉即可发展为癌, 而普通人群息肉恶变的时间>4年[15]. 但有研究发现LS结直肠癌患者的总体存活率高于散发性结直肠癌患者, 前者的5年生存率为82.5%, 而后者5年生存率为56.4%[16]. 为NCCN指南建议, 根据LS患者的家庭史和基因型, 应从20-25岁开始每年进行一次或两次结肠镜检查用以监视并切除息肉以减少结直肠癌的发生. 通常在LS家族中最早被诊断为结直肠癌的成员届时年龄的基础上推前5-10年时开始考虑结肠镜检查, 癌症在早期被识别可改善整体生存率[8,17]. 而AGA指南亦建议对LS患者进行结肠镜监测, 间隔时间则为1-2年.
LS患子宫内膜癌风险约为60%, 8%-9%子宫内膜癌源于LS, 7%-21%子宫内膜癌合并卵巢癌源于LS[6,18,19]. 在确诊后10年内幸存的LS结直肠癌女性患者中, 多达26%的人可能会在此期间患原发性子宫内膜癌[20]. MSH2或MSH6突变者患子宫内膜癌风险最高, 高达44%[21]. 子宫内膜癌的症状包括异常子宫出血和疼痛, 这通常是容易被患者察觉的早期指标. 约有2%的卵巢癌是源于LS, 女性LS患者患卵巢癌风险为6.7%-12.0%, 而55%的LS卵巢癌患者同时或异时合并另一LS相关癌症[19,22,23]. 在LS中诊断卵巢癌的平均年龄主要在40-50岁, 高达30%的LS相关卵巢癌在35岁之前被诊断[19,24]. 欧洲指南建议可从35-40岁开始每1-2年进行妇科检查、阴道超声或针吸活组织检查来监测子宫内膜癌和卵巢癌的癌前病变或早期癌症.
约5%-13%的胃癌源于LS, 男性多于女性, 平均年龄为55岁, 肠型胃癌及弥漫型胃癌均有可能出现[23,25-27]. 多于6%的由MLH1和MSH2突变引起的LS发展为小肠癌的中位年龄为50岁左右, MSH6突变为54岁[23]. 肾盂移行细胞癌在普通人群是极其罕见的. 相比之下, LS患者在70岁左右患肾盂移行细胞癌的概率为8%, 中位年龄为58-62岁. 最近的数据显示LS患者患膀胱癌的风险为正常人的2-4倍, MSH2突变的LS患者在70岁左右患尿道癌的概率可能接近或超过20%[28,29]. 在LS患者中, 皮脂肿瘤的发病率已高达9%, 发病年龄更常出现在60岁之前(中位年龄56岁), 病灶多个而非孤立[30]. 对LS患者的死亡原因分析发现, 61%癌症相关死亡与结直肠癌和子宫内膜癌无关, 而是其他癌症(胃癌、小肠癌、泌尿系恶性肿瘤等)引起的死亡[31]. 有些医生可能会考虑行小肠X线和/或胃镜检查排查上消化道癌症, 尿液细胞学检查或超声排查移行细胞癌, 但缺乏有效的循证依据. 欧洲指南只推荐胃癌发病率较高国家中的LS家族或者幽门螺杆菌感染史>25年的LS患者, 可从30-35岁开始每1-2年进行胃镜监测; 而通过尿脱落细胞学检查和超声检查来监测泌尿道肿瘤只推荐用于研究.
LS患者结直肠癌治疗应该行部分结肠切除术还是全结肠切除术至今仍存在争议. 研究[32]表明, 部分结肠切除术后10年内再发结直肠癌概率为16%. 因此有外科医生可能会选择给LS并诊断结直肠癌的患者行全/次全结肠切除术, 但由此会影响患者术后生存质量[33]. 欧洲指南建议在行全/次全结肠切除术前应进行优点及缺点的讨论研究.
完成生育后预防性切除子宫和双侧卵巢基本被大众认可. 因为对卵巢癌和子宫内膜癌缺乏有效的监督, 并且在预防手术后该两种肿瘤风险明显降低[34]. LS子宫内膜癌的平均发病年龄是55岁, 有建议行经腹全子宫切除术和双边输卵管卵巢切除术的年龄为50岁[35]. 而欧洲指南建议预防性切除子宫和双侧卵巢手术年龄为40岁, 可与结直肠癌手术同台进行.
Rothwell等[36]一项包含8个预防心血管疾病随机试验的荟萃分析发现, 服用阿司匹林5年后结直肠癌风险降低. Burn等[37]一项包含1009例LS患者的随机对照试验发现服用阿司匹林至少2年后LS相关癌症发病率降低, 且服药剂量与癌症发病率呈负相关. AGA及欧洲指南均建议LS患者每日服用阿司匹林预防癌症发生. 但推荐服用阿司匹林的最佳开始年龄及剂量尚未提出. 普通人群中避孕药可降低子宫内膜癌和卵巢癌的风险, 而对部分LS的女性也有该效果[38].
LS患者患癌症风险受生活方式的影响, 多项研究表明吸烟与高体质量指数(body mass index, BMI)的LS患者患结直肠癌风险更高, 而水果和膳食纤维饮食会降低腺癌风险[39-42]. 另有前瞻性研究[43]发现高热量的饮食习惯者腺癌风险增加. 故欧洲指南建议LS患者体质量保持在正常范围内, 避免吸烟.
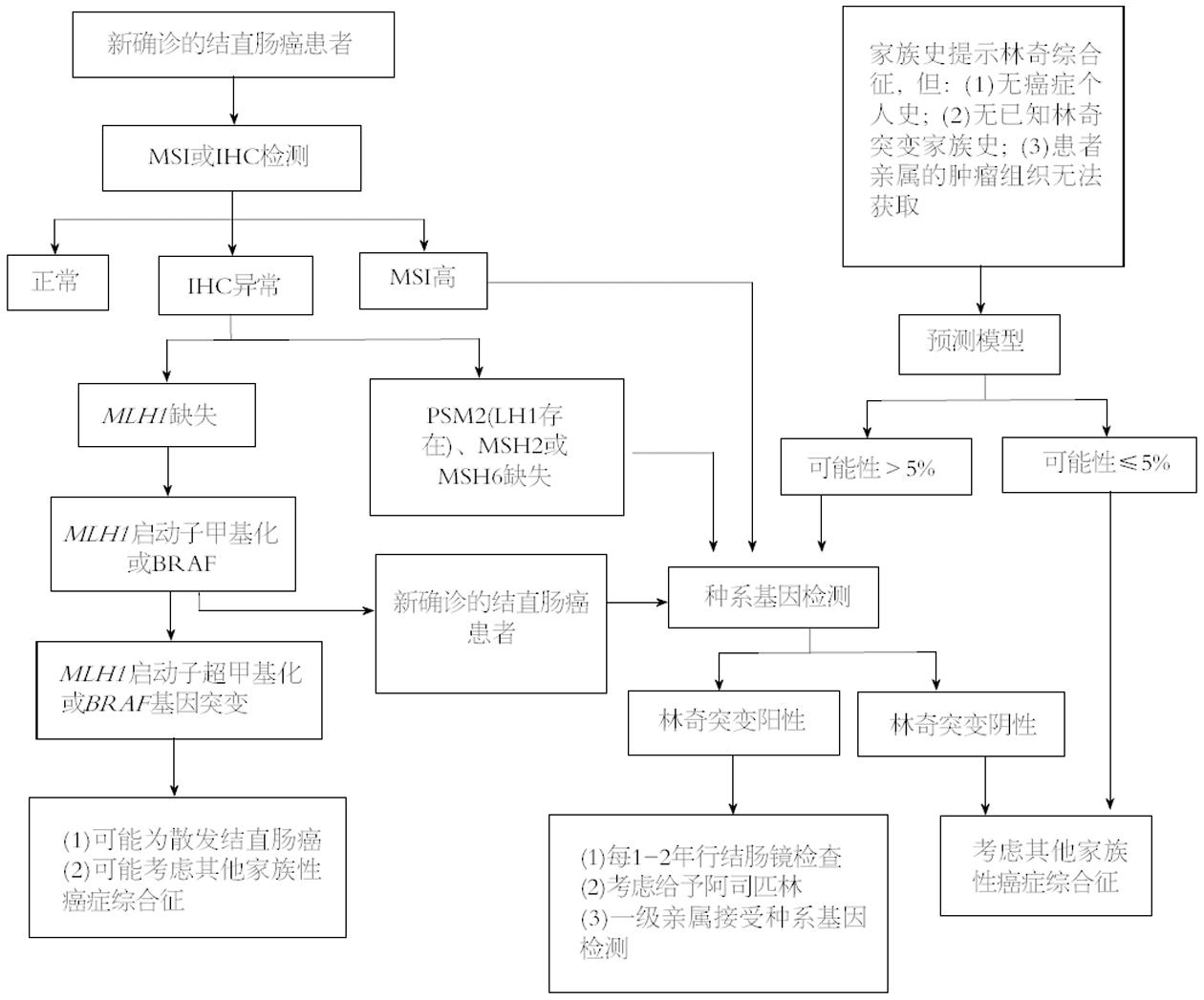
LS的识别需要多个步骤, 包括典型特征的识别和适当的测试和/或转介遗传学提供者. 系统收集、记录和评估家族史是高度可变的, 极少病理学家能认出LS的组织学特征. 考虑到这些局限性, 普遍筛查成为一种有效识别个人和高危家庭成员LS的办法. LS筛查是通过评价所有结直肠癌和/或子宫内膜癌患者是否有MMR缺陷. 指南均建议对结直肠癌和子宫内膜癌患者进行微卫星不稳定性(microsatellite instability, MSI)或免疫组织化学(immunological histological chemistry, IHC)检测, 来筛查LS. NCCN指南建议的筛查流程是用改良的Bethesda指南先筛选患者, 然后通过MSI或IHC检测MMR基因表达状态, 最后进行基因测序验证. AGA指南附有临床决策流程图(图1), 指导临床医生对LS患者进行管理. 普遍筛查结直肠癌和子宫内膜癌患者的研究表明, 多达70%的LS患者不符合Amsterdam或Bethesda的诊断标准[44]. 普遍筛查LS被认为符合成本效益[45-48].
LS的遗传学复杂, 而基因与环境相互作用仍需进一步探究. 如何有效识别和适当管理LS患者的指南和共识将继续完善. 而临床医生需不断学习相关知识和进展, 从而能有效识别LS患者和管理他们的健康状况, 由此改善其预后及生存质量.
林奇综合征是一种显性遗传性癌症综合征, 表现为肿瘤倾向特征的综合征, 包括患某些特定癌症的患病风险明显升高, 多种原发肿瘤的高发病率, 并且没有典型危险因素. 但临床上重视度不高, 误诊漏诊率极高, 了解林奇综合征的风险管理可为临床上提供理论基础, 具有十分重要的临床及科研意义.
张鹏, 副研究员, 同济大学附属第十人民医院普外科; 陈光, 教授, 吉林大学第一医院消化器官外科
本文就林奇综合征监测、手术、药物、生活方式、筛查等风险管理的最新进展做报道, 让读者对林奇综合征新的研究进展有了一定的认识, 为进一步指导临床和科研奠定了较好的理论基础.
目前, 关于林奇综合征的遗传基础的研究较多, 林奇综合征患者患肿瘤风险受到细胞错配修复基因突变的强烈影响, 同时也受到基因-环境相互作用的广泛影响.
本文分5部分系统地介绍林奇综合征的风险管理, 且附录林奇综合征的临床决策流程图, 有效指导临床实践过程中林奇综合征患者的管理.
本文详细介绍林奇综合征的风险管理, 为临床实践提供有力的理论基础, 加强临床医生对林奇综合征的认识, 使临床医生能有效识别林奇综合征患者和管理他们的健康状况, 由此改善患者预后及生存质量.
本文全面地介绍了林奇综合征的监测、手术、药物、生活方式、筛查等风险管理方面的最新进展, 内容丰富, 且提供美国指南建议的临床决策流程图, 指导林奇综合征患者的管理.
编辑: 郭鹏 电编:闫晋利
| 1. | Weissman SM, Burt R, Church J, Erdman S, Hampel H, Holter S, Jasperson K, Kalady MF, Haidle JL, Lynch HT. Identification of individuals at risk for Lynch syndrome using targeted evaluations and genetic testing: National Society of Genetic Counselors and the Collaborative Group of the Americas on Inherited Colorectal Cancer joint practice guideline. J Genet Couns. 2012;21:484-493. [PubMed] [DOI] |
| 2. | Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009;11:42-65. [PubMed] [DOI] |
| 3. | Egoavil C, Alenda C, Castillejo A, Paya A, Peiro G, Sánchez-Heras AB, Castillejo MI, Rojas E, Barberá VM, Cigüenza S. Prevalence of Lynch syndrome among patients with newly diagnosed endometrial cancers. PLoS One. 2013;8:e79737. [PubMed] [DOI] |
| 4. | Liu T, Wahlberg S, Burek E, Lindblom P, Rubio C, Lindblom A. Microsatellite instability as a predictor of a mutation in a DNA mismatch repair gene in familial colorectal cancer. Genes Chromosomes Cancer. 2000;27:17-25. [PubMed] |
| 5. | Syngal S, Fox EA, Eng C, Kolodner RD, Garber JE. Sensitivity and specificity of clinical criteria for hereditary non-polyposis colorectal cancer associated mutations in MSH2 and MLH1. J Med Genet. 2000;37:641-645. [PubMed] |
| 6. | Rubenstein JH, Enns R, Heidelbaugh J, Barkun A. American Gastroenterological Association Institute Guideline on the Diagnosis and Management of Lynch Syndrome. Gastroenterology. 2015;149:777-782; quiz e16-e17. [PubMed] [DOI] |
| 7. | Hampel H. NCCN increases the emphasis on genetic/familial high-risk assessment in colorectal cancer. J Natl Compr Canc Netw. 2014;12:829-831. [PubMed] |
| 8. | Vasen HF, Blanco I, Aktan-Collan K, Gopie JP, Alonso A, Aretz S, Bernstein I, Bertario L, Burn J, Capella G. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut. 2013;62:812-823. [PubMed] [DOI] |
| 9. | Barrow E, Hill J, Evans DG. Cancer risk in Lynch Syndrome. Fam Cancer. 2013;12:229-240. [PubMed] [DOI] |
| 10. | Pérez-Cabornero L, Infante M, Velasco E, Lastra E, Miner C, Durán M. Genotype-phenotype correlation in MMR mutation-positive families with Lynch syndrome. Int J Colorectal Dis. 2013;28:1195-1201. [PubMed] [DOI] |
| 11. | Ramsoekh D, Wagner A, van Leerdam ME, Dooijes D, Tops CM, Steyerberg EW, Kuipers EJ. Cancer risk in MLH1, MSH2 and MSH6 mutation carriers; different risk profiles may influence clinical management. Hered Cancer Clin Pract. 2009;7:17. [PubMed] [DOI] |
| 12. | Choi YH, Cotterchio M, McKeown-Eyssen G, Neerav M, Bapat B, Boyd K, Gallinger S, McLaughlin J, Aronson M, Briollais L. Penetrance of colorectal cancer among MLH1/MSH2 carriers participating in the colorectal cancer familial registry in Ontario. Hered Cancer Clin Pract. 2009;7:14. [PubMed] [DOI] |
| 13. | Win AK, Parry S, Parry B, Kalady MF, Macrae FA, Ahnen DJ, Young GP, Lipton L, Winship I, Boussioutas A. Risk of metachronous colon cancer following surgery for rectal cancer in mismatch repair gene mutation carriers. Ann Surg Oncol. 2013;20:1829-1836. [PubMed] [DOI] |
| 14. | Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Clendenning M, Sotamaa K, Prior T, Westman JA. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783-5788. [PubMed] [DOI] |
| 15. | Edelstein DL, Axilbund J, Baxter M, Hylind LM, Romans K, Griffin CA, Cruz-Correa M, Giardiello FM. Rapid development of colorectal neoplasia in patients with Lynch syndrome. Clin Gastroenterol Hepatol. 2011;9:340-343. [PubMed] [DOI] |
| 17. | Stupart DA, Goldberg PA, Algar U, Ramesar R. Surveillance colonoscopy improves survival in a cohort of subjects with a single mismatch repair gene mutation. Colorectal Dis. 2009;11:126-130. [PubMed] [DOI] |
| 18. | Walsh CS, Blum A, Walts A, Alsabeh R, Tran H, Koeffler HP, Karlan BY. Lynch syndrome among gynecologic oncology patients meeting Bethesda guidelines for screening. Gynecol Oncol. 2010;116:516-521. [PubMed] [DOI] |
| 19. | Kim MK, Song SY, Do IG, Kim SH, Choi CH, Kim TJ, Lee JW, Bae DS, Kim BG. Synchronous gynecologic malignancy and preliminary results of Lynch syndrome. J Gynecol Oncol. 2011;22:233-238. [PubMed] [DOI] |
| 20. | Obermair A, Youlden DR, Young JP, Lindor NM, Baron JA, Newcomb P, Parry S, Hopper JL, Haile R, Jenkins MA. Risk of endometrial cancer for women diagnosed with HNPCC-related colorectal carcinoma. Int J Cancer. 2010;127:2678-2684. [PubMed] [DOI] |
| 21. | Baglietto L, Lindor NM, Dowty JG, White DM, Wagner A, Gomez Garcia EB, Vriends AH, Cartwright NR, Barnetson RA, Farrington SM. Risks of Lynch syndrome cancers for MSH6 mutation carriers. J Natl Cancer Inst. 2010;102:193-201. [PubMed] [DOI] |
| 22. | Engel C, Loeffler M, Steinke V, Rahner N, Holinski-Feder E, Dietmaier W, Schackert HK, Goergens H, von Knebel Doeberitz M, Goecke TO. Risks of less common cancers in proven mutation carriers with lynch syndrome. J Clin Oncol. 2012;30:4409-4415. [PubMed] [DOI] |
| 23. | Pal T, Permuth-Wey J, Sellers TA. A review of the clinical relevance of mismatch-repair deficiency in ovarian cancer. Cancer. 2008;113:733-742. [PubMed] [DOI] |
| 24. | Malander S, Rambech E, Kristoffersson U, Halvarsson B, Ridderheim M, Borg A, Nilbert M. The contribution of the hereditary nonpolyposis colorectal cancer syndrome to the development of ovarian cancer. Gynecol Oncol. 2006;101:238-243. [PubMed] |
| 25. | Watson P, Vasen HF, Mecklin JP, Bernstein I, Aarnio M, Järvinen HJ, Myrhøj T, Sunde L, Wijnen JT, Lynch HT. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer. 2008;123:444-449. [PubMed] [DOI] |
| 26. | Chun N, Ford JM. Genetic testing by cancer site: stomach. Cancer J. 2012;18:355-363. [PubMed] [DOI] |
| 27. | Capelle LG, Van Grieken NC, Lingsma HF, Steyerberg EW, Klokman WJ, Bruno MJ, Vasen HF, Kuipers EJ. Risk and epidemiological time trends of gastric cancer in Lynch syndrome carriers in the Netherlands. Gastroenterology. 2010;138:487-492. [PubMed] [DOI] |
| 28. | van der Post RS, Kiemeney LA, Ligtenberg MJ, Witjes JA, Hulsbergen-van de Kaa CA, Bodmer D, Schaap L, Kets CM, van Krieken JH, Hoogerbrugge N. Risk of urothelial bladder cancer in Lynch syndrome is increased, in particular among MSH2 mutation carriers. J Med Genet. 2010;47:464-470. [PubMed] [DOI] |
| 29. | Skeldon SC, Semotiuk K, Aronson M, Holter S, Gallinger S, Pollett A, Kuk C, van Rhijn B, Bostrom P, Cohen Z. Patients with Lynch syndrome mismatch repair gene mutations are at higher risk for not only upper tract urothelial cancer but also bladder cancer. Eur Urol. 2013;63:379-385. [PubMed] [DOI] |
| 30. | Roberts ME, Riegert-Johnson DL, Thomas BC, Thomas CS, Heckman MG, Krishna M, DiCaudo DJ, Bridges AG, Hunt KS, Rumilla KM. Screening for Muir-Torre syndrome using mismatch repair protein immunohistochemistry of sebaceous neoplasms. J Genet Couns. 2013;22:393-405. [PubMed] [DOI] |
| 31. | Pylvänäinen K, Lehtinen T, Kellokumpu I, Järvinen H, Mecklin JP. Causes of death of mutation carriers in Finnish Lynch syndrome families. Fam Cancer. 2012;11:467-471. [PubMed] [DOI] |
| 32. | Parry S, Win AK, Parry B, Macrae FA, Gurrin LC, Church JM, Baron JA, Giles GG, Leggett BA, Winship I. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut. 2011;60:950-957. [PubMed] [DOI] |
| 33. | Vasen HF, de Vos tot Nederveen Cappel WH. Cancer: Lynch syndrome--how should colorectal cancer be managed? Nat Rev Gastroenterol Hepatol. 2011;8:184-186. [PubMed] [DOI] |
| 34. | Schmeler KM, Lynch HT, Chen LM, Munsell MF, Soliman PT, Clark MB, Daniels MS, White KG, Boyd-Rogers SG, Conrad PG. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354:261-269. [PubMed] |
| 35. | Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, Fix D, Comeras I, La Jeunesse J, Nakagawa H, Westman JA. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66:7810-7817. [PubMed] |
| 36. | Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31-41. [PubMed] [DOI] |
| 37. | Burn J, Gerdes AM, Macrae F, Mecklin JP, Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081-2087. [PubMed] [DOI] |
| 38. | Lu KH, Loose DS, Yates MS, Nogueras-Gonzalez GM, Munsell MF, Chen LM, Lynch H, Cornelison T, Boyd-Rogers S, Rubin M. Prospective multicenter randomized intermediate biomarker study of oral contraceptive versus depo-provera for prevention of endometrial cancer in women with Lynch syndrome. Cancer Prev Res (Phila). 2013;6:774-781. [PubMed] [DOI] |
| 39. | Pande M, Lynch PM, Hopper JL, Jenkins MA, Gallinger S, Haile RW, LeMarchand L, Lindor NM, Campbell PT, Newcomb PA. Smoking and colorectal cancer in Lynch syndrome: results from the Colon Cancer Family Registry and the University of Texas M.D. Anderson Cancer Center. Clin Cancer Res. 2010;16:1331-1339. [PubMed] [DOI] |
| 40. | Botma A, Nagengast FM, Braem MG, Hendriks JC, Kleibeuker JH, Vasen HF, Kampman E. Body mass index increases risk of colorectal adenomas in men with Lynch syndrome: the GEOLynch cohort study. J Clin Oncol. 2010;28:4346-4353. [PubMed] [DOI] |
| 41. | Win AK, Dowty JG, English DR, Campbell PT, Young JP, Winship I, Macrae FA, Lipton L, Parry S, Young GP. Body mass index in early adulthood and colorectal cancer risk for carriers and non-carriers of germline mutations in DNA mismatch repair genes. Br J Cancer. 2011;105:162-169. [PubMed] [DOI] |
| 42. | Winkels RM, Botma A, Van Duijnhoven FJ, Nagengast FM, Kleibeuker JH, Vasen HF, Kampman E. Smoking increases the risk for colorectal adenomas in patients with Lynch syndrome. Gastroenterology. 2012;142:241-247. [PubMed] [DOI] |
| 43. | Botma A, Vasen HF, van Duijnhoven FJ, Kleibeuker JH, Nagengast FM, Kampman E. Dietary patterns and colorectal adenomas in Lynch syndrome: the GEOLynch cohort study. Cancer. 2013;119:512-521. [PubMed] [DOI] |
| 44. | Dinh TA, Rosner BI, Atwood JC, Boland CR, Syngal S, Vasen HF, Gruber SB, Burt RW. Health benefits and cost-effectiveness of primary genetic screening for Lynch syndrome in the general population. Cancer Prev Res (Phila). 2011;4:9-22. [PubMed] [DOI] |
| 45. | Ramsoekh D, Wagner A, van Leerdam ME, Dinjens WN, Steyerberg EW, Halley DJ, Kuipers EJ, Dooijes D. A high incidence of MSH6 mutations in Amsterdam criteria II-negative families tested in a diagnostic setting. Gut. 2008;57:1539-1544. [PubMed] [DOI] |
| 46. | Gudgeon JM, Williams JL, Burt RW, Samowitz WS, Snow GL, Williams MS. Lynch syndrome screening implementation: business analysis by a healthcare system. Am J Manag Care. 2011;17:e288-e300. [PubMed] |
| 47. | Mvundura M, Grosse SD, Hampel H, Palomaki GE. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med. 2010;12:93-104. [PubMed] [DOI] |
| 48. | Ladabaum U, Wang G, Terdiman J, Blanco A, Kuppermann M, Boland CR, Ford J, Elkin E, Phillips KA. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a cost-effectiveness analysis. Ann Intern Med. 2011;155:69-79. [PubMed] [DOI] |