修回日期: 2015-06-22
接受日期: 2015-07-06
在线出版日期: 2015-08-08
目的: 探讨血清中诱骗受体3(decoy receptor 3, DcR3)和CA19-9联合检测对胰腺癌诊断的临床价值.
方法: 收集90例胰腺癌、20例胰腺良性肿瘤患者及20例健康对照者血清, 应用酶联免疫吸附试验(ELISA)方法测定DcR3水平, 电化学发光免疫分析法测定血清CA19-9水平, 采用受试者工作特征(receiver operating characteristic, ROC)曲线评估他们对胰腺癌诊断和可切除性判断的临床价值.
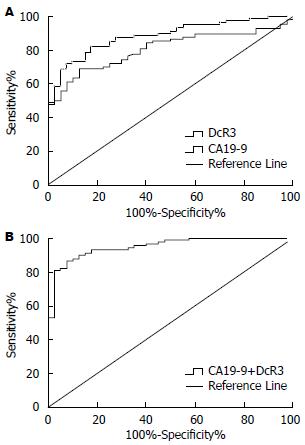
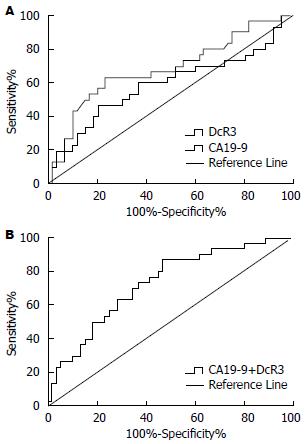
结果: 胰腺癌患者血清DcR3和CA19-9水平分别为37.75 pg/mL和202.29 kU/L, 明显高于胰腺良性肿瘤组和健康对照组, 差异均有统计学意义(P<0.01). DcR3和CA19-9诊断胰腺癌的曲线下面积(area under ROC curves, AUC)分别为0.81和0.89, 两者联合检测的AUC为0.95. DcR3和CA19-9对胰腺癌科切除性判断的AUC分别为0.68和0.59, 两者联合检测的AUC为0.73.
结论: DcR3和CA19-9在胰腺癌患者血清中升高, 两者联合检测有助于提高胰腺癌诊断价值和可切除性的判断.
核心提示: 本研究探讨血清中诱骗受体3(decoy receptor 3, DcR3)和CA19-9联合检测对胰腺癌的诊断及可切除性的价值, 结果显示DcR3和CA19-9在胰腺癌患者血清中升高, 两者联合检测有助于提高胰腺癌诊断价值和可切除性的判断.
引文著录: 杨健, 张旭, 张立峰, 朱东明, 张子祥, 张逸, 李德春, 周健. DcR3、CA19-9联合检测在胰腺癌诊断中的临床价值. 世界华人消化杂志 2015; 23(22): 3629-3633
Revised: June 22, 2015
Accepted: July 6, 2015
Published online: August 8, 2015
AIM: To assess the clinical value of combined detection of serum decoy receptor 3 (DcR3) and CA19-9 in the diagnosis of pancreatic cancer.
METHODS: Serum samples were collected from 90 pancreatic cancer patients, 20 pancreatic benign tumor patients and 20 healthy persons. Serum DcR3 levels were detected by ELISA, and serum CA19-9 levels were detected by eletro-chemiluminescent immunoassay. The receiver operating characteristic (ROC) curve was constructed to evaluate the diagnostic value and predict the resectability of pancreatic cancer.
RESULTS: The median serum levels of DcR3 and CA19-9 were 37.75 pg/mL and 202.29 kU/L, respectively, in the pancreatic cancer group, and they were significantly higher than those in patients with benign tumors or healthy persons (P < 0.01 for both). The area under ROC curves (AUC) of DcR3 and CA19-9 were 0.81 and 0.89, respectively, and AUC of combined detection of two markers produced the highest diagnostic yield (AUC = 0.95). For predicting resectability of pancreatic cancer, AUC values of DcR3 and CA19-9 were 0.68 and 0.59, respectively, however, AUC of combined detection of the two markers was 0.73.
CONCLUSION: The levels of serum DcR3 and CA19-9 are significantly elevated in pancreatic cancer patients. The combined detection of DcR3 and CA19-9 may be helpful to the diagnosis and predicting resectability of pancreatic cancer.
- Citation: Yang J, Zhang X, Zhang LF, Zhu DM, Zhang ZX, Zhang Y, Li DC, Zhou J. Clinical value of combined detection of serum decoy receptor 3 and CA19-9 in diagnosis of pancreatic cancer. Shijie Huaren Xiaohua Zazhi 2015; 23(22): 3629-3633
- URL: https://www.wjgnet.com/1009-3079/full/v23/i22/3629.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v23.i22.3629
胰腺癌是恶性程度最高的消化系肿瘤之一, 手术切除率仅为15%-20%, 5年生存率为5%左右[1,2]. 临床上常用CA19-9、CA50、CA12-5等肿瘤标志物诊断胰腺癌, 以CA19-9意义较大, 但受个体差异及机体内环境的影响, 其敏感性和特异性均不高. 诱骗受体3(decoy receptor 3, DcR3)是肿瘤坏死因子受体(tumor necrosis factor receptor, TNFR)超家族成员, 其氨基酸序列中缺乏跨膜结构, 是一种分泌性蛋白, 在正常组织和血清中不表达或微量表达, 但在多种肿瘤组织和血清中可以出现高表达[3-5]. DcR3具有抑制凋亡和促进肿瘤细胞免疫逃逸的双重功能, 在肿瘤的发生、发展中发挥着重要的作用. 本研究检测了90例胰腺癌患者血清中DcR3和CA19-9水平, 旨在探讨DcR3、CA19-9联合检测对胰腺癌的诊断价值和可切除性的评估.
选择2010-01/2013-12苏州大学附属第一医院普外科的胰腺癌患者90例, 其中男57例, 女33例, 年龄39-77岁, 平均61岁±12岁. 根据美国癌症联合委员会(American Joint Committee on Cancer, AJCC)TNM分期(2010), Ⅰ期23例, Ⅱ期37例, Ⅲ期12例, Ⅳ期18例. 可手术切除经病理证实60例, 通过术中探查判断肿瘤无法切除9例, 通过影像学计算机断层扫描(computed tomography, CT)或磁共振成像(magnetic resonance imaging, MRI)发现存在转移或其他远处转移而不可切除21例. 20例胰腺良性肿瘤包括黏液性囊腺瘤10例, 浆液性囊腺瘤6例, 乳头状瘤2例, 胰岛细胞瘤2例, 另选择20例健康志愿者作为健康对照组.
收集各组患者术前及健康者的血清, DcR3水平测定采用酶联免疫吸附试验(ELISA)方法, ELISA试剂盒购自BD公司, 按照试剂盒说明书操作. CA19-9水平的测定采用电化学发光免疫分析法, 正常参考范围0-37 kU/L.
统计学处理 采用SPSS17.0软件进行统计学处理, DcR3和CA19-9水平以中位数表示, 采用Kruskal-Wallis H检验、Spearman分析进行组间比较及相关性分析. 应用受试者工作特征(receiver operating characteristic, ROC)曲线和逐步Logistic回归结果的ROC曲线计算曲线下面积(area under ROC curves, AUC), 分析单指标及联合检测的效能. 以P<0.05为差异有统计学意义.
表1显示, 胰腺癌患者血清DcR3水平明显高于各胰腺良性肿瘤以及健康对照组(P<0.01), 胰腺良性肿瘤患者组和健康对照组血清DcR3水平比较, 差异无统计学意义(P>0.05). 胰腺癌患者血清CA19-9水平明显高于胰腺良性肿瘤组和健康对照组(P<0.01), 同时胰腺良性肿瘤组血清CA19-9水平与健康对照组比较, 差异有统计学意义(P<0.05). 相关性分析显示, 胰腺癌患者血清DcR3和CA19-9水平无明显相关性(r = 0.041, P>0.05).
| 分组 | n | DcR3(pg/mL) | CA19-9(kU/L) | ||
| 中位数 | 范围 | 中位数 | 范围 | ||
| 胰腺癌组 | 90 | 37.75 | 3.18-98.58 | 202.29 | 6.23-877.48 |
| 胰腺良性肿瘤组 | 20 | 8.64 | 3.31-36.49 | 13.57 | 3.45-211.25 |
| 健康对照组 | 20 | 8.17 | 3.18-17.47 | 8.30 | 2.15-30.46 |
基于Logistic回归的ROC曲线分析单个标志物及联合检测的评价结果示, 两种标志物单项检测的结果表明CA19-9的敏感度82.22%, 特异度82.50%, DcR3的敏感度68.89%, 特异度87.50%, 联合检测的敏感度87.78%, 特异度90.00%, 提示联合检测较为理想. DcR3、CA19-9诊断胰腺癌的AUC分别为0.81(95%CI: 0.74-0.88), 0.89(95%CI: 0.83-0.94), DcR3、CA19-9联合检测的AUC为0.95(95%CI: 0.91-0.98), 以两者联合检测的效能最佳(图1).
60例可切除患者中DcR3和CA19-9的水平分别为31.66 pg/mL和163.72 kU/L, 明显低于不可切除的患者(DcR3: 72.22 pg/mL; CA19-9: 366.99 kU/L), 差异均有统计学意义(P<0.01). 根据ROC曲线分析DcR3、CA19-9单指标对胰腺癌可切除性判断的AUC分别为0.68(95%CI: 0.56-0.81), 0.59(95%CI: 0.46-0.73)诊断价值较低, 而联合检测对胰腺癌可切除性判断的AUC为0.73(95%CI: 0.62-0.84), 对胰腺癌可切除性判断的意义为中等(图2).
DcR3最早是Pitti等[6]于1998年发现的与TNFR高度同源的凋亡抑制因子, 其氨基酸序列中缺乏跨膜结构, 是一种分泌性蛋白. DcR3基因定位于染色体20q13.3, 全长11.14 kb, 由271个氨基酸残基组成, 包括4个半胱氨酸残基富集区和1个N-糖基化位点. DcR3能竞争性结合Fas配体(Fas ligand, FasL)、肿瘤坏死因子样配体1A(tumor necrosis factor ligand-like 1A, TL1A)和T细胞上可诱导表达的、与单纯疱疹病毒糖蛋白D竞争结合单纯疱疹病毒侵入介体的淋巴毒素类似物(lymphotoxin analogues, LIGHT), 发挥抑制凋亡和促进肿瘤细胞免疫逃逸的双重作用[7-9]. Wu等[10]研究发现急性感染和健康者血清中DcR3阴性率为96.9%, 而恶性肿瘤患者血清中DcR3阳性率为55.0%, 提示DcR3可作为鉴别肿瘤和炎症的标志物之一. 我们前期的研究[11]发现胰腺癌患者血清中DcR3水平明显高于正常健康者和良性肿瘤组, 并且胰腺癌组织中DcR3的表达与血清中DcR3的表达呈正相关关系.
CA19-9是唾液酸化的Lewisa血型抗原, 正常胰腺组织分泌极少, 当Lewisa抗原阳性的胰管、胆管、胃肠道上皮细胞发生恶变时分泌亢进, 是针对胰腺癌临床应用最多和最有价值的肿瘤标志物, 但在急性炎症、胆道梗阻、肝硬化等疾病时也有不同程度的升高[12]. 此外, 在分化差的肿瘤中CA19-9的表达水平也较低. 因此, 选择包括CA19-9在内的两种及以上的血清肿瘤标志物进行联合检测, 有助于胰腺癌的诊断和术前评估[13,14].
本研究结果显示, DcR3、CA19-9单项检测以及联合检测的AUC分别为0.81、0.89和0.95, 表明联合检测能提高胰腺癌的诊断效能. 同时研究表明DcR3和CA19-9水平在胰腺癌患者血清中分别独立升高, 并无相关性, 可能与这两种标志物在细胞中不同的合成途径有关, 因此在诊断上具有互补性, 为两者的联合检测的合理性提供了理论依据. 此外, 通过ROC曲线对胰腺癌可切除性进行评估, DCR3和CA19-9单项检测的AUC分别为0.68和0.59, 诊断价值较低, 而两者联合检测AUC为0.73, 诊断价值中等. CA19-9对胰腺癌可切除性判断的效能较低, 与CA19-9的蛋白骨架有关, 其抗原决定簇是Lewisa抗原的衍生物, 而人群中Lewisa血型抗原阴性率在10%左右, 这部分胰腺癌患者血清中CA19-9水平较低[15]. DcR3作为一种分泌性蛋白, 在血清中长期稳定表达, 是鉴别肿瘤和炎症的标志物之一, 与CA19-9的表达特性无重叠性. 因此, 联合检测相对单项检测能获得更多的信息, 一方面互相补充, 支持诊断; 另一方面增加术前评估的手段, 通过结合影像学资料, 减少手术的盲目性.
胰腺癌是一种发病隐匿、发展迅速、预后极差的消化系统肿瘤. 针对胰腺癌诊断及术后评估指标一直在研究中, CA19-9在胰腺癌的诊治中发挥重要作用, 但仍存在许多不足. 因此, 临床上需要发现新的肿瘤标志物, 通过联合检测来提高胰腺癌诊断及手术切除率的敏感性和特异性.
江建新, 教授, 主任医师, 湖北省肿瘤医院肝胆胰腺外科
胰腺癌具有早期诊断困难、手术切除率低的特点, 血清肿瘤标志物检测是一种无创而且有效的诊断和评估方法, 单指标检测在胰腺癌诊断方面存在很多不足. 诱骗受体3(decoy receptor 3, DcR3)是一种分泌性蛋白, 在血清中长期稳定存在, 因此联合检测胰腺癌血清中DcR3和CA19-9对胰腺癌诊断及手术可切除性具有重要的评估作用.
DcR3具有抑制凋亡和促进肿瘤细胞免疫逃逸的双重功能, 在肿瘤的发生、发展中发挥着重要的作用. 研究表明DcR3在胰腺癌组织和血清中均高表达, 并在血清中可与胰腺良性肿瘤及正常对照组区分.
有学者提出胰腺癌生物学特性与术前评估可切除性的思考, 以及文献报道血清CA19-9水平对胰腺癌可切除性判断的临床意义, 但多数是单一性指标去判定, 本文探讨在胰腺癌患者血清中联合检测DcR3、CA19-9对胰腺癌诊断和可切除性的评估价值.
DcR3在国外已经被用于多种恶性肿瘤的临床诊治过程、治疗前后判断疗效和新的相关药物的研发. 由于血清中DcR3的检测具有操作简单方便, 创伤小, 费用低的优势, 是潜在的临床诊断指标.
诱骗受体: 诱骗受体家族包括3个成员, DcR1、DcR2和DcR3, 通过阻断凋亡信号发挥抑制凋亡的作用, 与DcR3在肿瘤中高表达不同, DcR1和DcR2主要分布在正常组织和细胞中.
本文探讨在胰腺癌患者血清中联合检测DcR3、CA19-9的表达水平, 并分析与胰腺癌诊断及可切除性的相关性, 得出结论DcR3和CA19-9在胰腺癌患者血清中升高, 两者联合检测有助于提高胰腺癌诊断价值和可切除性的判断. 实验设计及结果处理合理, 具有一定的创新性和临床意义.
编辑: 韦元涛 电编:闫晋利
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [PubMed] [DOI] |
| 2. | Del Chiaro M, Segersvärd R, Lohr M, Verbeke C. Early detection and prevention of pancreatic cancer: is it really possible today? World J Gastroenterol. 2014;20:12118-12131. [PubMed] [DOI] |
| 3. | Tong J, Ao R, Wang Y, Chang B, Wang BY. Prognostic and clinicopathological differences of DcR3 in gastrointestinal cancer: evidence from meta-analysis. Int J Clin Exp Med. 2014;7:3096-3105. [PubMed] |
| 4. | Bedewy AM, Elgammal MM, Bedewy MM, El-Maghraby SM. Assessing DcR3 expression in relation to survivin and other prognostic factors in B cell non-Hodgkin's lymphoma. Ann Hematol. 2013;92:1359-1367. [PubMed] [DOI] |
| 5. | Zong L, Chen P, Wang DX. Death decoy receptor overexpression and increased malignancy risk in colorectal cancer. World J Gastroenterol. 2014;20:4440-4445. [PubMed] [DOI] |
| 6. | Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, Dowd P, Huang A, Donahue CJ, Sherwood SW, Baldwin DT. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396:699-703. [PubMed] [DOI] |
| 7. | Gill RM, Hunt JS. Soluble receptor (DcR3) and cellular inhibitor of apoptosis-2 (cIAP-2) protect human cytotrophoblast cells against LIGHT-mediated apoptosis. Am J Pathol. 2004;165:309-317. [PubMed] [DOI] |
| 8. | Takahashi M, Miura Y, Hayashi S, Tateishi K, Fukuda K, Kurosaka M. DcR3-TL1A signalling inhibits cytokine-induced proliferation of rheumatoid synovial fibroblasts. Int J Mol Med. 2011;28:423-427. [PubMed] [DOI] |
| 9. | Zhou J, Song S, He S, Wang Z, Zhang B, Li D, Zhu D. Silencing of decoy receptor 3 (DcR3) expression by siRNA in pancreatic carcinoma cells induces Fas ligand-mediated apoptosis in vitro and in vivo. Int J Mol Med. 2013;32:653-660. [PubMed] [DOI] |
| 10. | Wu Y, Han B, Sheng H, Lin M, Moore PA, Zhang J, Wu J. Clinical significance of detecting elevated serum DcR3/TR6/M68 in malignant tumor patients. Int J Cancer. 2003;105:724-732. [PubMed] |
| 12. | Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266-270. [PubMed] |
| 15. | Kim JE, Lee KT, Lee JK, Paik SW, Rhee JC, Choi KW. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J Gastroenterol Hepatol. 2004;19:182-186. [PubMed] [DOI] |