修回日期: 2015-07-01
接受日期: 2015-07-06
在线出版日期: 2015-09-18
目的: 探讨感染后肠易激综合征(post-infectious irritable bowel syndrome, PI-IBS)小鼠肠道热休克蛋白70(heat shock protein 70, HSP70)表达及其与肠道低度炎症的相关性.
方法: 旋毛虫感染制作PI-IBS小鼠模型. 免疫组织化学检测PI-IBS小鼠肠道HSP70蛋白质表达. 肠道组织HE染色, Dieleman LA肠道炎症评分系统评分, 腹壁撤退反射(abdominal withdrawal reflex, AWR)检测内脏高敏感性, 结肠传输试验(colon transportation test, CTT)观察其肠道动力改变. Spearman分析肠道HSP70表达与炎症评分、AWR及CTT的相关性.
结果: 感染旋毛虫第8周, PI-IBS小鼠肠道炎症评分高于对照组但无显著性差异(P>0.05). PI-IBS小鼠回肠末段HSP70蛋白质表达较对照组明显升高(P<0.01), 但结肠未见明显变化(P>0.05). PI-IBS小鼠回肠HSP70蛋白质表达与肠道炎症评分呈弱负相关(P>0.05), 与AWR和Bristol评分呈显著负相关(P<0.01), 与. 结肠HSP70表达与AWR及Bristol评分均无明显相关性(P>0.05).
结论: PI-IBS时肠道存在低度炎症, 回肠末段HSP70的表达异常可能与肠道低度炎症、内脏高敏感性和肠道动力改变有关.
核心提示: 肠易激综合征(irritable bowel syndrome, IBS)是消化系常见病多发病, 其发病机制尚未十分明确. 本研究发现感染后IBS(post-infectious IBS, PI-IBS)时回肠末段热休克蛋白70(heat shock protein 70)表达升高且与肠道低度炎症、内脏高敏感和肠道动力异常存在相关性, 这为进一步阐明PI-IBS时肠道免疫激活和炎症的调控机制提供了线索.
引文著录: 周旭春, 孙晓宁, 杨波, 黄白丽, 邓桃枝, 何周桃, 韩向阳, 蓝程. 感染后肠易激综合征小鼠肠道热休克蛋白70的表达及意义. 世界华人消化杂志 2015; 23(26): 4177-4183
Revised: July 1, 2015
Accepted: July 6, 2015
Published online: September 18, 2015
AIM: To detect the expression of heat shock protein 70 (HSP70) in intestinal tissue of mice with post-infectious irritable bowel syndrome (PI-IBS).
METHODS: A mouse model for PI-IBS was established by infection of mice with trichinella spiralis in vivo. The inflammatory score of the intestine of PI-IBS mice was calculated, and abdominal withdrawal reflex (AWR) and colon transportation test (CTT) were used to evaluate the clinical features of the PI-IBS mice. Intestinal expression of HSP70 was measured by immunohistochemistry. Spearman correlation analysis was carried out to assess the relationship between the expression of HSP70 and the clinical features of PI-IBS.
RESULTS: The PI-IBS mouse model was established successfully. On the 8th week, there was no visible inflammation in the intestine (ileal inflammation score: P > 0.05), whereas the clinical features significantly changed, including increased AWR score (P < 0.01) and decreased CTT score (P < 0.01). The expression of HSP70 significantly increased in the ileum but not in the colon of PI-IBS mice at the 8th week (ileum: P < 0.01; colon: P > 0.05). The expression of HSP70 in the ileum, but not in the colon, was remarkably related with the clinical features of PI-IBS mice, including the negative relationship with AWR (P < 0.01), and the positive relationship with CTT (P < 0.01).
CONCLUSION: HSP70 might be associated with low-grade intestinal inflammation induced visceral hypersensitivity and abnormal intestinal motility in mice with PI-IBS.
- Citation: Zhou XC, Sun XN, Yang B, Huang BL, Deng TZ, He ZT, Han XY, Lan C. Expression of heat shock protein 70 in intestinal tissue of mice with post-infectious irritable bowel syndrome. Shijie Huaren Xiaohua Zazhi 2015; 23(26): 4177-4183
- URL: https://www.wjgnet.com/1009-3079/full/v23/i26/4177.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v23.i26.4177
肠易激综合征(irritable bowel syndrome, IBS)是一组病因不明的以腹部不适伴排便改变为主要表现的综合征[1-4]. IBS的发病机制尚未完全阐明, 大量证据[5-7]提示感染和炎症可能是IBS的重要发病机制之一, 即感染后肠易激综合征(post-infectious irritable bowel syndrome, PI-IBS). 热休克蛋白70(heat shock protein 70, HSP70)是分子伴侣热休克蛋白家族的一员, 在胃肠黏膜的炎症和损伤中起重要作用[8-11]. 然而, 有关PI-IBS中肠道HSP70的表达与肠道炎症的关系尚未见报道, 本文就此作初步探讨.
26只无病原环境出生的♀57BL/6小鼠(6-8周龄, 体质量13-15 g)购自中国科学院昆明动物所, 正常饮食饮水, 随机分为PI-IBS组13只和对照组13只. 旋毛虫包囊购自兰州兽医研究所. 胃蛋白酶为Invitrogen公司产品. 免疫组织化学、Western blot试剂盒为武汉博士德公司产品. 所有抗体均来自美国BD Bioscience公司. DU530分光光度计为美国Beckman公司产品.
1.2.1 PI-IBS小鼠模型的建立: 采用旋毛虫感染法[12,13]制作PI-IBS模型. 旋毛虫包囊感染60 d的SD大鼠以1.5%胃蛋白酶人工消化法分离肌幼虫, 悬于生理盐水中. 每只小鼠饲服0.2 mL含300个幼虫的生理盐水, 对照组给予等量生理盐水灌胃. 造模成功的标准为: 感染后第8周腹壁撤退反射(abdominal withdrawal reflex, AWR)及结肠传输功能试验(colon transportation test, CTT)明显异常, 肠道组织病理学无明显异常.
1.2.2 AWR实验: 动物实验前24 h禁食不禁水, 2%戊巴比妥钠腹腔注射麻醉, 带气囊的导尿管经肛门插入. 每只小鼠以容量0.25/0.5/0.75 mL×l5 min扩张3次, 每次间隔30 s. AWR评分标准[14]: 结直肠扩张刺激时情绪基本稳定0分; 刺激时不稳定, 偶尔扭动头部1分; 腹背部肌肉轻微收缩但腹部未抬离地面2分; 腹背部肌肉较强烈收缩并把腹部抬离地面3分; 腹部肌肉强烈收缩, 腹部呈¹形并把腹部﹑会阴部抬离地面4分.
1.2.3 CTT: 每只小鼠予0.4 mL活性炭悬液灌胃后开始记录首次排黑便时间, 评价小鼠整个肠道的传输功能. 连续采集8 h, 记录每只小鼠的粪便总粒数、总湿质量, 并进行Bristol评分标准[15]: 1分: 正常粪便; 2分: 柔软或不成形粪便; 3分: 水样粪便.
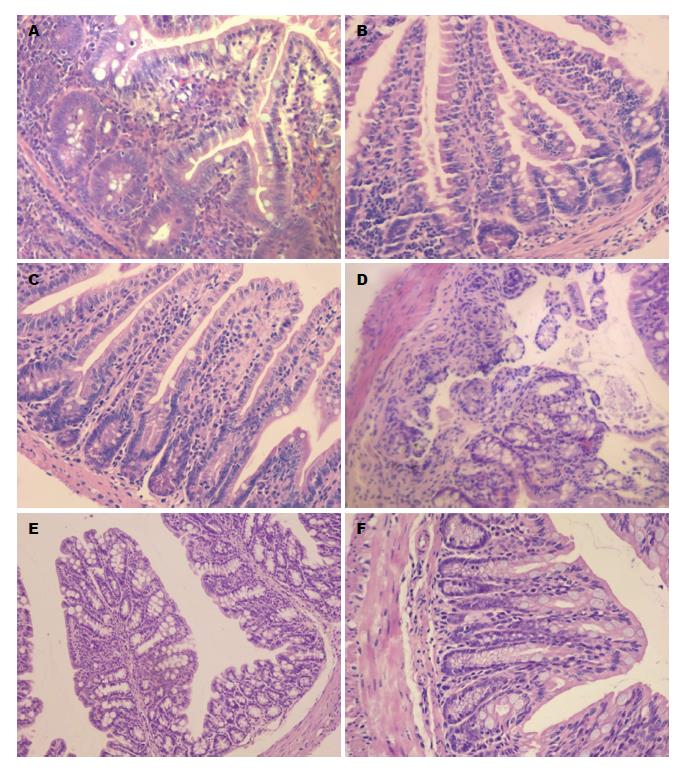
1.2.4 组织病理学观察: 分别取末端回肠和近端结肠组织, 4%多聚甲醛固定, 常规石蜡包埋切片, HE染色, 显微镜下观察各段肠黏膜组织炎症情况. 按Dieleman等[16]报道的方法, 由两名病理学家进行双盲方法评分.
1.2.5 肠黏膜HSP70表达: 肠组织切片脱蜡至水, 一抗为大鼠抗小鼠HSP70单克隆IgG抗体, 二抗为HRP polymer酶标兔抗大鼠IgG抗体, DAB显色, 光镜观察.
参照Zagzag等[17]的方法对染色强度和阳性细胞比例进行综合评分, 并对两组的结果进行半定量比较. 染色强度评分: 0分: 未见黄色; 1分: 浅黄色; 2分: 棕黄色; 3分: 棕褐色. 阳性细胞比例评分: 0分: 无阳性细胞; 1分: <1%; 2分: 1%-10%; 3分: 11%-50%; 4分: >50%.
统计学处理 所有数据采用SPSS13.0统计软件进行分析, 计量资料符合正态分布的以mean±SD表示, 采用两样本均数t检验, 两组变量的相关性分析采用Spearman分析, 以P<0.05为差异有统计学意义.
2.1.1 肠组织病理变化: PI-IBS小鼠第2周起回肠末端及结肠均出现显著的中性粒细胞等炎症细胞浸润和间质充血水肿(炎症评分分别为7.85分±1.21分 vs 0.69分±1.32分, 7.31分±0.95分 vs 0.84分±1.34分, P<0.01), 第8周时PI-IBS小鼠回肠末端及结肠炎症细胞浸润明显减少, 间质充血水肿明显减轻(炎症评分分别为1.38分±1.56分 vs 0.69分±1.32分, 1.23分±1.42分 vs 0.84分±1.34分, P>0.05), 高于对照组但无统计学意义, 提示可能存在低度炎症(图1).
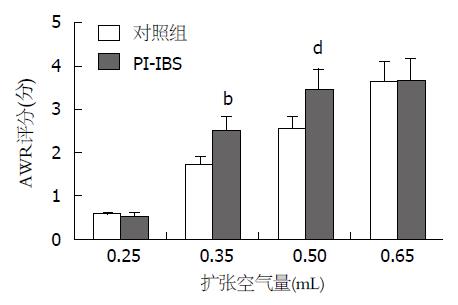
2.1.2 AWR评分: 结直肠的扩张空气量为0.25 mL和0.65 mL时, PI-IBS组小鼠的AWR评分与对照组无显著差异(P>0.05); 但当结直肠的扩张空气量为0.35 mL和0.50 mL时, PI-IBS组小鼠的AWR评分与对照组有显著差异(P>0.05)(图2).
2.1.3 肠道动力检测: PI-IBS组小鼠首次排黑便的时间明显少于对照组(87.68 min±9.32 min vs 109.85 min±9.81 min, P<0.01); 连续收集8 h小鼠粪便, PI-IBS组小鼠Bristol评分明显高于对照组(2.08分±0.30分 vs 1.02分±0.19分, P<0.01).
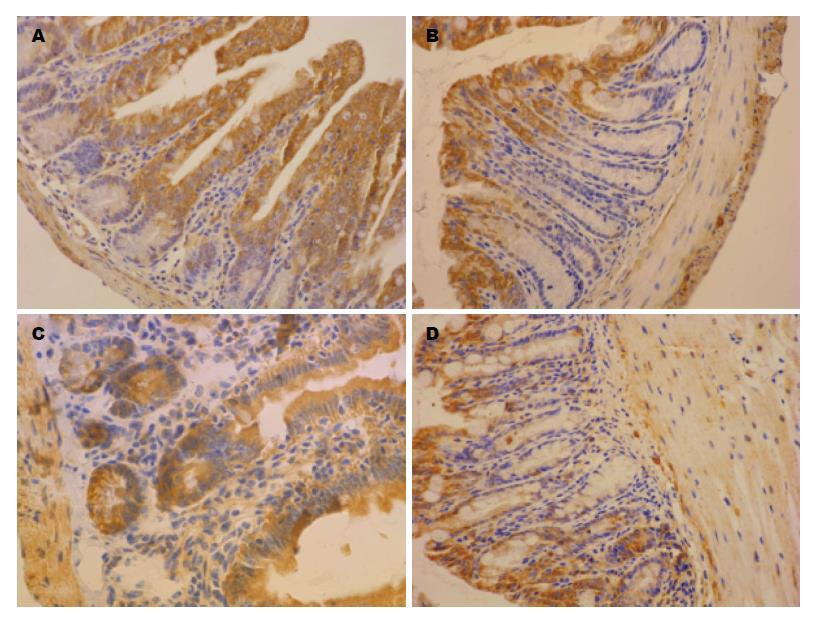
HSP70染色呈黄棕色颗粒, 主要表达于肠道黏膜上皮细胞的胞浆, 在固有层有部分表达, 在黏膜下层及黏膜肌层表达相对较少(图3). PI-IBS组小鼠回肠末端HSP70免疫组织化学染色评分为显著高于对照组(4.75分±0.69分 vs 2.92分±0.64分, P<0.01), 但结肠HSP70染色评分与对照组无显著差异(2.77分±0.60分 vs 2.69分±0.63分, P>0.05).
Spearman分析表明, PI-IBS小鼠回肠HSP70蛋白质表达与肠道炎症评分呈弱的负相关(r = -0.302, P>0.05), 与AWR呈显著负相关(r = -0.957, P<0.01), 与Bristol评分呈显著负相关(r = -0.863, P<0.01). 结肠HSP70表达与AWR及Bristol评分均无明显相关性(r = 0.076, 0.083, P>0.05).
IBS是一种常见的功能性肠病, 表现为腹痛或腹部不适伴有排便习惯改变和/或大便性状异常. 本病发病率高, 症状迁延反复, 严重影响患者生活质量, 加大了医疗费用开支. 由于缺乏可解释症状的形态学改变和生化异常, 病因不明, 目前尚无特异而有效的治疗手段[18-21].
大量临床和实验室证据[22-25]表明, IBS是胃肠动力异常、内脏敏感性增高、局部炎症免疫反应、心理社会因素等多种因素共同作用的结果. 关于IBS发病机制的研究主要集中在两个方面: 神经递质、神经肽介导的IBS内脏敏感性及肠道动力异常机制, 感染后肠道低度炎症和持续免疫激活机制. 感染后或PI-IBS的概念现已得到大多数学者的公认[7,26,27]. 为了探讨黏膜免疫异常在IBS中的作用, 本项目选择旋毛虫感染的PI-IBS小鼠模型作为研究载体.
本研究中, 小鼠感染旋毛虫后第2周即出现明显的肠道炎症, 第8周时炎症逐渐消退, 肠道炎症评分尽管与对照组无显著差异, 但仍高于对照组. 此时AWR评分和肠道动力变化则明显高于对照组. 提示PI-IBS小鼠存在肠道低度炎症.
HSP70是一组结构高度保守的分子伴侣蛋白, 在多数原核和真核细胞中呈结构性表达或由热休克及其他刺激诱导表达. HSP70可以协助由于应激或炎症损伤而改变折叠结构的重要蛋白重新折叠, 从而使损伤得到修复[28]. 应急和炎症时表面表达HSP分子的炎症效应细胞可以被免疫细胞识别并清除[29-31]. 本研究观察到在感染后第8周, 回肠末段的HSP70表达明显高于正常对照组. 目前关于HSP70在胃肠道中的作用的研究大多集中在胃黏膜损伤, 而肠道, 特别是IBS时HSP70的变化及其作用则未见报道. 进一步的相关分析显示, PI-IBS小鼠回肠HSP70表达与肠道炎症评分呈弱负相关, 这可能是由于HSP70并非炎症的独立机制. 但HSP70表达与AWR和Bristol评分呈显著负相关, 这提示HSP70在PI-IBS的内脏高敏感和肠道动力变化中可能起保护作用, 其机制不清楚, 是否因为HSP70对肠道炎症的负调控作用还需进一步研究.
一个有趣的结果是, PI-IBS时结肠HSP70表达并未显著升高, 其与肠道炎症、内脏高敏感和肠道动力变化也无相关性, 这与我们最初的推测并不一致, 原因并不清楚. 可能回肠末段的免疫组织丰富, 是PI-IBS时肠道炎症的启动和调控部位, 结肠则可能是IBS的效应部位, 这些情况有待进一步研究.
总之, 本研究发现PI-IBS时回肠末段HSP70表达升高且与肠道低度炎症、内脏高敏感和肠道动力异常存在相关性. 这为进一步阐明PI-IBS时肠道免疫激活和炎症的调控机制提供了线索.
感染和炎症可能是肠易激综合征(irritable bowel syndrome, IBS)的重要发病机制之一, 即感染后肠易激综合征(post-infectious IBS, PI-IBS). 热休克蛋白70(heat shock protein 70, HSP70)是分子伴侣热休克蛋白家族的一员, 在胃肠黏膜的炎症和损伤中起重要作用, 有关PI-IBS中肠道HSP70的表达与肠道炎症的关系尚未见报道.
潘秀珍, 教授, 主任医师, 福建省立医院消化科
PI-IBS的发病机制尚未完全阐明, 该文探讨PI-IBS小鼠肠道HSP70表达及其与肠道低度炎症的相关性, 为从炎症和免疫紊乱的角度研究PI-IBS的发病机制提供了实验依据.
目前关于PI-IBS发病机制的报道主要集中在两个方面: 神经递质、神经肽介导的IBS内脏敏感性及肠道动力异常机制, 感染后肠道低度炎症和持续免疫激活机制, 包括食物、肠道各种细胞的相互作用.
本研究立题有依据, 设计严谨, 方法先进, 统计正确, 结果可信, 观点明确, 对PI-IBS的发病机制和临床诊疗有参考价值.
编辑: 郭鹏 电编: 闫晋利
| 1. | Soares RL. Irritable bowel syndrome: a clinical review. World J Gastroenterol. 2014;20:12144-12160. [PubMed] [DOI] |
| 2. | Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949-958. [PubMed] [DOI] |
| 3. | Sperber AD, Drossman DA, Quigley EM. The global perspective on irritable bowel syndrome: a Rome Foundation-World Gastroenterology Organisation symposium. Am J Gastroenterol. 2012;107:1602-1609. [PubMed] [DOI] |
| 4. | Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:237-241. [PubMed] |
| 5. | Rajilić-Stojanović M, Jonkers DM, Salonen A, Hanevik K, Raes J, Jalanka J, de Vos WM, Manichanh C, Golic N, Enck P. Intestinal microbiota and diet in IBS: causes, consequences, or epiphenomena? Am J Gastroenterol. 2015;110:278-287. [PubMed] [DOI] |
| 6. | Futagami S, Itoh T, Sakamoto C. Systematic review with meta-analysis: post-infectious functional dyspepsia. Aliment Pharmacol Ther. 2015;41:177-188. [PubMed] [DOI] |
| 7. | Neal KR, Barker L, Spiller RC. Prognosis in post-infective irritable bowel syndrome: a six year follow up study. Gut. 2002;51:410-413. [PubMed] [DOI] |
| 8. | Duncan EJ, Cheetham ME, Chapple JP, van der Spuy J. The role of HSP70 and its co-chaperones in protein misfolding, aggregation and disease. Subcell Biochem. 2015;78:243-273. [PubMed] [DOI] |
| 9. | Shiber A, Ravid T. Chaperoning proteins for destruction: diverse roles of Hsp70 chaperones and their co-chaperones in targeting misfolded proteins to the proteasome. Biomolecules. 2014;4:704-724. [PubMed] [DOI] |
| 10. | Mattoo RU, Goloubinoff P. Molecular chaperones are nanomachines that catalytically unfold misfolded and alternatively folded proteins. Cell Mol Life Sci. 2014;71:3311-3325. [PubMed] [DOI] |
| 11. | Afrazi A, Sodhi CP, Good M, Jia H, Siggers R, Yazji I, Ma C, Neal MD, Prindle T, Grant ZS. Intracellular heat shock protein-70 negatively regulates TLR4 signaling in the newborn intestinal epithelium. J Immunol. 2012;188:4543-4557. [PubMed] [DOI] |
| 12. | Yang B, Zhou X, Lan C. Changes of cytokine levels in a mouse model of post-infectious irritable bowel syndrome. BMC Gastroenterol. 2015;15:43. [PubMed] [DOI] |
| 13. | Long Y, Wang W, Wang H, Hao L, Qian W, Hou X. Characteristics of intestinal lamina propria dendritic cells in a mouse model of postinfectious irritable bowel syndrome. J Gastroenterol Hepatol. 2012;27:935-944. [PubMed] [DOI] |
| 14. | Distrutti E, Cipriani S, Mencarelli A, Renga B, Fiorucci S. Probiotics VSL#3 protect against development of visceral pain in murine model of irritable bowel syndrome. PLoS One. 2013;8:e63893. [PubMed] [DOI] |
| 15. | Caroff DA, Edelstein PH, Hamilton K, Pegues DA. The Bristol stool scale and its relationship to Clostridium difficile infection. J Clin Microbiol. 2014;52:3437-3439. [PubMed] [DOI] |
| 16. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. [PubMed] [DOI] |
| 17. | Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL. Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000;88:2606-2618. [PubMed] [DOI] |
| 18. | Hungin AP, Molloy-Bland M, Claes R, Heidelbaugh J, Cayley WE, Muris J, Seifert B, Rubin G, de Wit N. Systematic review: the perceptions, diagnosis and management of irritable bowel syndrome in primary care--a Rome Foundation working team report. Aliment Pharmacol Ther. 2014;40:1133-1145. [PubMed] [DOI] |
| 19. | Dai C, Zheng CQ, Jiang M, Ma XY, Jiang LJ. Probiotics and irritable bowel syndrome. World J Gastroenterol. 2013;19:5973-5980. [PubMed] [DOI] |
| 20. | Barbara G, Zecchi L, Barbaro R, Cremon C, Bellacosa L, Marcellini M, De Giorgio R, Corinaldesi R, Stanghellini V. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2012;46 Suppl:S52-S55. [PubMed] [DOI] |
| 21. | De Ponti F. Drug development for the irritable bowel syndrome: current challenges and future perspectives. Front Pharmacol. 2013;4:7. [PubMed] [DOI] |
| 22. | Lindfors P, Törnblom H, Sadik R, Björnsson ES, Abrahamsson H, Simrén M. Effects on gastrointestinal transit and antroduodenojejunal manometry after gut-directed hypnotherapy in irritable bowel syndrome (IBS). Scand J Gastroenterol. 2012;47:1480-1487. [PubMed] [DOI] |
| 23. | Bian ZX. Novel insights about the mechanism of visceral hypersensitivity in maternally separated rats. Neurogastroenterol Motil. 2012;24:593-596. [PubMed] [DOI] |
| 24. | Ishihara S, Tada Y, Fukuba N, Oka A, Kusunoki R, Mishima Y, Oshima N, Moriyama I, Yuki T, Kawashima K. Pathogenesis of irritable bowel syndrome--review regarding associated infection and immune activation. Digestion. 2013;87:204-211. [PubMed] [DOI] |
| 25. | Qin HY, Cheng CW, Tang XD, Bian ZX. Impact of psychological stress on irritable bowel syndrome. World J Gastroenterol. 2014;20:14126-14131. [PubMed] [DOI] |
| 26. | Schwille-Kiuntke J, Frick JS, Zanger P, Enck P. Post-infectious irritable bowel syndrome--a review of the literature. Z Gastroenterol. 2011;49:997-1003. [PubMed] [DOI] |
| 27. | Barbara G, Cremon C, Pallotti F, De Giorgio R, Stanghellini V, Corinaldesi R. Postinfectious irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 2009;48 Suppl 2:S95-S97. [PubMed] [DOI] |
| 28. | Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Antonelli E, Roviezzo F, Morelli A, Cirino G, Wallace JL. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J Pharmacol Exp Ther. 2006;316:325-335. [PubMed] [DOI] |
| 29. | Multhoff G. Activation of natural killer cells by heat shock protein 70. 2002. Int J Hyperthermia. 2009;25:169-175. [PubMed] [DOI] |
| 30. | Hirsh MI, Hashiguchi N, Chen Y, Yip L, Junger WG. Surface expression of HSP72 by LPS-stimulated neutrophils facilitates gammadeltaT cell-mediated killing. Eur J Immunol. 2006;36:712-721. [PubMed] [DOI] |