修回日期: 2015-07-25
接受日期: 2015-09-07
在线出版日期: 2015-10-08
目的: 通过探究肝癌单纯手术和手术后辅助化疗对于患者血清肿瘤标志物、细胞分化抑制因子2(differentiation inhibitory factor 2, ID2)、ID3、生存时间的影响, 分析ID2、ID3表达与肝癌患者血清中重要肿瘤标志物甲胎蛋白(α-fetoprotein, AFP)的相关性以及ID2、ID3对肝癌侵袭转移力的影响, 从而阐明分化抑制因子表达水平检测在评价肝癌术后辅助化疗效果的可行性.
方法: 采用1:1病例对照研究方法, 采用ELISA方法检测患者血清中肿瘤标志物、ID2、ID3含量; 采用Western blot方法检测ID2、ID3的蛋白表达水平; 采用Transwell法检测ID2、ID3对肿瘤侵袭转移力的影响; 采用SPSS软件分析ID2、ID3表达量与血清中肿瘤标志物AFP含量的相关性.
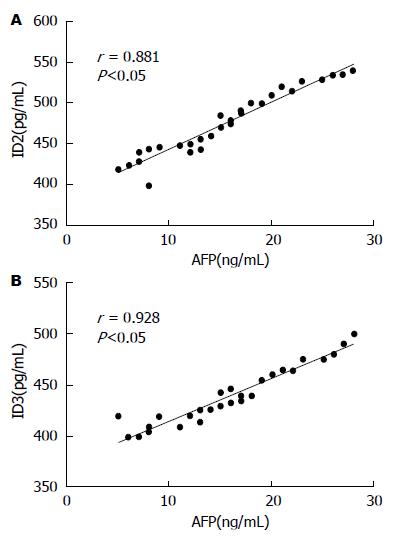
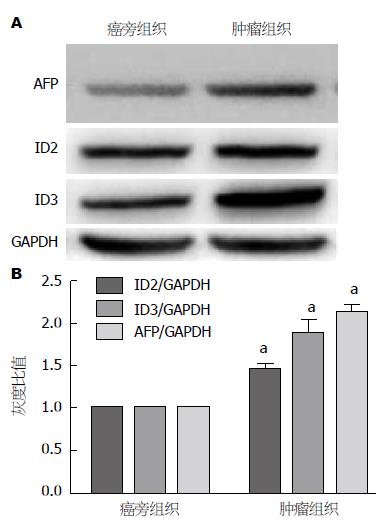
结果: 术后给予辅助化疗的患者血清中肿瘤标志物癌胚抗原(carcino-embryonic antigen, CEA)、糖链抗原50(carbohydrate antigen 50, CA50)、AFP、CA242含量明显低于单纯手术组患者(P<0.05), 生存时间明显长于单纯手术患者(P<0.05), 血清中ID2、ID3的表达水平均低于单纯手术患者(P<0.05), 肿瘤组织中ID2、ID3的蛋白表达水平高于癌旁组织(P<0.05), 血清肿瘤标志物AFP的与ID2、ID3基因表达量呈正相关(rID2 = 0.881, rID3 = 0.928, P<0.05). 在细胞水平沉默ID2、ID3可以削弱肿瘤的侵袭转移能力, 过表达ID2、ID3可以增强肿瘤的侵袭转移能力(P<0.05).
结论: ID2、ID3与肝癌肿瘤标志物有类似作用, 其表达上调提示肿瘤的侵袭转移能力增强, 患者生存时间缩短, 这可为临床上评价肝癌术后辅助化疗效果提供新的参考依据.
核心提示: 细胞分化抑制因子2(differentiation inhibitory factor 2, ID2)、ID3表达的上调或下调提示肿瘤的侵袭转移能力以及患者生存时间的改变, 对于预测肿瘤细胞的浸润转移有重要意义.
引文著录: 禹梅, 马玉梅, 陈花莲, 刘婕, 方晓蕾. 分化抑制因子在肝癌化疗疗效检验中的应用. 世界华人消化杂志 2015; 23(28): 4499-4506
Revised: July 25, 2015
Accepted: September 7, 2015
Published online: October 8, 2015
AIM: To explore the influence of surgery alone and in combination with postoperative adjuvant chemotherapy on tumor markers, inhibitor of differentiation 2 (ID2), ID3 and survival time in patients with liver cancer, analyze the influence of ID2 and ID3 on the invasion and metastasis of liver cancer, and explore the feasibility of detection of ID2 and ID3 expression in evaluating efficacy of postoperative adjuvant chemotherapy.
METHODS: This was a 1:1 matched case-control study. ELISA was used to detect the levels of tumor markers, ID2 and ID3 in the serum of patients. Western blot was used to detect the protein expression levels of ID2 and ID3 in tumor tissues and adjacent tissues. Transwell assay was used to detect the invasion and metastasis of liver cancer cells. The correlation between the content of AFP and the expression levels of ID2 and ID3 was statistically analyzed.
RESULTS: The tumor markers CEA, CA50, AFP, and CA242 as well as ID2 and ID3 in the serum decreased significantly and the survival time was longer in patients receiving surgery with postoperative adjuvant chemotherapy when compared with patients receiving surgery alone (P < 0.05). The protein expression levels of ID2 and ID3 were decreased in the adjacent normal tissues compared with the liver cancer tissues (P < 0.05). Transwell analysis indicated that ID2 and ID3 knockdown inhibited the invasion and metastasis ability of HepG2 cells while overexpression of ID2 and ID3 promoted the invasion and metastasis of HepG2 cells (P < 0.05). There was a positive correlation between the content of AFP and the expression levels of ID2 and ID3 (rID2 = 0.881, rID3 = 0.928, P < 0.05).
CONCLUSION: ID2 and ID3 have similar effects to liver tumor markers, and the increased expression of ID2 and ID3 indicates greater invasion and metastasis ability of HepG2 cells and shorter survival time in patients with liver cancer. ID2 and ID3 expression might be used for clinical evaluation of efficacy of postoperative adjuvant chemotherapy.
- Citation: Yu M, Ma YM, Chen HL, Liu J, Fang XL. Application of inhibitors of differentiation 2 and 3 for evaluation of chemotherapy efficacy in liver cancer. Shijie Huaren Xiaohua Zazhi 2015; 23(28): 4499-4506
- URL: https://www.wjgnet.com/1009-3079/full/v23/i28/4499.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v23.i28.4499
肝癌可分为原发性和继发性两大类. 原发性肝脏恶性肿瘤起源于肝脏的上皮或间叶组织, 是我国高发的, 危害极大的恶性肿瘤; 继发性或称转移性肝癌系指全身多个器官起源的恶性肿瘤侵犯至肝脏. 一般多见于胃、胆道、胰腺、结直肠、卵巢、子宫、肺、乳腺等器官恶性肿瘤的肝转移, 与原发性肝癌相比较较为少见[1-3]. 流行病学及实验研究[4-6]资料表明, 乙型肝炎病毒(hepatitis B virus, HBV)和丙型肝炎病毒(hepatitis C virus, HCV)感染、黄曲霉素、饮水污染、酒精、肝硬化、性激素、亚硝胺类物质、微量元素等都与肝癌发病相关. 继发性肝癌(转移性肝癌)可通过不同途径, 如随血液、淋巴液转移或直接浸润肝脏而形成疾病.
原发性肝癌的实验室检验项目一般有肝癌血清标志物的检测, 例如: 血清甲胎蛋白; γ-谷氨酰转肽酶及其同功酶、异常凝血酶原、碱性磷酸酶、乳酸脱氢酶同功酶等水平的检测; 癌胚抗原(carcino-embryonic antigen, CEA)高有助于肝转移癌的诊断[7-10]. 手术是治疗肝癌的首选, 也是最有效的方法. 手术方法有根治性肝切除, 姑息性肝切除等. 患者手术后属于术后康复期. 在康复期的治疗上也是尤为重要的. 因为存在的复发和转移几率是很高的, 术后残余的癌细胞会不定时的向各部位转移. 所以术后要加强巩固以防止他的复发和转移, 所以化疗是术后较为有效的治疗办法[11-13]. 化疗疗效的评价也是肝癌患者临床一项重要的监测指标.
分化抑制因子又称DNA结合抑制因子, 是一类广泛表达的转录调控因子, 属于螺旋-环-螺旋转录因子家族成员之一, 细胞分化抑制因子(differentiation inhibitory factor, ID)在细胞周期调控中发挥重要作用, 其细胞表达模式及其调控机制具有多样性和复杂性. 一般认为, ID基因家族成员的行为与致癌基因相似[14]. 某些ID基因的过度表达可延迟细胞衰老, 与肿瘤的发生及其严重程度有关联[15].
本研究通过探究肝癌单纯手术和手术后辅助化疗对于患者血清肿瘤标志物、ID2、ID3、生存时间的影响, 并检测肿瘤组织以及癌旁组织分化抑制因子ID2、ID3的mRNA表达水平, 分析ID2、ID3表达与肝癌患者血清中重要肿瘤标志物AFP的相关性以及ID2、ID3对肝癌侵袭转移力的影响, 从而阐明分化抑制因子ID2、ID3表达水平检测在评价肝癌术后辅助化疗效果的参考价值.
(1)随机选取2008-01-01/2009-01-01西安市北方医院、安康市中心医院、渭南市妇幼保健院收治的进行单纯手术治疗的早期肝癌患者30例(平均年龄45.25岁±3.75岁), 以及2008-01-01/2009-01-01安康市中心医院收治的进行手术治疗并在术后接受辅助化疗的患者30例(平均年龄46.38岁±2.24岁); (2)根据医学论理学原则, 研究前应对研究对象及其家属进行告知和讲解, 确保所有研究对象充分了解相关信息, 并签署知情同意书; (3)对于病例进行随访, 随访时间为10年, 每年1次. ELISA检测试剂盒, 上海邦奕生物科技有限公司; ID2、ID3抗体, Anti-Rabbit二抗抗体, 人肝癌细胞株HepG2、HDMEM培养基、胎牛血清, 上海拜力生物科技有限公司; BD Matrigel基质胶, 上海慧颖生物科技有限公司; 结晶紫染色试剂, 上海研生生化试剂有限公司; CULTEX® LTC全自动Transwell细胞培养系统, 北京佰乐良成科技有限公司; Simon Western blot自动分析仪, 北京佰乐良成科技有限公司; ID2、ID3干扰腺病毒、过表达病毒载体构建, 上海生博生物医药科技有限公司.
1.2.1 标本采集与保存: 所有实验对象在手术后化疗4个疗程(一个疗程2 d)后的清晨10:00点抽取空腹肘静脉血5 mL(实验前10 h禁食), 装于有肝素抗凝的离心管中, 混匀, 离心(5000 r/min×10 min), 收集上层血清, 一部分用于ELISA检测肿瘤标志物; 另一部分用于血清中ID2、ID3的水平检测. 在手术中, 分别钳取距癌灶2 cm的肿瘤组织以及癌旁组织, 经常规处理后, 一部分进行病理组织学观察, 一部分冻存于-80 ℃保存待用.
1.2.2 ELISA检测术前、术后血清中肿瘤标志物CEA、糖链抗原50(carbohydrate antigen 50, CA50)、AFP、CA242以及分化抑制因子ID2、ID3的含量: 将不同浓度的标准品各0.1 mL依次加入一排7孔中, 加入稀释的待检测样品0.1 mL, 置于37 ℃孵育90 min, 甩去酶标板中的液体. 将准备好的抗体工作液按每孔0.1 mL依次加入, 置于37 ℃孵育60 min 用PBS洗涤3次, 每次浸泡1 min左右; 将准备好的ABC工作液按每孔0.1 mL依次加入, 置于37 ℃孵育30 min, 用PBS洗涤, 5次; 按每孔90 µL依次加入TMB显色液, 置于37 ℃避光反应15-20 min; 按每孔100 µL依次加入TMB终止液, 此时蓝色立刻转为黄色, 用酶标仪在450 nm处测定吸光度[16].
1.2.3 组织中分化抑制因子ID2、ID3的蛋白水平检测: 测定组织中待测蛋白样品的浓度, 为确保每个蛋白样品上样体积的一致, 采用碧云天生产的BCA工作液对样品中的蛋白进行定量. 在样品中加入适量碧云天生产的5×SDS-PAGE蛋白上样缓冲液. 沸水浴中3 min后上样, 电泳(80 V电压30 min, 100 V电压60 min), 电转膜仪转膜(200 mA 90 min), 用5%脱脂牛奶封闭, 随后于摇床上孵育一抗1 h, 4 ℃冰箱过夜, 第2天继续孵育一抗1 h, 洗膜, 3次, 5 min/次, 孵育二抗, 2 h, 洗膜3次, 10 min/次. 用自动显影仪显影, 并分析条带灰度[17].
1.2.4 病毒转染: 将50 MOI的ID2、ID3干扰腺病毒加入含2%胎牛血清、无双抗的培养皿中(细胞密度2.5×105个/mL), 转染6 h后, 换含10%胎牛血清和1 mL双抗的培养基培养48 h; 将100 MOI ID2、ID3过表达腺病毒加入含2%胎牛血清、无双抗的培养皿中(细胞密度2.5×105个/mL), 转染6 h后, 换含10%胎牛血清和1 mL双抗的培养基培养48 h[18].
1.2.5 Transwell检测分化抑制因子ID2、ID3对肝癌细胞(HepG2)侵袭转移力的影响: 构建ID2、ID3干扰腺病毒(ID2、ID3 sh-RNA), 以及ID2、ID3的过表达腺病毒(Ad-ID2、ID3), 采用肝癌细胞株HepG2进行实验. 将实验分为3个组别, 分别为: GFP组、ID2 sh-RNA组、Ad- ID2组; GFP组、ID3 sh-RNA组、Ad- ID3组. 转染病毒48 h后, 进行Transwel实验. Matrigel胶预冷为液态, 在冰上将无血清培养基与Matrigel胶1:8混合稀释, 混匀后按每孔加入Transwell小室中. 在室温条件下风干5 h. 每孔加入100 µL无血清培养基水化基底膜, 在37 ℃温箱中放置30 min. 调整细胞密度为2.5×105个/mL, 取200 µL加入小室内, 底部培养室内加入500 µL含20%FBS的高糖完全培养基. 在37 ℃、 50 mL/L CO2条件培养36 h. 弃去小室内液体, 用棉棒擦掉小室底部内面细胞, 将小室侵入950 mL/L乙醇固定5 min, 加入4 g/L结晶紫200 µL染色10 min. 计算每个视野细胞平均数[19].
统计学处理 采用SPSS19.0统计软件分析, 计量数据以mean±SD示, 计数资料采用χ2检验, 计量资料采用t检验. 以P<0.05表示差异有统计学意义.
对两组患者临床一般资料的分析结果显示, 单纯手术组30例, 男女比例为2.78:1, 术前病程为3.58 mo±0.66 mo; 手术后辅助化疗组30例, 男女比例为2.62:1, 术前病程为3.25 mo±0.71 mo; 两组患者在男女比例、平均年龄、术前病程方面差异均无明显的统计学意义(P>0.05).
由ELISA结果可知, 两组患者术前和术后CEA、CA50、AFP、CA242、ID2、ID3含量无明显统计学差异(P>0.05); 与单纯手术治疗组相比, 术后辅助化疗组血清中肿瘤标志物CEA、CA50、AFP、CA242含量明显降低(P<0.05), 血清中分化抑制因子ID2、ID3的含量明显降低(P<0.05), 术后未化疗组患者平均生存时间为4.41年±2.86年, 术后化疗组患者平均生存时间明显延长, 为6.62年±1.23年(P<0.05)(表1).
| 检测项目 | 单纯手术组 | 术后化疗组 | |||
| 手术前 | 手术后 | 手术前 | 手术后 | 化疗后 | |
| CEA(ng/mL) | 26.37±2.17 | 6.25±1.14a | 23.26±1.99 | 6.32±1.79c | 3.34±1.08e |
| CA50(U/mL) | 116.76±20.92 | 87.96±25.42a | 122.83±21.65 | 85.24±24.21c | 65.62±22.77e |
| AFP(ng/mL) | 50.37±1.55 | 18.24±2.56a | 57.79±2.24 | 19.61±1.04c | 13.74±3.38e |
| CA242(U/mL) | 107.36±21.55 | 80.25±20.14a | 96.25±14.87 | 83.09±17.75c | 46.76±19.49e |
| ID2(pg/mL) | 880.44±32.16 | 520.23±19.86a | 859.21±29.88 | 525.11±31.78c | 470.26±30.57e |
| ID3(pg/mL) | 677.27±23.25 | 495.76±25.44a | 653.21±25.42 | 500.09±27.34c | 420.22±31.25e |
ELISA结果显示, 血清中分化抑制因子ID2与ID3的表达与肿瘤标志物AFP含量呈正相关(rID2 = 0.881, P<0.05; rID3 = 0.928, P<0.05)(图1).
Western blot结果显示, 肿瘤组织中ID2、ID3蛋白表达的灰度密度明显高于癌旁组织, 提示肿瘤组织中ID2、ID3蛋白表达水平高于癌旁组(P<0.05)(图2).
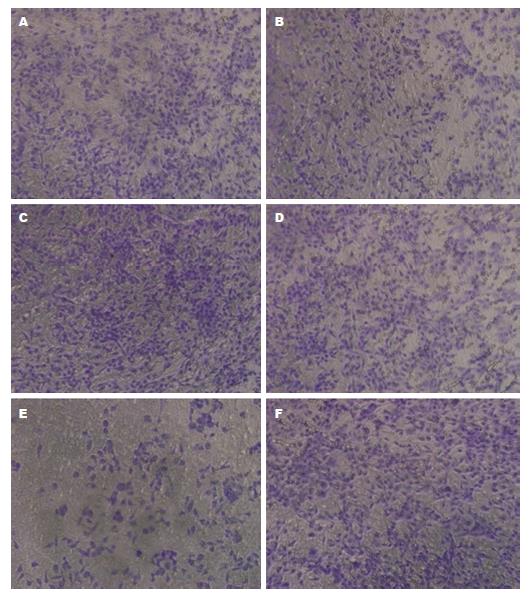
实验结果表明, GPF组细胞穿膜数为45.2个/mL±3.4个/mL, 干扰ID2组细胞穿膜数为33.7个/mL±1.3个/mL、干扰ID3基因表达组细胞穿膜数为37.4个/mL±0.9个/mL, 说明干扰ID2、ID3后, 胃癌细胞的侵袭、转移能力减弱(P<0.05); 过表达GFP组细胞穿膜数为42.6个/mL±3.3个/mL, 过表达ID2组细胞穿膜数为50.4个/mL±1.2个/mL、过表达ID3基因表达组细胞穿膜数为49.8个/mL±1.5个/mL(图3).
早期肝癌常症状无特异性, 中晚期肝癌的症状则较多, 常见的临床表现有肝区疼痛、腹胀、纳差、乏力、消瘦, 进行性肝大或上腹部包块等; 部分患者有低热、黄疸、腹泻、上消化道出血; 肝癌破裂后出现急腹症表现等. 也有症状不明显或仅表现为转移灶的症状[20-22].
原发性肝癌的实验室检测指标主要有肝癌血清标志物的检测、血液酶学、超声检查、CT检查、MRI检查、选择性腹腔动脉或肝动脉造影检查、肝穿刺行针吸细胞学检查等[23-25]. 大多数继发性肝癌患者肿瘤标志物在正常范围内, 但少数来自胃、食管、胰腺及卵巢的肝转移癌则可有AFP的升高. 有症状者多伴有ALP、GGT升高. 癌胚抗原CEA升高有助于肝转移癌的诊断[26-29]. 由此可见, 对肝癌血清标志物的检测是确诊、复查的一个重要的指标[30,31].
本研究探究肝癌单纯手术和手术后辅助化疗对于患者血清肿瘤标志物、ID2、ID3、生存时间的影响, 并检测肿瘤组织以及癌旁组织分化抑制因子ID2、ID3的mRNA表达水平, 分析ID2、ID3表达与肝癌患者血清中重要肿瘤标志物AFP的相关性以及ID2、ID3对肝癌侵袭转移力的影响, 为评价肝癌术后辅助化疗效果提供一个重要的标志物.
研究结果显示, 术后给予辅助化疗的患者血清中肿瘤标志物CEA、CA50、AFP、CA242含量明显低于单纯手术组患者(P<0.05); 术后辅助化疗患者的生存时间明显长于单纯手术患者(P<0.05); 术后辅助化疗患者血清中ID2、ID3的表达水平均低于单纯手术患者(P<0.05); 肿瘤组织中ID2、ID3的蛋白表达水平高于癌旁组织(P<0.05); 患者血清肿瘤标志物AFP的与ID2、ID3基因表达量呈正相关(rID2 = 0.881, rID3 = 0.928, P<0.05); 在细胞水平沉默ID2、ID3可以削弱肿瘤的侵袭转移能力, 过表达ID2、ID3可以增强肿瘤的侵袭转移能力(P<0.05). 过去有报道称ID基因家族成员的行为与致癌基因相似[14,31]. 某些ID基因的过度表达可延迟细胞衰老, 与肿瘤的发生及其严重程度有关联, 而我们的研究结果证实, 分化抑制因子ID2、ID3与肝癌肿瘤标志物有类似作用, ID2、ID3的升高说明肿瘤细胞的侵袭转移能力提高, ID2、ID3水平的下降说明肿瘤细胞侵袭转移能力的下降.
本研究在预测肿瘤细胞的浸润转移方面有重要意义, 这可为临床上评价肝癌术后辅助化疗效果提供了新的参考依据.
分化抑制因子又称DNA结合抑制因子, 是一类广泛表达的转录调控因子, 属于螺旋-环-螺旋转录因子家族成员之一, 细胞分化抑制因子(differentiation inhibitory factor, ID)在细胞周期调控中发挥重要作用, 其细胞表达模式及其调控机制具有多样性和复杂性. 一般认为, ID基因家族成员的行为与致癌基因相似.
邓安梅, 教授, 主任医师, 中国人民解放军第二军医大学长海医院实验诊断科
肝癌可分为原发性和继发性两大类. 原发性肝脏恶性肿瘤起源于肝脏的上皮或间叶组织, 是我国高发的, 危害极大的恶性肿瘤. 对于癌症早期的检验诊断学的研究对于患者生存率和生活质量的提高具有重要作用, 目前研究主要是集中在肝癌血清标志物的检测, 例如: 血清甲胎蛋白; γ-谷氨酰转肽酶及其同功酶、异常凝血酶原、碱性磷酸酶、乳酸脱氢酶同功酶等水平的检测. 有报道提示, 分化抑制因子与肿瘤有密切相关, 因此, 本文的研究从分化抑制因子和肿瘤的相关性入手.
ID分子在细胞周期调控中发挥重要作用, 其细胞表达模式及其调控机制具有多样性和复杂性. 一般认为, ID基因家族成员的行为与致癌基因相似, 某些ID基因的过度表达可延迟细胞衰老, 与肿瘤的发生及其严重程度有关联.
本研究通过采用相关性分析, Western blot半定量分析以及肿瘤侵袭转移能力分析等方法学, 证明分化抑制因子ID2、ID3与肝癌肿瘤标志物有类似作用, 其表达上调提示肿瘤的侵袭转移能力增强, 患者生存时间缩短, 在预测肿瘤细胞的浸润转移方面有重要意义, 为临床提供了新的肿瘤预测点.
在预测肿瘤细胞的浸润转移方面有重要意义, 这可为临床上评价肝癌术后辅助化疗效果提供新的参考依据.
分化抑制因子: DNA结合抑制因子, 是一类广泛表达的转录调控因子, 属于螺旋-环-螺旋转录因子家族成员之一, ID分子在细胞周期调控中发挥重要作用.
本文利用ELISA、Western blot以及Transwell技术检测了术后化疗与单纯手术疗法对肝患者血清中癌多种指标的影响、分析了ID2、ID3表达与肝癌标志物AFP之间的相关性以及ID2、ID3对肝癌侵袭转移力的影响, 揭示了分化抑制因子ID2、ID3在评价肝癌术后辅助化疗效果方面有一定的参考价值.
编辑: 郭鹏 电编:闫晋利
| 1. | Tsang CM, Cheung KC, Cheung YC, Man K, Lui VW, Tsao SW, Feng Y. Berberine suppresses Id-1 expression and inhibits the growth and development of lung metastases in hepatocellular carcinoma. Biochim Biophys Acta. 2015;1852:541-551. [PubMed] [DOI] |
| 2. | Nittka S, Krueger MA, Shively JE, Boll H, Brockmann MA, Doyon F, Pichler BJ, Neumaier M. Radioimmunoimaging of liver metastases with PET using a 64Cu-labeled CEA antibody in transgenic mice. PLoS One. 2014;9:e106921. [PubMed] [DOI] |
| 3. | Vandewynckel YP, Laukens D, Bogaerts E, Paridaens A, Van den Bussche A, Verhelst X, Van Steenkiste C, Descamps B, Vanhove C, Libbrecht L. Modulation of the unfolded protein response impedes tumor cell adaptation to proteotoxic stress: a PERK for hepatocellular carcinoma therapy. Hepatol Int. 2015;9:93-104. [PubMed] [DOI] |
| 4. | Sham JG, Kievit FM, Grierson JR, Chiarelli PA, Miyaoka RS, Zhang M, Yeung RS, Minoshima S, Park JO. Glypican-3-targeting F(ab')2 for 89Zr PET of hepatocellular carcinoma. J Nucl Med. 2014;55:2032-2037. [PubMed] [DOI] |
| 5. | Zhao ZM, Jin Y, Gan Y, Zhu Y, Chen TY, Wang JB, Sun Y, Cao ZG, Qian GS, Tu H. Novel approach to identifying the hepatitis B virus pre-S deletions associated with hepatocellular carcinoma. World J Gastroenterol. 2014;20:13573-13581. [PubMed] [DOI] |
| 6. | Vandewynckel YP, Laukens D, Geerts A, Vanhove C, Descamps B, Colle I, Devisscher L, Bogaerts E, Paridaens A, Verhelst X. Therapeutic effects of artesunate in hepatocellular carcinoma: repurposing an ancient antimalarial agent. Eur J Gastroenterol Hepatol. 2014;26:861-870. [PubMed] [DOI] |
| 7. | Wang G, Zhuo Z, Xia H, Zhang Y, He Y, Tan W, Gao Y. Investigation into the impact of diagnostic ultrasound with microbubbles on the capillary permeability of rat hepatomas. Ultrasound Med Biol. 2013;39:628-637. [PubMed] [DOI] |
| 8. | Knoop K, Schwenk N, Dolp P, Willhauck MJ, Zischek C, Zach C, Hacker M, Göke B, Wagner E, Nelson PJ. Stromal targeting of sodium iodide symporter using mesenchymal stem cells allows enhanced imaging and therapy of hepatocellular carcinoma. Hum Gene Ther. 2013;24:306-316. [PubMed] [DOI] |
| 9. | Grünwald GK, Klutz K, Willhauck MJ, Schwenk N, Senekowitsch-Schmidtke R, Schwaiger M, Zach C, Göke B, Holm PS, Spitzweg C. Sodium iodide symporter (NIS)-mediated radiovirotherapy of hepatocellular cancer using a conditionally replicating adenovirus. Gene Ther. 2013;20:625-633. [PubMed] [DOI] |
| 10. | Lesmana LA, Gani RA, Hasan I, Waspodo A, Boedi P, Luwia MS, Ganggaiswari A. Influence of the sorafenib patients assistance program on treatment compliance and overall survival of unresectable hepatocellular carcinoma patients. Acta Med Indones. 2012;44:228-232. [PubMed] |
| 11. | Zhang H, Cai H, Lu X, Muzik O, Peng F. Positron emission tomography of human hepatocellular carcinoma xenografts in mice using copper (II)-64 chloride as a tracer with copper (II)-64 chloride. Acad Radiol. 2011;18:1561-1568. [PubMed] [DOI] |
| 12. | Arita J, Takahashi M, Hata S, Shindoh J, Beck Y, Sugawara Y, Hasegawa K, Kokudo N. Usefulness of contrast-enhanced intraoperative ultrasound using Sonazoid in patients with hepatocellular carcinoma. Ann Surg. 2011;254:992-999. [PubMed] [DOI] |
| 13. | Golcher H, Brunner TB, Witzigmann H, Marti L, Bechstein WO, Bruns C, Jungnickel H, Schreiber S, Grabenbauer GG, Meyer T. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther Onkol. 2015;191:7-16. [PubMed] [DOI] |
| 14. | Kanda M, Fujii T, Suenaga M, Takami H, Inokawa Y, Yamada S, Kobayashi D, Tanaka C, Sugimoto H, Nomoto S. Pancreatoduodenectomy with portal vein resection is feasible and potentially beneficial for elderly patients with pancreatic cancer. Pancreas. 2014;43:951-958. [PubMed] [DOI] |
| 15. | Bachellier P, Rosso E, Fuchshuber P, Addeo P, David P, Oussoultzoglou E, Lucescu I. Use of a temporary intraoperative mesentericoportal shunt for pancreatic resection for locally advanced pancreatic cancer with portal vein occlusion and portal hypertension. Surgery. 2014;155:449-456. [PubMed] [DOI] |
| 16. | Valle JW, Palmer D, Jackson R, Cox T, Neoptolemos JP, Ghaneh P, Rawcliffe CL, Bassi C, Stocken DD, Cunningham D. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol. 2014;32:504-512. [PubMed] [DOI] |
| 17. | Ishida J, Matsumoto I, Shinzeki M, Asari S, Goto T, Tanaka M, Yamashita H, Kido M, Ajiki T, Fukumoto T. [A case of metachronous pancreatic cancer that developed 4 years after initial pancreatectomy]. Gan To Kagaku Ryoho. 2013;40:1893-1896. [PubMed] |
| 18. | Murakami S, Ajiki T, Shinozaki K, Yoshida Y, Ohtsubo I, Okazaki T, Goto T, Asari S, Shinzeki M, Kido M. [A case of initially unresectable gallbladder cancer with surgical resection after chemotherapy with gemcitabine]. Gan To Kagaku Ryoho. 2013;40:1744-1746. [PubMed] |
| 19. | Merkow RP, Bilimoria KY, Tomlinson JS, Paruch JL, Fleming JB, Talamonti MS, Ko CY, Bentrem DJ. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260:372-377. [PubMed] [DOI] |
| 20. | Gangl O, Sahora K, Kornprat P, Margreiter C, Primavesi F, Bareck E, Schindl M, Längle F, Öfner D, Mischinger HJ. Preparing for prospective clinical trials: a national initiative of an excellence registry for consecutive pancreatic cancer resections. World J Surg. 2014;38:456-462. [PubMed] |
| 21. | Hotz B, Erben U, Arndt M, Buhr HJ, Hotz HG. Taurolidine induces epithelial-mesenchymal transition via up-regulation of the transcription factor Snail in human pancreatic cancer cell lines. Int J Colorectal Dis. 2014;29:1339-1348. [PubMed] [DOI] |
| 22. | Knab LM, Ebine K, Chow CR, Raza SS, Sahai V, Patel AP, Kumar K, Bentrem DJ, Grippo PJ, Munshi HG. Snail cooperates with Kras G12D in vivo to increase stem cell factor and enhance mast cell infiltration. Mol Cancer Res. 2014;12:1440-1448. [PubMed] [DOI] |
| 23. | Chen J, Xu H, Zou X, Wang J, Zhu Y, Chen H, Shen B, Deng X, Zhou A, Chin YE. Snail recruits Ring1B to mediate transcriptional repression and cell migration in pancreatic cancer cells. Cancer Res. 2014;74:4353-4363. [PubMed] [DOI] |
| 24. | Cheng ZX, Wang DW, Liu T, Liu WX, Xia WB, Xu J, Zhang YH, Qu YK, Guo LQ, Ding L. Effects of the HIF-1α and NF-κB loop on epithelial-mesenchymal transition and chemoresistance induced by hypoxia in pancreatic cancer cells. Oncol Rep. 2014;31:1891-1898. [PubMed] [DOI] |
| 25. | Li Y, Wang H, Sun T, Chen J, Guo L, Shen H, Du Z, Zhou Y. Biological characteristics of a new human glioma cell line transformed into A2B5(+) stem cells. Mol Cancer. 2015;14:75. [PubMed] [DOI] |
| 26. | Shi QY, Zhang SJ, Liu L, Chen QS, Yu LN, Zhang FJ, Yan M. Sevoflurane promotes the expansion of glioma stem cells through activation of hypoxia-inducible factors in vitro. Br J Anaesth. 2015;114:825-830. [PubMed] [DOI] |
| 27. | Zhang S, Xie R, Zhao T, Yang X, Han L, Ye F, Lei T, Wan F. Neural stem cells preferentially migrate to glioma stem cells and reduce their stemness phenotypes. Int J Oncol. 2014;45:1989-1996. [PubMed] [DOI] |
| 28. | Li Q, Qiao G, Ma J, Li Y. Downregulation of VEGF expression attenuates malignant biological behavior of C6 glioma stem cells. Int J Oncol. 2014;44:1581-1588. [PubMed] [DOI] |
| 29. | Liu Z, Jiang Z, Huang J, Huang S, Li Y, Sheng F, Yu S, Yu S, Liu X. Mesenchymal stem cells show little tropism for the resting and differentiated cancer stem cell-like glioma cells. Int J Oncol. 2014;44:1223-1232. [PubMed] [DOI] |
| 30. | Yang X, Cui W, Yu S, Xu C, Chen G, Gu A, Li T, Cui Y, Zhang X, Bian X. A synthetic dl-nordihydroguaiaretic acid (Nordy), inhibits angiogenesis, invasion and proliferation of glioma stem cells within a zebrafish xenotransplantation model. PLoS One. 2014;9:e85759. [PubMed] [DOI] |
| 31. | Shin JH, Lee YS, Hong YK, Kang CS. Correlation between the prognostic value and the expression of the stem cell marker CD133 and isocitrate dehydrogenase1 in glioblastomas. J Neurooncol. 2013;115:333-341. [PubMed] [DOI] |