修回日期: 2015-04-21
接受日期: 2015-04-24
在线出版日期: 2015-07-18
甲胎蛋白(alpha-fetoprotein, AFP)是一种胚胎特异性α-球蛋白, 是临床诊断胎儿缺陷和肝细胞癌(hepatocellular carcinoma, HCC)的肿瘤标志物. 近年来不断有研究证实, AFP在HCC中发挥信号分子样作用, 尤其在乙型肝炎病毒(hepatitis B virus, HBV)诱导的肝癌中, AFP和HBV及HBV编码的x蛋白质(hepatitis B virus protein x, HBx)之间存在相互协同作用, 调控并且促进肝癌的发生发展过程.
核心提示: 甲胎蛋白(alpha-fetoprotein, AFP)不仅是肿瘤标志物, 胞浆中的AFP还可以发挥信号分子的作用, 在肝癌的发生发展过程中起促进作用. AFP的表达与乙型肝炎病毒(hepatitis B virus, HBV)的感染密切相关, 并能和HBV协同作用, 共同促进癌症的进程.
引文著录: 张超, 李刚. 甲胎蛋白在乙型肝炎病毒诱导肝癌发生中的作用及临床应用展望. 世界华人消化杂志 2015; 23(20): 3171-3181
Revised: April 21, 2015
Accepted: April 24, 2015
Published online: July 18, 2015
Mammalian alpha-fetoprotein (AFP) as a fetal specific alpha-globulin that has been used as a serum fetal defect/tumor marker for diagnosis and prediction of liver disease. Over the past decade, research indicates that AFP as an intracellular signal molecule is not only a biomarker but also interacts with hepatitis B virus (HBV) and hepatitis B virus protein x and plays multifarious roles in the development of hepatocellular carcinoma, especially in HBV-induced liver cancer.
- Citation: Zhang C, Li G. Role of alpha-fetoprotein in hepatitis B virus-induced hepatocellular carcinoma: Prospect in clinical application. Shijie Huaren Xiaohua Zazhi 2015; 23(20): 3171-3181
- URL: https://www.wjgnet.com/1009-3079/full/v23/i20/3171.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v23.i20.3171
甲胎蛋白(alpha-fetoprotein, AFP)在临床常被用作检测胎儿缺陷和肝脏肿瘤发生的标志物[1]. 我国是肝癌高发区, 患者占全球55%以上[2], 临床统计, 80%以上的肝癌患者由乙型肝炎病毒(hepatitis B virus, HBV)的慢性感染发展而来, 肝癌的发生和HBV感染密不可分[3,4]. 成人中, 约70%-80%的肝细胞癌(hepatocellular carcinoma, HCC)患者血清中AFP升高, AFP水平的升高与HCC各种恶性特征相关联[5,6], 且血清中高AFP值的患者相对于低AFP值的患者, 预后极差, 无病生存率(disease free survival, DFS)和总生存率(overall survival, OS)等指标远低于后者[7]. 由此可知, 在HBV诱导肝癌的发生发展过程中, AFP必定发挥了重要的作用. 本文将从以下几个方面展开.
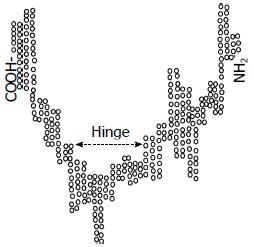
AFP基因位于4号染色体, 由14个外显子和15个内含子组成[8], 剪切成熟的mRNA编码609个氨基酸, 其中位于N末端的19个氨基酸是信号肽, 分子量约为69 kDa, 属于白蛋白基因超家族的成员. 这一家族的结构特点为: 由半胱氨酸的残基形成二硫键, 而内折成为一个U形的三级结构域, 具有这一结构的还包括: 白蛋白、维生素D蛋白和α-球蛋白等(图1)[9].
使用GCG(Wisconsin GCG software)软件对AFP的分子结构进行分析, 结果显示其第三个结构域中包含有转录调节功能的氨基酸序列. 这一序列中, 有9个疏水氨基酸重复序列, 形成一个亮氨酸拉链样的结构, 是经典的与顺式作用元件结合的结构域[10]. 对AFP的几段氨基酸序列: 447-480、339-362、445-457、458-470、471-480分析发现, AFP可与多种核受体或核转录因子序列匹配(表1)[10,11], 有这样的结构基础为前提, 为AFP在细胞内发挥生物学功能, 提供了理论依据和研究方向.
| Protein | Amino-acidnumbers | Amino-acid sequence | PercentIdentity/Similarity |
| Growth factors | |||
| Human AFP | 447-480 | L S E D K L L A C G E G A A DI I I G H L C I R H E M T P V N P G V G | 100/100 |
| Human TGF-β | 106-117 | L S E D Q L L L I Q I P | 50/8 |
| Human ATV | 955-968 | L S E Q R L L P R G E G | 58/17 |
| Human IL2 | 1245-1256 | I X S I I V G H L G X F | 42/8 |
| Transcription-associated factors | |||
| Human AFP | 339-362 | Y S R R H P Q L A V S V I L R V A K G Y Q E L L | 100/100 |
| Human RAR | 235-254 | F A K R L P G F R X G X X L R I A D Q X I T L L | 35/40 |
| Human RXR | 346-365 | W A K R I P H F S X S X X L P L D D Q X V I L L | 35/35 |
| Human T3R | 286-305 | F A K K L P M F C X E X X L P C E D Q X I I L L | 20/45 |
| Human ER | 360-379 | F A K R V P G F V X D X X L R L H D Q X V H L L | 25/35 |
| Human AFP | 445-457 | L S E D K L L A C G E G | 100/100 |
| Human HGMP | 10-22 | L S E D K L L A V A R E | 66/8 |
| Human Zn-Fg | 725-736 | L S D H K L L E S T C K | 42/25 |
| Human Rev-erb | 523-535 | F S E X K L N A L T E E | 50/30 |
| Human RAR | 307-319 | F A N Q X L L X P L E M | 30/40 |
| Huamn Coup | 305-317 | V X E X K L K A L V D S | 30/30 |
| Human AFP | 458-470 | A A D I I I G H L C I R | 100/100 |
| Human HGMP | 12-21 | V A Q I I I G H L C I R | 83/7 |
| Human Zn-Fg | 330-341 | A G G T L V G H L C V R | 50/25 |
| Human C-myc | 217-226 | V Q S I I V G H L C W F | 50/17 |
| Human Rb-p | 2612-2623 | L Q S I I V G H L G C F | 42/25 |
| Human Coup | 489-502 | A A G V A P G H V L R I | 33/25 |
| Human AFP | 471-480 | E M T P V N P G V G | 100/100 |
| Human Crumbs | 1652-1661 | Q W T P V N P G V Q | 70/10 |
| Human PAX-3 | 512-521 | I M T P V N P G V P | 70/10 |
| Human HOXG2 | 633-642 | E M T P S T P G L Q | 60/30 |
| Human TF11D | 163-174 | P M T P A T P G S A | 50/20 |
| Human Src-TK | 1280-1289 | E M A P I W P G A L | 50/20 |
| Human Kid-TS | 1145-1154 | K S T G A N P G V P | 50/10 |
| Human Cad-TS | 3718-3727 | E M T P V L E A I I | 50/0 |
| Human FTZ-F1 | 628-637 | K P T P I S P G Y Q | 40/30 |
| Human I-Rel TF | 528-537 | E A S P S T P G R Q | 40/30 |
在HCC中, AFP基因重新表达, 癌细胞较之正常细胞增殖能力和抗凋亡能力都大为提高, AFP在其中发挥作用[12]. 在体外培养的人肝癌细胞中, 加入适当剂量的AFP时, 他可直接促进肝癌细胞的增殖[13].
临床研究[14]发现, 血清AFP值高的患者较之AFP低值或阴性的患者凋亡指数显著降低, 其预后、存活率及对药物的敏感性等方面也远远低于后者[13]. 在胚胎发育过程中, AFP可以有效抑制T淋巴细胞介导的细胞毒性, 使胎儿逃逸母体的免疫排斥[15]. 在肝癌细胞中, 分泌的AFP同样可以保护癌细胞免受免疫细胞的攻击, 如AFP可以抑制免疫细胞的增殖、诱导抑制性的T淋巴细胞、降低自然杀伤细胞的活性, 以及导致淋巴细胞和树突状细胞(dendritic cells, DCs)凋亡[16,17]. 将AFP作用于肝癌患者的单核细胞, 发现AFP不仅能抑制单核细胞转变为成熟的DCs, 并且能损伤DCs的功能、抑制DCs对T细胞的激活、抑制DCs合成肿瘤坏死因子(tumor necrosis factor, TNF)-α和白介素(interleukin, IL)-12并诱导DCs凋亡, 使TNF受体(tumor necrosis factor receptor, TNFR)的表达停止或者丢失. AFP的所有这些功能, 都明显地导致了肿瘤细胞逃避免疫监视, 细胞凋亡减少[18,19].
有研究[12,20]证实, 胞浆中的AFP可以选择性地和Caspase3结合形成复合物, 阻断来自Caspase8凋亡级联信号的传递, 进而阻断了Fas/FasL、TNF/TNFR等凋亡通路, 促进细胞增殖. 胞浆中AFP还可以与PTEN相互作用, 干扰磷脂酰肌醇3激酶(phosphatidylinositol 3 kinase, PI3K)/Akt信号通路. PTEN可以维护基因稳定性, 通过调控细胞增殖、迁移、侵袭和微血管生成等来发挥抑制肿瘤的功能. PTEN突变或缺失时, 会导致Akt的激活和肿瘤的发生, 而Akt非正常激活在癌症转移中发挥着关键作用[21]. 此外, 胞浆AFP可以与维甲酸受体(retinoic acid receptor, RAR)相互作用, 干扰RA-RAR信号通路, 导致RAR调控的下游基因的表达变化, 甚至影响到整个细胞中生物学行为的改变[22]. 已得到证实的RAR下游基因有GADD153、Fn14、GADD45α和Bcl-2[23-25]. 这些蛋白质在促进或抑制凋亡的过程中都发挥着重要的作用[26-28]. 这也可以部分地解释, 为什么临床上在使用全反式维甲酸(all-trans-retinoic acid, ATRA)治疗AFP阳性的肝癌患者时很容易出现耐药性. 胞浆中AFP可直接参与生物过程, 而分泌到胞外的AFP不可能直接进入细胞内调节细胞的生命活动, 因而预测有AFP受体(AFP receptor, AFPR)的存在, 并且可能是跨膜的G蛋白偶联受体(G-protein-coupled receptor, GPCR)[29,30], AFP可能通过AFP-GPCR-cAMP-PKA途径来调节肝癌细胞的生长. AFP还可以通过酪氨酸蛋白激酶TPK-Ras-MAPK途径促进肝癌细胞生长, 研究[31]发现AFP作用于Bel7402细胞可显著地提高胞内Ca2+浓度, 而Ca2+是重要的第二信使物质, 他能调节许多细胞内酶的活性, 可以通过AFP-Ca2+-PKC/CaM等途径来影响肝细胞代谢.
总之, AFP是肝癌细胞中促进细胞增殖, 血管生成和抑制细胞凋亡的关键分子. AFP疫苗已被证明可启动AFP相关免疫反应, 尽管针对单一抗原的疫苗的应用价值有待讨论, 但该疫苗对于肝硬化晚期及存在HCC风险的患者可能是一个很有价值的预防途径. 这也表明在HCC患者中AFP发挥着一种有效的调节肝癌进程的作用, 可能会成为未来用以治疗癌症的靶标.
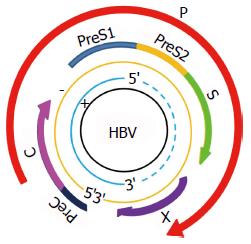
乙型肝炎病毒(hepatitis B virus)简称HBV, 是一种嗜肝DNA病毒, 分为外衣壳和核心两部分. 基因组全长约3.2 kb, 为不完全的双链环状DNA, 4个开放读码框(open reading frame, ORF)均位于负链, 分别为S区、C区、P区、X区(图2)[4]. S区编码乙型肝炎表面抗原(hepatitis B surface antigen, HBsAg), 可诱导机体产生特意保护性的抗-HBs[32]. C区编码e抗原(hepatitis B e antigen, HBeAg)和核心抗原(hepatitis B core antigen, HBcAg), HBcAg可诱导体液免疫和细胞免疫, 刺激机体产生抗-HBc, HBeAg传染性强, 是HBV复制以具有强感染性的指标. P区最长, 编码DNA聚合酶等. X基因编码x蛋白, HBx具有反式激活作用, 可激活HBV本身和细胞的多种调控基因, 促进HBV的复制[33,34].
慢性HBV感染是一个全球性的主要健康问题, 慢性乙型肝炎感染者发展为肝癌的终生风险是未感染者的10-25倍之多, 即便是隐匿的HBV携带者或清除HBsAg之后, 发展为肝癌的风险较之未感染人群依然是增高的[35]. 世界卫生组织肝癌预防会议指出: HBV与肝癌关系密切, 肝癌患者中HBsAg阳性率高达81.82%.
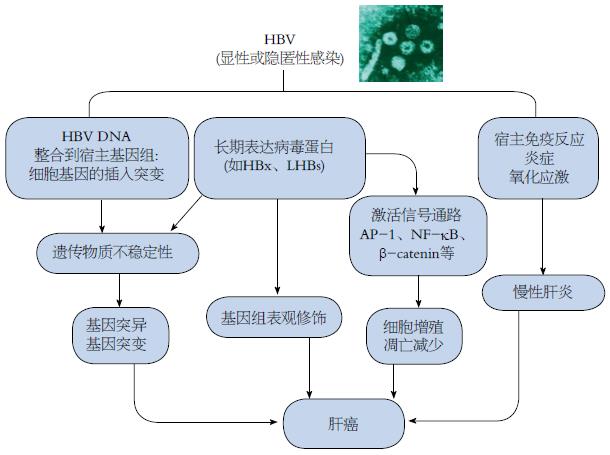
在肝癌发病机制的研究方面, 由于HBV病毒感染和HCC之间有着很长的潜伏期, 并且肝癌的发病率和年龄也密切相关, 人们一度错误地认为病毒只是起到一个间接作用. 但更多的证据证明, HBV可以通过改变宿主基因的表达和细胞表型, 促进长期肝损伤、慢性炎症、细胞死亡、再生和氧化应激性DNA损伤, 直接和间接地促进肝癌的发生和进程[36]. HBV相关肝癌的基因组整合率不但高达85%-90%, 且通常先于肝癌的发生[37], 整合事件一般好发生在人类基因组不稳定的位点及其周围, 癌症相关的区域, 或者调节细胞信号、增殖和活力的位点[38], 与整合相关的遗传不稳定性可能改变癌基因及肿瘤抑制基因和microRNA的表达. HBV的存在还可使信号通路失去正常的秩序和管制, 例如Wnt/β-catenin信号通路、转化生长因子β(transforming growth factor-β, TGF-β)信号通路、Ras/MAPK信号通路、PTEN/Akt和雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR)信号通路以及p14ARF/p53信号通路等[39,40], 出现异常激活或者抑制. 在HBV感染中, 病毒的直接"贡献"可归纳如图3所示[37,41].
HBx蛋白质是一种多功能的调节因子. 调节转录、信号转导、细胞周期进程、细胞凋亡、蛋白质降解通路以及通过整合到宿主基因组而影响遗传物质的稳定性[42]. 研究[43]证实, 即使不存在HBV复制, HCC患者体内HBx在mRNA和蛋白质水平都是升高的. 而且慢性乙型肝炎患者和HCC患者相比, 在后者体内HBx的抗体更容易被检测到. 转基因小鼠模型中, HBx的表达不受控制与肝癌的发生密切相关, 这些都暗示了在肝癌的发展过程中HBx的作用[44].
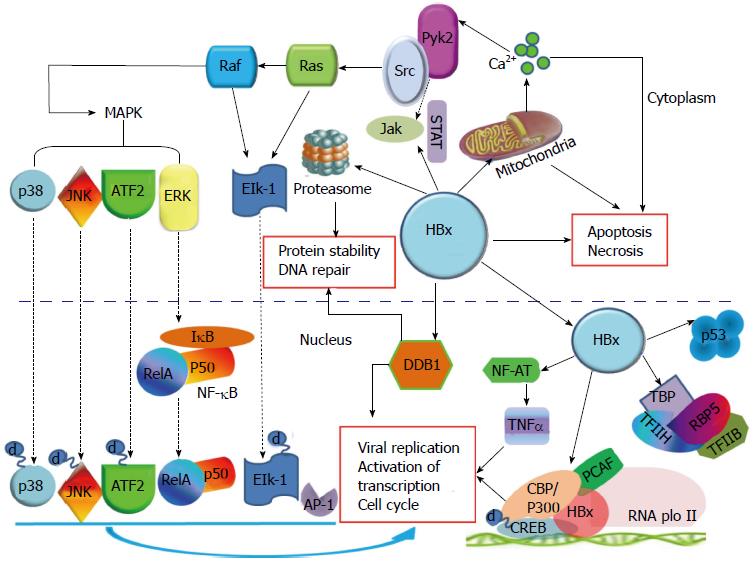
HBx可以参与转录调控, 目前的报道都认为HBx并不直接与DNA结合, 但他可通过与胞核中的众多转录因子结合, 如核因子κB(nuclear factor-κB, NF-κB)、激活蛋白-1(activator protein-1, AP-1)、激活转录因子(activating transcription factor, ATF)、cAMP反应元间结合转录因子(cAMP response element binding protein, CREB)、RNA聚合酶和TATA结合蛋白(TATA binding protein, TBP)等, 来间接干扰转录过程[45-48]. HBx还可以转录激活一些与细胞增殖相关的基因启动子, 如IL-8、TGF-β、EGRF等. 研究[42,49]还发现, 在早期癌变过程中, HBx可以转变肝细胞的TGF-β信号, 使之从肿瘤抑制途径pSmad3C转化为致癌途径pSmad3L. HBx可以调节c-Jun的转录, c-Jun和c-fos相互作用组成异源二聚体, 被认为在控制真核基因表达和细胞增殖上发挥核心作用[50]. 位于胞浆中的HBx可以激活信号转导通路, 如Ras-Raf-MAPK通路、Jak-STAT和Src蛋白激酶通路等. Ras-Raf-MAPK级联通路的激活又可以活化转录因子AP-1和NF-κB. HBx还可以抑制细胞周期蛋白依赖激酶2(cyclin dependent kinase 2, CDK2)的活性, 与Wnt-1互作激活Wnt/β-catenin通路, 最终导致细胞周期监测点的失控. 结合p53并使其失活, 与损伤特异性DNA结合蛋白-1(damage-specific DNA binding protein-1, DDB1)结合影响DNA的损伤修复, 使基因的改变得以积累[51]. 通过激活c-Myc, 使其在热休克蛋白90α(heat shock protein 90α, HSP90α)启动子区特定位点的结合增强, 促进HSP90α的表达, 在肿瘤细胞中HSP90α可作为AFP依赖的一个分子伴侣, 使癌蛋白保持活性构象[52]. HBx还可以影响基因的表观修饰, 上调甲基转移酶(DNA methyltransferase, DNMT)1、DNMT3a1和DNMT3a的表达来提高细胞内总体甲基转移酶活性, 使一些肿瘤抑制因子的启动子发生高甲基化, 如p16/INK4A等, 下调染色质修饰蛋白Suz12和Znf198, 使组蛋白3第27位赖氨酸发生3甲基化(H3K27Me3)而导致抑癌基因沉默, 而失去抑制肿瘤的功能[53-55]. 图4对HBx在胞内的作用机制进行了汇总描述.
在肝癌的发病和发展过程中AFP和HBV都发挥了各自的作用, 那么两个如此重要的因素之间是否存在某些联系, 这是一个值得探究的问题. 临床统计, 在HBsAg阳性肝癌中, AFP阳性率显著高于HBsAg阴性肝癌; 同理, 循环中AFP的mRNA为阳性的肝癌中, 87.5%为HBV感染者, 而在AFP的mRNA水平为阴性的肝癌中, HBV感染者的比例仅有33.3%[56], 这提示HBV感染与肝癌患者AFP升高有关, HBV可能通过与肝癌细胞的整合, 而使肝癌细胞合成AFP的能力增强, 也可能是HBx结合转录因子、干扰信号通路等途径促使AFP的表达增强. 临床队列研究[57], 对93例HBV肝硬化的患者使用恩替卡韦治疗, 并严密监控结果, 其中16(17.2%)例发展为肝癌, 这些患者的共同点是, 均有AFP不可控的持续性增高. 故而, 乙型肝炎肝硬化患者AFP水平升高是发展为HCC的一个极高危因素[57].
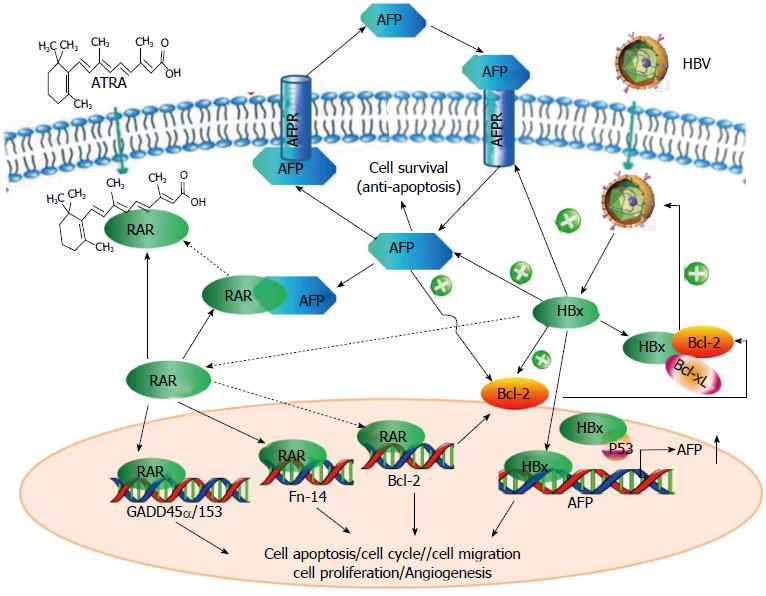
早在20世纪90年代, 就有研究[58]指出, AFP的表达受两个互斥的反式作用元件肝核因子(hepatocyte nuclear factor, HNF)-3和p53控制. HNF-3促进AFP表达, p53抑制AFP的表达, 当p300组蛋白乙酰转移酶使p53发生乙酰化后, 抑制作用增强. 在正常的成年人肝细胞中, p53的抑制作用占主导, 猜测当肝发生再生、病毒刺激或恶性转变时, 在某些因素的作用下, 可使p53的抑制作用削弱, 甚至完全解除. 2000年时预测就得到印证, 有研究[59]解释HBx和肿瘤抑制蛋白p53之间存在相互作用, HBx存在可使p53的抑制作用削弱而增强AFP的表达. 之后Arima等[60]又进一步分别在含有野生型、突变型和缺失p53基因的3个肝癌细胞系HepG2、HuH-7和Hep3B中使用HBx表达质粒和AFP报告基因共转染的方法证实了, HBx可以与p53相互作用, 进而解除p53对AFP基因启动子区活性的抑制, 使AFP表达上升. 除了和p53结合, HBx也能够激活AFP的5'上游序列, 使AFP的表达上升, 主要作用位点分别位于-5.0--3.0 kb、-2.9--1.0 kb以及C/EBP、AP-1、HNF-1等转录因子的结合位点[50], 其中中间的位点作用相对较弱. 我们实验室也分别将不同长度的AFP报告基因与HBx的表达质粒共转染, 结果显示, HBx作用于AFP基因的-1.8--1.0 kb和启动子区域, 使AFP基因的转录活性增强, 促进AFP表达. 在我们的实验中发现了AFP可以上调Bcl-2. 我们证实胞浆中的AFP能够和核转录因子RAR相互作用, 阻碍RAR入核与基因转录调控区结合, 而在Bcl-2的负调控区恰好存在RAR的结合位点[23], 并且Bcl-2基因的启动子可以被全反式维甲酸的拮抗剂所激活[61], 故而AFP可以上调Bcl-2的表达. 有学者[62]在对HBx稳转的HepG2细胞系进行蛋白组学分析发现, HBx可以也促进Bcl-2的表达. 这一点通过HBx可上调AFP的表达就可以得到解释, 同时还有报道[63]指出, HBx可以上调甲基转移酶DNMT1和DNMT3a, 而导致RAR-β2启动子区的过度甲基化, 致其表达下降. HBx的这一效应不但可以进一步解释, 他可使Bcl-2表达上升, 而且可以解释在HCC患者中, 因为缺少了RAR-β2这个关键执行者, 加之AFP又与ATRA竞争结合RAR, 故而ATRA不能发挥其抗肿瘤作用. HBx不但可以促进AFP的表达, 还可以促进AFPR的表达, AFPR的表达增加可促使对外泌AFP的吸收, 研究[64]认为AFPR可作为HBx蛋白驱动肝癌的早期指标及应用于追踪癌细胞转移. HBx还可以通过结构中Bcl-2 homology 3 (BH3)-like基序与Bcl-2和Bcl-xL相互作用, 升高细胞内的Ca2+浓度,促进HBV复制[65]. 如此, HBV-HBx-(AFPR)-AFP-Bcl-2-HBV在细胞内就如同形成一个正反馈的通路, 不断加速细胞的恶性进程. 近期研究[66]发现, HBx对AFP的表达影响早于很多原癌基因, 如Src、CXCR4和Ras, AFP与PTEN结合, 激活PI3K/Akt通路且促进mTOR介导的对转录因子缺氧诱导因子1α(hypoxia-inducible factor-1α, HIF-1α)的刺激, 因而导致Src、CXCR4和Ras等原癌基因激活, 这让AFP在HBV诱导肝癌的发生过程中的作用, 显得愈发重要.
针对已知HBV(主要是HBx)和AFP关系的研究, 以及我们实验室对其二者的研究成果, 总结绘图如图5.
2015年, 加拿大Partnership抗癌中心的Allemani博士等在LANCET杂志在线版发文, 通过对以人口为基础的登记数据进行中心分析得出全球癌症生存率监测数据. 所有国家肝癌和肺癌仍是致命性疾病, 这两种癌症的5年生存率欧洲均低于20%, 北美则为15%-19%, 蒙古和泰国低至7%-9%, 可见肝癌的形势非常严峻. 由上述可知, AFP在HBV诱导的肝癌发生发展过程中发挥了重要作用是毋庸置疑的. 如能加深对AFP作用机制的研究, 必将成为未来治疗肝癌的一个新靶点. 换言之, 如果能够直接降低HCC患者体内的AFP水平, 或对AFP基因加以沉默是否会有助于疾病的治疗呢?
研究[67-72]发现, 从中药淫羊藿中提取得到的一种单一有效成分, 命名为阿可拉定(Icaritin), 化学名为3,5,7-三羟基-2-(4-羟甲基苯基)-8-(3-甲基丁烯基-2)-1,2-苯并吡喃酮-4, 已被证实对多种肿瘤具有抑制生长和促进凋亡的作用. 他可通过抑制JAK/STAT3信号, 降低受STAT3调节的Bcl-xL、Mcl-1、Survivin和CyclinD1等蛋白质的水平; 导致细胞周期S期阻滞; 激活Fas、Caspase3、Caspase8、Caspase9、DNA修复酶(poly ADP-ribose polymerase, PARP), 降低Bcl-2、c-Myc、基质金属蛋白酶(matrix metalloproteinases, MMP)、CDK2等蛋白质水平; 以及改变Bcl-2/Bax比例等途径, 来抑制肾癌细胞、Burkitt淋巴瘤细胞、肝癌细胞、肺癌细胞、胶质母细胞瘤细胞、骨肉瘤细胞等的生长并促进凋亡. 这种药物经过一定的修饰、改造, 应用于临床试验(现已进入临床Ⅲ期)发现, 其能明显降低部分肝癌患者的AFP的水平, 在保守治疗下达到良好的预后效果, 而AFP水平不能降低的患者, 预后较差. 这一过程中, 阿可拉定是如何降低AFP, 以及为什么只有一部分患者对药物处理有反应, 其中的机制我们尚不清楚, 但我们有理由相信, 随着对AFP研究的不断深入, 对AFP生物学效应和作用机制的不断清晰明确, 一定能够为临床HCC的治疗提供更多更好的方法.
甲胎蛋白(alpha-fetoprotein, AFP)在临床被用作诊断肝癌肿瘤标志物, 但胞浆内的AFP的生物学功能却不甚明了, 在乙型肝炎病毒(hepatitis B virus, HBV)诱导的肝癌中, AFP的存在可使患者容易产生耐药性, 且预后更差、死亡率更高.
秦建民, 主任医师, 上海中医药大学附属普陀医院普外科
本文介绍HBV诱导肝癌发生的分子机制、AFP在肝癌细胞中重新表达的机制, 能够有效降低AFP可望成为临床治疗肝癌的新思路.
本文最大创新点就在于将HBV和AFP在肝癌发生发展中的关系及相互作用联系起来, 发掘了胞浆中AFP在HBV诱导肝癌中的作用以及临床应用前景.
实用价值即为临床肝癌治疗提供了一种新的思考途径, 在现有治疗的基础上, 尝试降低AFP的水平, 可以达到更佳的治疗效果.
本文为深入了解肝细胞癌发生机制和分子靶向治疗提供了重要的理论依据, 具有较高的学术价值.
编辑: 韦元涛 电编:闫晋利
| 1. | Jeng LB, Lee WC, Wang CC, Chen MF, Hsieh TT. Hepatocellular carcinoma in a pregnant woman detected by routine screening of maternal alpha-fetoprotein. Am J Obstet Gynecol. 1995;172:219-220. [PubMed] [DOI] |
| 2. | Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15 Suppl 4:5-13. [PubMed] [DOI] |
| 3. | Iavarone M, Colombo M. HBV-related HCC, clinical issues and therapy. Dig Liver Dis. 2011;43 Suppl 1:S32-S39. [PubMed] [DOI] |
| 4. | Neuveut C, Wei Y, Buendia MA. Mechanisms of HBV-related hepatocarcinogenesis. J Hepatol. 2010;52:594-604. [PubMed] [DOI] |
| 5. | Tangkijvanich P, Anukulkarnkusol N, Suwangool P, Lertmaharit S, Hanvivatvong O, Kullavanijaya P, Poovorawan Y. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol. 2000;31:302-308. [PubMed] [DOI] |
| 6. | Suriapranata IM, Sudania WM, Tjong WY, Suciptan AA, Gani RA, Hasan I, Sanityoso A, Budihusodo U, Miskad UA, Akil F. Alpha-fetoprotein gene polymorphisms and risk of HCC and cirrhosis. Clin Chim Acta. 2010;411:351-358. [PubMed] [DOI] |
| 7. | Mitsuhashi N, Kobayashi S, Doki T, Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, Nozawa S. Clinical significance of alpha-fetoprotein: involvement in proliferation, angiogenesis, and apoptosis of hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:e189-e197. [PubMed] [DOI] |
| 8. | Morinaga T, Sakai M, Wegmann TG, Tamaoki T. Primary structures of human alpha-fetoprotein and its mRNA. Proc Natl Acad Sci U S A. 1983;80:4604-4608. [PubMed] |
| 9. | Mizejewski GJ. Alpha-fetoprotein structure and function: relevance to isoforms, epitopes, and conformational variants. Exp Biol Med (Maywood). 2001;226:377-408. [PubMed] |
| 10. | Mizejewski GJ. An apparent dimerization motif in the third domain of alpha-fetoprotein: molecular mimicry of the steroid/thyroid nuclear receptor superfamily. Bioessays. 1993;15:427-432. [PubMed] |
| 11. | Dauphinée MJ, Mizejewski GJ. Human alpha-fetoprotein contains potential heterodimerization motifs capable of interaction with nuclear receptors and transcription/growth factors. Med Hypotheses. 2002;58:453-461. [PubMed] [DOI] |
| 12. | Li M, Zhou S, Liu X, Li P, McNutt MA, Li G. alpha-Fetoprotein shields hepatocellular carcinoma cells from apoptosis induced by tumor necrosis factor-related apoptosis-inducing ligand. Cancer Lett. 2007;249:227-234. [PubMed] [DOI] |
| 13. | Li P, Wang SS, Liu H, Li N, McNutt MA, Li G, Ding HG. Elevated serum alpha fetoprotein levels promote pathological progression of hepatocellular carcinoma. World J Gastroenterol. 2011;17:4563-4571. [PubMed] [DOI] |
| 14. | Wang XW, Xie H. Alpha-fetoprotein enhances the proliferation of human hepatoma cells in vitro. Life Sci. 1999;64:17-23. [PubMed] [DOI] |
| 15. | Semeniuk DJ, Boismenu R, Tam J, Weissenhofer W, Murgita RA. Evidence that immunosuppression is an intrinsic property of the alpha-fetoprotein molecule. Adv Exp Med Biol. 1995;383:255-269. [PubMed] [DOI] |
| 16. | Wang XW, Xu B. Stimulation of tumor-cell growth by alpha-fetoprotein. Int J Cancer. 1998;75:596-599. [PubMed] |
| 17. | Yamada A, Hayami M. Suppression of natural killer cell activity by chicken alpha-fetoprotein in Japanese quails. J Natl Cancer Inst. 1983;70:735-738. [PubMed] |
| 18. | Evdokimova VN, Liu Y, Potter DM, Butterfield LH. AFP-specific CD4+ helper T-cell responses in healthy donors and HCC patients. J Immunother. 2007;30:425-437. [PubMed] [DOI] |
| 19. | Li MS, Ma QL, Chen Q, Liu XH, Li PF, Du GG, Li G. Alpha-fetoprotein triggers hepatoma cells escaping from immune surveillance through altering the expression of Fas/FasL and tumor necrosis factor related apoptosis-inducing ligand and its receptor of lymphocytes and liver cancer cells. World J Gastroenterol. 2005;11:2564-2569. [PubMed] [DOI] |
| 20. | Li M, Li H, Li C, Zhou S, Guo L, Liu H, Jiang W, Liu X, Li P, McNutt MA. Alpha fetoprotein is a novel protein-binding partner for caspase-3 and blocks the apoptotic signaling pathway in human hepatoma cells. Int J Cancer. 2009;124:2845-2854. [PubMed] [DOI] |
| 21. | Li M, Li H, Li C, Wang S, Jiang W, Liu Z, Zhou S, Liu X, McNutt MA, Li G. Alpha-fetoprotein: a new member of intracellular signal molecules in regulation of the PI3K/AKT signaling in human hepatoma cell lines. Int J Cancer. 2011;128:524-532. [PubMed] [DOI] |
| 22. | Li M, Li H, Li C, Guo L, Liu H, Zhou S, Liu X, Chen Z, Shi S, Wei J. Cytoplasmic alpha-fetoprotein functions as a co-repressor in RA-RAR signaling to promote the growth of human hepatoma Bel 7402 cells. Cancer Lett. 2009;285:190-199. [PubMed] [DOI] |
| 23. | Young RL, Korsmeyer SJ. A negative regulatory element in the bcl-2 5'-untranslated region inhibits expression from an upstream promoter. Mol Cell Biol. 1993;13:3686-3697. [PubMed] |
| 24. | Wang S, Jiang W, Chen X, Zhang C, Li H, Hou W, Liu Z, McNutt MA, Lu F, Li G. Alpha-fetoprotein acts as a novel signal molecule and mediates transcription of Fn14 in human hepatocellular carcinoma. J Hepatol. 2012;57:322-329. [PubMed] [DOI] |
| 25. | Li C, Wang S, Jiang W, Li H, Liu Z, Zhang C, McNutt MA, Li G. Impact of intracellular alpha fetoprotein on retinoic acid receptors-mediated expression of GADD153 in human hepatoma cell lines. Int J Cancer. 2012;130:754-764. [PubMed] [DOI] |
| 26. | Bocaneti F, Altamura G, Corteggio A, Velescu E, Borzacchiello G. Expression of bcl-2 and p53 in bovine cutaneous fibropapillomas. Infect Agent Cancer. 2015;10:2. [PubMed] [DOI] |
| 27. | Hoffman B, Liebermann DA. Gadd45 in modulation of solid tumors and leukemia. Adv Exp Med Biol. 2013;793:21-33. [PubMed] [DOI] |
| 28. | Peck AB, Murgita RA, Wigzell H. Cellular and genetic restrictions in the immunoregulatory activity of alpha-fetoprotein. III. Role of the MLC-stimulating cell population in alpha-fetoprotein-induced suppression of T cell-mediated cytotoxicity. J Immunol. 1982;128:1134-1140. [PubMed] |
| 29. | Mizejewski GJ. Review of the adenocarcinoma cell surface receptor for human alpha-fetoprotein; proposed identification of a widespread mucin as the tumor cell receptor. Tumour Biol. 2013;34:1317-1336. [PubMed] [DOI] |
| 30. | Mizejewski GJ. The adenocarcinoma cell surface mucin receptor for alpha-fetoprotein: is the same receptor present on circulating monocytes and macrophages? A commentary. Tumour Biol. 2014;35:7397-7402. [PubMed] [DOI] |
| 31. | Li MS, Li PF, He SP, Du GG, Li G. The promoting molecular mechanism of alpha-fetoprotein on the growth of human hepatoma Bel7402 cell line. World J Gastroenterol. 2002;8:469-475. [PubMed] |
| 32. | Fang ZL, Sabin CA, Dong BQ, Wei SC, Chen QY, Fang KX, Yang JY, Huang J, Wang XY, Harrison TJ. Hepatitis B virus pre-S deletion mutations are a risk factor for hepatocellular carcinoma: a matched nested case-control study. J Gen Virol. 2008;89:2882-2890. [PubMed] [DOI] |
| 33. | Parvez MK. HBV and HIV co-infection: Impact on liver pathobiology and therapeutic approaches. World J Hepatol. 2015;7:121-126. [PubMed] [DOI] |
| 34. | Olawumi HO, Olanrewaju DO, Shittu AO, Durotoye IA, Akande AA, Nyamngee A. Effect of hepatitis-B virus co-infection on CD4 cell count and liver function of HIV infected patients. Ghana Med J. 2014;48:96-100. [PubMed] [DOI] |
| 35. | Sherman M. Risk of hepatocellular carcinoma in hepatitis B and prevention through treatment. Cleve Clin J Med. 2009;76 Suppl 3:S6-S9. [PubMed] [DOI] |
| 36. | Guerrieri F, Belloni L, Pediconi N, Levrero M. Molecular mechanisms of HBV-associated hepatocarcinogenesis. Semin Liver Dis. 2013;33:147-156. [PubMed] [DOI] |
| 37. | Bouchard MJ, Navas-Martin S. Hepatitis B and C virus hepatocarcinogenesis: lessons learned and future challenges. Cancer Lett. 2011;305:123-143. [PubMed] [DOI] |
| 38. | Hai H, Tamori A, Kawada N. Role of hepatitis B virus DNA integration in human hepatocarcinogenesis. World J Gastroenterol. 2014;20:6236-6243. [PubMed] [DOI] |
| 39. | Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, Tovar V, Roayaie S, Minguez B, Sole M. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972-1983, 1983.e1-11. [PubMed] [DOI] |
| 40. | de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1998;95:8847-8851. [PubMed] [DOI] |
| 41. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [PubMed] [DOI] |
| 42. | Ng SA, Lee C. Hepatitis B virus X gene and hepatocarcinogenesis. J Gastroenterol. 2011;46:974-990. [PubMed] [DOI] |
| 43. | Su Q, Schröder CH, Hofmann WJ, Otto G, Pichlmayr R, Bannasch P. Expression of hepatitis B virus X protein in HBV-infected human livers and hepatocellular carcinomas. Hepatology. 1998;27:1109-1120. [PubMed] [DOI] |
| 44. | Zhang H, Wu LY, Zhang S, Qiu LY, Li N, Zhang X, Zhang XZ, Shan CL, Ye LH, Zhang XD. Anti-hepatitis B virus X protein in sera is one of the markers of development of liver cirrhosis and liver cancer mediated by HBV. J Biomed Biotechnol. 2009;2009:289068. [PubMed] [DOI] |
| 45. | Kim HR, Lee SH, Jung G. The hepatitis B viral X protein activates NF-kappaB signaling pathway through the up-regulation of TBK1. FEBS Lett. 2010;584:525-530. [PubMed] [DOI] |
| 46. | Qadri I, Fatima K, AbdeL-Hafiz H. Hepatitis B virus X protein impedes the DNA repair via its association with transcription factor, TFIIH. BMC Microbiol. 2011;11:48. [PubMed] [DOI] |
| 47. | Tanaka Y, Kanai F, Ichimura T, Tateishi K, Asaoka Y, Guleng B, Jazag A, Ohta M, Imamura J, Ikenoue T. The hepatitis B virus X protein enhances AP-1 activation through interaction with Jab1. Oncogene. 2006;25:633-642. [PubMed] [DOI] |
| 48. | Tang H, Delgermaa L, Huang F, Oishi N, Liu L, He F, Zhao L, Murakami S. The transcriptional transactivation function of HBx protein is important for its augmentation role in hepatitis B virus replication. J Virol. 2005;79:5548-5556. [PubMed] [DOI] |
| 49. | Murata M, Matsuzaki K, Yoshida K, Sekimoto G, Tahashi Y, Mori S, Uemura Y, Sakaida N, Fujisawa J, Seki T. Hepatitis B virus X protein shifts human hepatic transforming growth factor (TGF)-beta signaling from tumor suppression to oncogenesis in early chronic hepatitis B. Hepatology. 2009;49:1203-1217. [PubMed] [DOI] |
| 50. | Zhou MX, Watabe M, Watabe K. The X-gene of human hepatitis B virus transactivates the c-jun and alpha-fetoprotein genes. Arch Virol. 1994;134:369-378. [PubMed] [DOI] |
| 51. | Zhang X, Zhang H, Ye L. Effects of hepatitis B virus X protein on the development of liver cancer. J Lab Clin Med. 2006;147:58-66. [PubMed] [DOI] |
| 52. | Li W, Miao X, Qi Z, Zeng W, Liang J, Liang Z. Hepatitis B virus X protein upregulates HSP90alpha expression via activation of c-Myc in human hepatocarcinoma cell line, HepG2. Virol J. 2010;7:45. [PubMed] [DOI] |
| 53. | Park IY, Sohn BH, Yu E, Suh DJ, Chung YH, Lee JH, Surzycki SJ, Lee YI. Aberrant epigenetic modifications in hepatocarcinogenesis induced by hepatitis B virus X protein. Gastroenterology. 2007;132:1476-1494. [PubMed] [DOI] |
| 54. | Zheng DL, Zhang L, Cheng N, Xu X, Deng Q, Teng XM, Wang KS, Zhang X, Huang J, Han ZG. Epigenetic modification induced by hepatitis B virus X protein via interaction with de novo DNA methyltransferase DNMT3A. J Hepatol. 2009;50:377-387. [PubMed] [DOI] |
| 55. | Wang WH, Studach LL, Andrisani OM. Proteins ZNF198 and SUZ12 are down-regulated in hepatitis B virus (HBV) X protein-mediated hepatocyte transformation and in HBV replication. Hepatology. 2011;53:1137-1147. [PubMed] [DOI] |
| 56. | Montaser LM, Abbas OM, Saltah AM, Waked IA. Circulating AFP mRNA as a Possible Indicator of Hematogenous Spread of HCC Cells: A Possible Association with HBV Infection. J Egypt Natl Canc Inst. 2007;19:48-60. [PubMed] |
| 57. | Luo K, Liu Z, Karayiannis P. Effect of antiviral treatment on alfa-fetoprotein levels in HBV-related cirrhotic patients: early detection of hepatocellular carcinoma. J Viral Hepat. 2010;17:511-517. [PubMed] [DOI] |
| 58. | Lee KC, Crowe AJ, Barton MC. p53-mediated repression of alpha-fetoprotein gene expression by specific DNA binding. Mol Cell Biol. 1999;19:1279-1288. [PubMed] |
| 59. | Ogden SK, Lee KC, Barton MC. Hepatitis B viral transactivator HBx alleviates p53-mediated repression of alpha-fetoprotein gene expression. J Biol Chem. 2000;275:27806-27814. [PubMed] [DOI] |
| 60. | Arima T, Nakao K, Nakata K, Ishikawa H, Ichikawa T, Hamasaki K, Ishii N, Eguchi K. Transactivation of human alpha-fetoprotein gene by X-gene product of hepatitis B virus in human hepatoma cells. Int J Mol Med. 2002;9:397-400. [PubMed] [DOI] |
| 61. | Rasooly R, Schuster GU, Gregg JP, Xiao JH, Chandraratna RA, Stephensen CB. Retinoid x receptor agonists increase bcl2a1 expression and decrease apoptosis of naive T lymphocytes. J Immunol. 2005;175:7916-7929. [PubMed] [DOI] |
| 62. | Niu D, Feng H, Chen WN. Proteomic analysis of HBV-associated HCC: insights on mechanisms of disease onset and biomarker discovery. J Proteomics. 2010;73:1283-1290. [PubMed] [DOI] |
| 63. | Jung JK, Park SH, Jang KL. Hepatitis B virus X protein overcomes the growth-inhibitory potential of retinoic acid by downregulating retinoic acid receptor-beta2 expression via DNA methylation. J Gen Virol. 2010;91:493-500. [PubMed] [DOI] |
| 64. | Li M, Zhu M, Li W, Lu Y, Xie X, Wu Y, Zheng S. Alpha-fetoprotein receptor as an early indicator of HBx-driven hepatocarcinogenesis and its applications in tracing cancer cell metastasis. Cancer Lett. 2013;330:170-180. [PubMed] [DOI] |
| 65. | Geng X, Huang C, Qin Y, McCombs JE, Yuan Q, Harry BL, Palmer AE, Xia NS, Xue D. Hepatitis B virus X protein targets Bcl-2 proteins to increase intracellular calcium, required for virus replication and cell death induction. Proc Natl Acad Sci U S A. 2012;109:18471-18476. [PubMed] [DOI] |
| 66. | Zhu M, Guo J, Li W, Lu Y, Fu S, Xie X, Xia H, Dong X, Chen Y, Quan M. Hepatitis B virus X protein induces expression of alpha-fetoprotein and activates PI3K/mTOR signaling pathway in liver cells. Oncotarget. 2015; Jan 21. [Epub ahead of print]. [PubMed] |
| 67. | Li S, Priceman SJ, Xin H, Zhang W, Deng J, Liu Y, Huang J, Zhu W, Chen M, Hu W. Icaritin inhibits JAK/STAT3 signaling and growth of renal cell carcinoma. PLoS One. 2013;8:e81657. [PubMed] [DOI] |
| 68. | Li ZJ, Yao C, Liu SF, Chen L, Xi YM, Zhang W, Zhang GS. Cytotoxic effect of icaritin and its mechanisms in inducing apoptosis in human burkitt lymphoma cell line. Biomed Res Int. 2014;2014:391512. [PubMed] [DOI] |
| 69. | Wang XF, Wang J. Icaritin suppresses the proliferation of human osteosarcoma cells in vitro by increasing apoptosis and decreasing MMP expression. Acta Pharmacol Sin. 2014;35:531-539. [PubMed] [DOI] |
| 70. | Zheng Q, Liu WW, Li B, Chen HJ, Zhu WS, Yang GX, Chen MJ, He GY. Anticancer effect of icaritin on human lung cancer cells through inducing S phase cell cycle arrest and apoptosis. J Huazhong Univ Sci Technolog Med Sci. 2014;34:497-503. [PubMed] [DOI] |
| 71. | Han H, Xu B, Hou P, Jiang C, Liu L, Tang M, Yang X, Zhang Y, Liu Y. Icaritin Sensitizes Human Glioblastoma Cells to TRAIL-Induced Apoptosis. Cell Biochem Biophys. 2015; Jan 11. [Epub ahead of print]. [PubMed] [DOI] |
| 72. | Sun L, Peng Q, Qu L, Gong L, Si J. Anticancer agent icaritin induces apoptosis through caspase-dependent pathways in human hepatocellular carcinoma cells. Mol Med Rep. 2015;11:3094-3100. [PubMed] [DOI] |