修回日期: 2015-06-08
接受日期: 2015-06-29
在线出版日期: 2015-07-28
目的: 观察胰岛素样生长因子1(insulin-like growth factor 1, IGF-1)在结直肠癌中的表达情况, 评价其与结直肠癌血管生成指标及临床病理参数之间的关系.
方法: 选择于首都医科大学附属北京世纪坛医院经病理确诊的56例散发性结直肠癌患者病变组织, 免疫组织化学染色观察IGF-1在结直肠癌组织的表达情况, CD34免疫组织化学染色后计数微血管密度(microvessel density, MVD).
结果: IGF-1在结直肠癌与正常黏膜中高表达率分别为85.71%和35.00%(P<0.05), IGF-1的高表达与淋巴结转移明显相关(P<0.05). 结直肠癌与正常黏膜的MVD值分别为35.55±7.78和8.76±2.67(P<0.05). IGF-1的表达强度与MVD呈正相关(P<0.05).
结论: IGF-1在结直肠癌组织中高表达, 并可能通过参与结直肠癌血管生成促进其进展.
核心提示: 胰岛素样生长因子-1(insulin-like growth factor 1, IGF-1)参与了多种肿瘤的发生发展及侵袭转移过程, 并可能影响肿瘤血管生成. 本研究对结直肠癌中IGF-1与血管生成的关系进行了初步评价.
引文著录: 刘揆亮, 余瑞金, 封国生, 吴静. 胰岛素样生长因子-1在结直肠癌中的表达及其与血管生成的关系. 世界华人消化杂志 2015; 23(21): 3384-3389
Revised: June 8, 2015
Accepted: June 29, 2015
Published online: July 28, 2015
AIM: To investigate the relationship between the expression of insulin-like growth factor 1 (IGF-1) and clinicopathological parameters, as well as tumor angiogenesis in colorectal cancer.
METHODS: The expression of IGF-1 was detected using immunohistochemical method in 56 colorectal carcinoma and 20 normal colon tissues. Microvessel density (MVD) was counted by evaluating the expression of endothelial marker CD34.
RESULTS: The positive rates of IGF-1 in colorectal carcinoma and normal mucosa were 85.71% and 35%, respectively. The expression of IGF-1 correlated with lymph node metastasis significantly (P < 0.05). MVD values were 8.76±2.67 and 35.55 ± 7.78 in normal colon tissue and colorectal cancer, respectively. MVD correlated significantly with differentiation degree, invasion depth, Duke's stage and lymph node metastasis (P < 0.05 for all).
CONCLUSION: IGF-1 is highly expressed in colorectal adenocarcinoma and may be involved in the progression of colorectal cancer through enhancing tumor angiogenesis.
- Citation: Liu KL, Yu RJ, Feng GS, Wu J. Expression of insulin-like growth factor 1 in colorectal cancer: Relationship with angiogenesis. Shijie Huaren Xiaohua Zazhi 2015; 23(21): 3384-3389
- URL: https://www.wjgnet.com/1009-3079/full/v23/i21/3384.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v23.i21.3384
结直肠癌是常见的恶性肿瘤之一, 近年来我国的结直肠癌发病率也在逐渐上升, 从1982年的7/10万升至目前的30.7/10万[1,2]. 其发生发展是一个多阶段、多步骤的渐进过程, 与多种生长因子活性的表达与调控密切相关. 胰岛素样生长因子-1(insulin-like growth factor 1, IGF-1)是一类受生长激素调控的蛋白多肽, 在维持人体内环境稳定中起着重要作用[3], 参与了多种肿瘤的发生发展及侵袭转移过程[4-6]. IGF-1通过自分泌、旁分泌的方式与其受体结合后, 参与结直肠癌的发生发展, 并可能影响肿瘤血管生成[7]. 本研究通过免疫组织化学方法观察IGF-1及CD34在结直肠癌中的表达情况, 初步评价其与血管生成的关系.
收集2008-01/2011-12首都医科大学附属北京世纪坛医院临床资料完整的56例散发性结直肠癌及20例正常黏膜组织的石蜡标本, 患者年龄30-92岁(平均63.4岁±13.5岁), 结直肠癌者均为首次发现肿瘤, 未进行化疗及放疗等治疗. 免疫组织化学染色采用Envision二步法. 兔抗人IGF-1单克隆抗体购自英国abcam公司(ab9572, 1:100), 鼠抗人CD34单克隆抗体购自北京中杉金桥生物有限公司(ZM0046, 即用型). 二抗(工作液)购自丹麦Dako公司. 本研究经首都医科大学附属北京世纪坛医院伦理委员会评审通过, 并取得患者知情同意.
1.2.1 免疫组织化学检测及评分标准: 蜡块4 μm切片, 常规脱蜡置水, 枸橼酸缓冲液抗原修复, 3%过氧化氢室温避光封闭, 5%羊血清封闭后滴加IGF-1与CD34单克隆抗体及二抗工作液, DAB显色, 苏木素复染15 s. 用PBS液代替一抗作阴性对照. 染色完成后显微镜下随机选取5个高倍镜视野(×400)摄片, 由一名病理医师进行观察. 评分标准参照Koga等[8]进行判定: IGF-1染色判定如下: 染色强度分析: 分为0-3分, 0分表达缺失(无染色)、1分表达减弱(淡黄色)、2分中等表达(黄色)、3分强表达(黄褐色), 染色细胞范围分析: 0-3分, 0分无染色细胞、1分<10%、2分10-50%、3分>50%. 两个系统分数相加为总表达强度, 0分为(-), 1-2分为(+), 3-4分为(++), 5-6分为(+++). (-)与(+)记入低表达, (++)与(+++)记入高表达.
1.2.2 微血管密度计数: 采用CD34计数. 将CD34定位于血管内皮细胞的胞膜和胞浆, 阳性者呈黄色或棕色, CD34染色的单个内皮细胞或内皮细胞群形成的管状或窄隙状结构视为微血管. 先于低倍镜下确定血管密集区域, 之后高倍镜下计数微血管. 取3个视野的平均值作为微血管密度(microvessel density, MVD)值. 以MVD>30为高密度, MVD≤30为低密度[9].
统计学处理 采用SPSS15.0统计学软件. 计量资料以mean±SD表示, 比较采用χ2检验, 计数资料以率表示, 比较采用独立样本t检验. 采用Spearman相关性分析分析等级资料的相关性. P<0.05为差异有统计学意义.
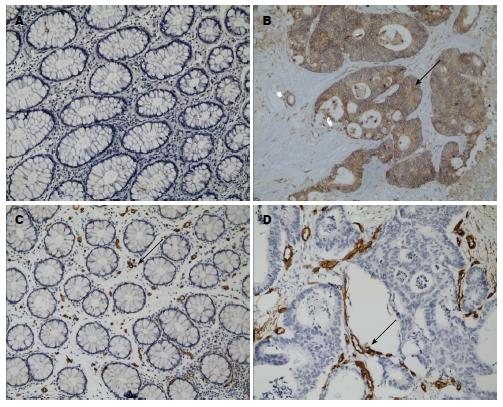
IGF-1主要表达于细胞浆, 在正常结直肠黏膜中呈低表达, 而在结直肠癌组织中呈高表达(图1A, B). IGF-1在结直肠癌及正常黏膜中高表达率分别为85.71%与35.00%(P<0.05). IGF-1表达强度与患者的性别、年龄、肿瘤大小、部位、浸润深度、分化程度、Duke's分期以及有无癌栓均无相关(P>0.05), 而与淋巴结转移有显著相关性(P<0.05)(表1).
| 项目 | n | IGF-1 | P值 | MVD值 | P值 | |
| 高表达 | 低表达 | |||||
| 性别 | 0.387 | 0.415 | ||||
| 男 | 29 | 26 | 3 | 35.30±6.88 | ||
| 女 | 27 | 22 | 5 | 35.82±8.76 | ||
| 年龄(岁) | 0.903 | 0.024 | ||||
| ≥60 | 41 | 35 | 6 | 34.33±6.49 | ||
| <60 | 15 | 13 | 2 | 38.89±10.05 | ||
| 肿瘤大小(cm) | 0.449 | 0.968 | ||||
| ≥5 | 28 | 25 | 3 | 35.99±7.76 | ||
| <5 | 28 | 23 | 5 | 35.10±7.90 | ||
| 部位 | 0.229 | 0.110 | ||||
| 结肠 | 32 | 29 | 3 | 35.31±8.64 | ||
| 直肠 | 24 | 19 | 5 | 35.87±6.62 | ||
| 分化程度 | 0.785 | 0.002 | ||||
| 高中分化 | 40 | 36 | 4 | 32.82±4.51 | ||
| 低分化 | 16 | 14 | 2 | 42.38±9.96 | ||
| Duke分期 | 0.277 | 0.028 | ||||
| A+B | 40 | 33 | 7 | 34.01±6.15 | ||
| C+D | 16 | 15 | 1 | 38.80±9.86 | ||
| 浸润深度 | 0.075 | 0.020 | ||||
| 未穿透浆膜 | 9 | 6 | 3 | 31.91±3.68 | ||
| 穿透浆膜层 | 47 | 42 | 5 | 36.25±8.18 | ||
| 淋巴结转移 | 0.038 | 0.018 | ||||
| 有 | 26 | 25 | 1 | 39.09±10.08 | ||
| 无 | 30 | 23 | 7 | 34.01±6.06 | ||
| 脉管癌栓 | 0.281 | 0.260 | ||||
| 有 | 27 | 24 | 3 | 39.24±7.66 | ||
| 无 | 29 | 21 | 8 | 32.35±6.43 | ||
CD34主要表达于细胞膜, 在正常结直肠黏膜中呈低表达, 而在结直肠癌组织中呈高表达(图1C, D). 正常结直肠黏膜组织中MVD值为8.76±2.67; 结直肠癌中MVD值为35.55±7.78(P<0.05). MVD值与肿瘤分化程度、肿瘤浸润深度、Duke's分期、淋巴结转移均显著相关(P<0.05). IGF-1高表达组中, 44例MVD呈高密度, 4例MVD呈低密度; IGF-1低表达组中, 高密度与低密度各有4例; IGF-1的表达强度与MVD显著相关(P<0.001).
IGF-1是一条由70个氨基酸组成的单链多肽, 一级结构与胰岛素有很高的同源性[3]. IGF-1与其受体结合后在细胞内外可启动多条信号转导途径, 促进肿瘤细胞的增殖与生长[10]. IGF-1的过度表达与结直肠癌、前列腺癌、胰腺癌、肝癌等多种肿瘤的有丝分裂、增殖、分化、凋亡、血管生成等生命活动密切相关[11-15]. IGF-1在结直肠癌的发生中具有重要作用, 通过丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)与磷酯酰肌醇-3-激酶(phosphatidylinositol 3-kinase, PI3K)/Akt途径参与结直肠癌的发生发展[16-18]. 一些研究显示在结直肠癌患者血清中IGF-1表达水平增高, 且可能参与了结直肠癌的进展及转移[19,20]. 本研究采用免疫组织化学方法检测IGF-1的表达, 发现其在结直肠癌中的表达较正常黏膜显著升高. 这与Hakam等[21]及Reinmuth等[22]报道一致. 结果还显示IGF-1的表达与结直肠癌淋巴结转移明显相关, 这与Shiratsuchi等[23]的研究结果一致. 提示IGF-1参与了结直肠癌的肿瘤进展过程.
Folkman等[24]提出肿瘤的生长可分为两个明显不同的阶段, 即初期无血管的缓慢生长阶段与之后有血管的快速增殖阶段. MVD 通过对肿瘤血管最密集的部位进行微小血管计数, 可反映肿瘤组织中的血管生成状况[9]. 本研究中采用CD34染色评价肿瘤血管生成状况, 证实结直肠癌组织中CD34的表达明显高于正常黏膜, 且MVD值与肿瘤分化程度、肿瘤浸润深度、Duke's分期、淋巴结转移均显著相关, 提示微血管生成在结直肠癌的进展具有重要推动作用. 并且, 我们的结果显示IGF-1的表达强度与MVD值存在显著相关(P<0.001). 研究[25]报道IGF-1可能具有促进血管生成的能力. IGF-1可刺激内皮祖细胞的分化, 迁移、归巢, 还可诱导角膜及视网膜内皮细胞的增殖和新生血管的形成[26], 在心血管系统中也有类似作用[27]. Fukuda等[28]研究发现, IGF-1可在结直肠癌肿瘤细胞中调控诱导血管内皮生长因子(vascular endothelial growth factor, VEGF)的表达, 从而促进血管生成, 导致肿瘤进展及转移. 还有研究[29]证实腺病毒介导的IGF-1转染可促进VEGF诱导的血管生成. 此外, IGF-1还可通过诱导缺氧诱导因子-1(hypoxia inducible factor-1, HIF-1)的表达而促进肿瘤的血管生成[30].
总之, 本研究发现, IGF-1在结直肠腺癌中呈较高水平表达, 且与MVD值显著相关, 其可能通过促进肿瘤血管生成而参与结直肠癌进展. IGF-1可能成为结直肠癌抗血管生成靶向治疗的新靶点.
胰岛素样生长因子-1(insulin-like growth factor 1, IGF-1)受到生长激素调控, 通过自分泌、旁分泌的方式与其受体结合后在多种肿瘤中发挥作用, 也参与结直肠癌的发生发展及侵袭转移, 并可能影响肿瘤血管生成. 但目前有关结直肠癌中IGF-1与血管生成之间关系的研究报道不多.
周建奖, 教授, 贵阳医学院分子生物学重点实验室
结直肠癌的发生发展是一个多阶段、多步骤的渐进过程, 与多种生长因子活性的表达与调控密切相关, 这些生长因子的作用一直受到较多关注.
研究认为IGF-1可刺激内皮祖细胞的分化, 迁移、归巢, 还可通过调控血管内皮生长因子及缺氧诱导因子1的表达而促进血管生成.
IGF-1在结直肠腺癌中呈较高水平表达, 且与微血管密度值显著相关, 提示IGF-1可能通过促进肿瘤血管生成而参与结直肠癌进展.
IGF-1可能通过促进肿瘤血管生成而参与结直肠癌进展,可能成为结直肠癌抗血管生成靶向治疗的新靶点.
本文用免疫组织化学染色检测结直肠癌组织标本中IGF-1及CD34的表达, 探讨结直肠癌中IGF-1与血管密度及临床病理参数之间的关系. 有一定新颖性, 也有一定的科学性和实用价值, 文章撰写逻辑性强, 图表规范, 可读性好.
编辑: 郭鹏 电编:都珍珍
| 3. | Rinderknecht E, Humbel RE. Primary structure of human insulin-like growth factor II. FEBS Lett. 1978;89:283-286. [PubMed] [DOI] |
| 4. | Key TJ, Appleby PN, Reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11:530-542. [PubMed] [DOI] |
| 5. | Rinaldi S, Cleveland R, Norat T, Biessy C, Rohrmann S, Linseisen J, Boeing H, Pischon T, Panico S, Agnoli C. Serum levels of IGF-I, IGFBP-3 and colorectal cancer risk: results from the EPIC cohort, plus a meta-analysis of prospective studies. Int J Cancer. 2010;126:1702-1715. [PubMed] [DOI] |
| 6. | Cui H. Loss of imprinting of IGF2 as an epigenetic marker for the risk of human cancer. Dis Markers. 2007;23:105-112. [PubMed] [DOI] |
| 7. | Sridhar SS, Goodwin PJ. Insulin-insulin-like growth factor axis and colon cancer. J Clin Oncol. 2009;27:165-167. [PubMed] [DOI] |
| 8. | Koga H, Sakisaka S, Ohishi M, Kawaguchi T, Taniguchi E, Sasatomi K, Harada M, Kusaba T, Tanaka M, Kimura R. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology. 1999;29:688-696. [PubMed] [DOI] |
| 9. | Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401-409. [PubMed] |
| 10. | Foulstone E, Prince S, Zaccheo O, Burns JL, Harper J, Jacobs C, Church D, Hassan AB. Insulin-like growth factor ligands, receptors, and binding proteins in cancer. J Pathol. 2005;205:145-153. [PubMed] [DOI] |
| 11. | Huang F, Xu LA, Khambata-Ford S. Correlation between gene expression of IGF-1R pathway markers and cetuximab benefit in metastatic colorectal cancer. Clin Cancer Res. 2012;18:1156-1166. [PubMed] [DOI] |
| 12. | Dong X, Javle M, Hess KR, Shroff R, Abbruzzese JL, Li D. Insulin-like growth factor axis gene polymorphisms and clinical outcomes in pancreatic cancer. Gastroenterology. 2010;139:464-473, 473.e1-e3. [PubMed] [DOI] |
| 13. | Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clin Cancer Res. 2010;16:2505-2511. [PubMed] [DOI] |
| 14. | Jaquish DV, Yu PT, Shields DJ, French RP, Maruyama KP, Niessen S, Hoover H, A Cheresh D, Cravatt B, Lowy AM. IGF1-R signals through the RON receptor to mediate pancreatic cancer cell migration. Carcinogenesis. 2011;32:1151-1156. [PubMed] [DOI] |
| 15. | Kaseb AO, Morris JS, Hassan MM, Siddiqui AM, Lin E, Xiao L, Abdalla EK, Vauthey JN, Aloia TA, Krishnan S. Clinical and prognostic implications of plasma insulin-like growth factor-1 and vascular endothelial growth factor in patients with hepatocellular carcinoma. J Clin Oncol. 2011;29:3892-3899. [PubMed] [DOI] |
| 16. | Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915-928. [PubMed] [DOI] |
| 17. | Sharon C, Baranwal S, Patel NJ, Rodriguez-Agudo D, Pandak WM, Majumdar AP, Krystal G, Patel BB. Inhibition of insulin-like growth factor receptor/AKT/mammalian target of rapamycin axis targets colorectal cancer stem cells by attenuating mevalonate-isoprenoid pathway in vitro and in vivo. Oncotarget. 2015; Mar 29. [Epub ahead of print]. [PubMed] |
| 18. | Navarro M, Baserga R. Limited redundancy of survival signals from the type 1 insulin-like growth factor receptor. Endocrinology. 2001;142:1073-1081. [PubMed] [DOI] |
| 19. | Jiang B, Zhang X, Du LL, Wang Y, Liu DB, Han CZ, Jing JX, Zhao XW, Xu XQ. Possible roles of insulin, IGF-1 and IGFBPs in initiation and progression of colorectal cancer. World J Gastroenterol. 2014;20:1608-1613. [PubMed] [DOI] |
| 20. | Zhang R, Xu GL, Li Y, He LJ, Chen LM, Wang GB, Lin SY, Luo GY, Gao XY, Shan HB. The role of insulin-like growth factor 1 and its receptor in the formation and development of colorectal carcinoma. J Int Med Res. 2013;41:1228-1235. [PubMed] [DOI] |
| 21. | Hakam A, Yeatman TJ, Lu L, Mora L, Marcet G, Nicosia SV, Karl RC, Coppola D. Expression of insulin-like growth factor-1 receptor in human colorectal cancer. Hum Pathol. 1999;30:1128-1133. [PubMed] [DOI] |
| 22. | Reinmuth N, Fan F, Liu W, Parikh AA, Stoeltzing O, Jung YD, Bucana CD, Radinsky R, Gallick GE, Ellis LM. Impact of insulin-like growth factor receptor-I function on angiogenesis, growth, and metastasis of colon cancer. Lab Invest. 2002;82:1377-1389. [PubMed] |
| 23. | Shiratsuchi I, Akagi Y, Kawahara A, Kinugasa T, Romeo K, Yoshida T, Ryu Y, Gotanda Y, Kage M, Shirouzu K. Expression of IGF-1 and IGF-1R and their relation to clinicopathological factors in colorectal cancer. Anticancer Res. 2011;31:2541-2545. [PubMed] |
| 24. | Folkman J. How is blood vessel growth regulated in normal and neoplastic tissue? G.H.A. Clowes memorial Award lecture. Cancer Res. 1986;46:467-473. [PubMed] |
| 25. | Piecewicz SM, Pandey A, Roy B, Xiang SH, Zetter BR, Sengupta S. Insulin-like growth factors promote vasculogenesis in embryonic stem cells. PLoS One. 2012;7:e32191. [PubMed] [DOI] |
| 26. | Haleagrahara N, Chakravarthi S, Mathews L. Insulin like growth factor-1 (IGF-1) causes overproduction of IL-8, an angiogenic cytokine and stimulates neovascularization in isoproterenol-induced myocardial infarction in rats. Int J Mol Sci. 2011;12:8562-8574. [PubMed] [DOI] |
| 27. | Lorier G, Touriño C, Kalil RA. Coronary angiogenesis as an endogenous response to myocardial ischemia in adults. Arq Bras Cardiol. 2011;97:e140-e148. [PubMed] [DOI] |
| 28. | Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem. 2002;277:38205-38211. [PubMed] [DOI] |
| 29. | Balaji S, LeSaint M, Bhattacharya SS, Moles C, Dhamija Y, Kidd M, Le LD, King A, Shaaban A, Crombleholme TM. Adenoviral-mediated gene transfer of insulin-like growth factor 1 enhances wound healing and induces angiogenesis. J Surg Res. 2014;190:367-377. [PubMed] [DOI] |
| 30. | Wahl P, Schmidt A, Demarees M, Achtzehn S, Bloch W, Mester J. Responses of angiogenic growth factors to exercise, to hypoxia and to exercise under hypoxic conditions. Int J Sports Med. 2013;34:95-100. [PubMed] [DOI] |