修回日期: 2015-04-03
接受日期: 2015-04-10
在线出版日期: 2015-05-28
非酒精性脂肪性肝病(non-alcoholic fatty liver disease, NAFLD)是西方发达国家常见的肝脏疾病, 其患病率在发展中国家也持续增高. NAFLD是一类肝脏疾病的总称, 其中单纯性脂肪肝属良性病变, 但非酒精性脂肪性肝炎(non-alcoholic steatohepatitis, NASH)可进一步发展为肝纤维化、肝硬化, 最终导致肝癌, 但NAFLD的发病机制并不完全清楚. 微小RNA(microRNA, miRNA)作为一类在转录后水平调控机体生长发育、细胞代谢、增殖、分化、凋亡及肿瘤形成等生理、病理过程的非编码RNA, 也参与了NAFLD的发病. miRNA种类繁多, 广泛参与NAFLD中胰岛素抵抗、脂代谢紊乱、内质网损伤和细胞凋亡等过程. 本文主要就miRNA在NAFLD细胞凋亡中的作用作一综述.
核心提示: 细胞凋亡是包括非酒精性脂肪性肝病(non-alcoholic fatty liver disease, NAFLD)在内的多种肝脏疾病发生、发展的重要环节, 并与其严重程度相关. NAFLD常和肥胖、胰岛素抵抗、2型糖尿病、脂代谢紊乱、高血压、炎症等合并存在. 微小RNA(microRNA)不但参与NAFLD脂质代谢、胰岛素抵抗等环节, 也通过调节受p53基因上调表达的凋亡调控蛋白(p53-upregulated modulator of apoptosis)、C/EBP同源蛋白(C/EBP homologous protein)、B细胞淋巴瘤基因(B cell lymphoma)家族、p27 Kip1、丝裂原激活/细胞外信号调节激酶(mitogen-activated/extracellular signal-regulated kinase)/细胞外调节蛋白激酶(extracellar regulated protein kinase)通路、程序性细胞死亡因子4(programmed cell death 4)、第10号染色体同源丢失磷酸酶张力蛋白(phosphatase and tensin homolog detected on chromosome ten)、回复引导半胱氨酸丰富蛋白Kazal基元(reversion inducing cysteine rich protein with Kazal motifs)等过程参与NAFLD肝细胞及脂肪细胞凋亡的调控.
引文著录: 聂娇, 李昌平. 非酒精性脂肪性肝病中microRNA调控细胞凋亡的机制. 世界华人消化杂志 2015; 23(15): 2389-2396
Revised: April 3, 2015
Accepted: April 10, 2015
Published online: May 28, 2015
Non-alcoholic fatty liver disease (NAFLD) has become a common liver disease in Western developed countries, and the prevalence is also continuously increasing in developing countries. NAFLD comprises a spectrum of disease stages, in which simple steatosis is a benign course and steatohepatitis can progress to liver fibrosis, cirrhosis and even hepatocellular carcinoma. The pathogenesis of NAFLD has not been fully understood. As noncoding RNA molecules, microRNAs (miRNAs) regulate the pathophysiological processes including development, metabolism, cell proliferation, differentiation, apoptosis and carcinogenesis, as well as the pathogenesis of NAFLD. miRNAs extensively participate in insulin resistance, lipid metabolic disorder, endoplasmic injury and cell apoptosis in NAFLD. This review highlights the roles of miRNAs in cell apoptosis in NAFLD.
- Citation: Nie J, Li CP. Mechanisms of microRNAs in regulation of apoptosis in non-alcoholic fatty liver disease. Shijie Huaren Xiaohua Zazhi 2015; 23(15): 2389-2396
- URL: https://www.wjgnet.com/1009-3079/full/v23/i15/2389.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v23.i15.2389
非酒精性脂肪性肝病(non-alcoholic fatty liver disease, NAFLD)是指肝细胞脂肪沉积超过肝脏重量的5%, 除外过量酒精摄入(折合乙醇量, 男性>40 g/d, 女性>20 g/d)、病毒感染及其他明确病因所导致的临床病理综合症[1]. NAFLD是一类疾病的总称, 包括非酒精性单纯性脂肪肝、非酒精性脂肪性肝炎(non-alcoholic steatohepatitis, NASH)及肝硬化, 后者可进一步发展为肝细胞癌(hepatocellular carcinoma, HCC)及其他终末期的肝脏疾病[2]. 有研究[3,4]报道, 西方国家, 尤其是希腊等地中海国家, 成人NAFLD的患病率达20%-30%, 在诸如中国、印度等亚洲国家, 其患病率约为15%-20%[5,6]. 在欧洲的2型糖尿病人群中, 其患病率高达42.6%-69.5%[7,8]. NAFLD常常和肥胖、胰岛素抵抗、2型糖尿病、脂代谢紊乱、高血压、炎症等合并存在[9,10], 给人类健康、经济等带来了严重的负担, 但其发病机制并不完全清楚. 近年研究表明细胞凋亡在NAFLD发病及进展中扮演了重要角色. 微小RNA(microRNA, miRNA)作为一类在转录后水平调控机体生长发育、细胞代谢、增殖、分化、肿瘤形成等生理、病理过程的非编码RNA, 不但参与NAFLD中脂质代谢、胰岛素抵抗等环节[11,12], 也参与了NAFLD中细胞凋亡的调控.
1993年, Lee等[13]在秀丽隐杆线虫中首次发现一类含有19-24个核苷酸的非编码单链RNA, 即miRNA. 他通过与之互补的mRNA配对, 在转录后水平调控机体各项病理生理过程, 如细胞增殖、代谢、分化、凋亡和肿瘤发生等[14]. 随着研究[15]的进一步深入, 现已识别出约2000个人类miRNA序列, 他们可能调节三分之二的人类基因组. miRNA最早在细胞核中, 由编码基因编码, 经RNA聚合酶Ⅱ转录成前体分子pri-miRNA[16]. 然后, pri-miRNA经过细胞核中核糖核酸酶Ⅲ(ribonuclease Ⅲ, RNaseⅢ)家族成员, 即Drosha酶(nuclear Drosha)、Dicer酶(nuclear Dicer)的作用, 最终形成双链miRNA. 双链miRNA在解螺旋酶的作用下, 其中一条与AGO蛋白(argonauts)结合而形成miRNA-诱导沉默复合体(miRNA-induced silencing complex, miRISC); 另一条被称为随从链的miRNA则被释放或降解. miRNA的5'端有一区域含有2-8个核苷酸, 被称为"种子区域", 这个区域与其靶mRNA的3'端非翻译区(3' untranslated region, 3'UTR)多以不完全互补配对的方式结合, 从而抑制蛋白质的翻译或引起靶mRNA降解[17].
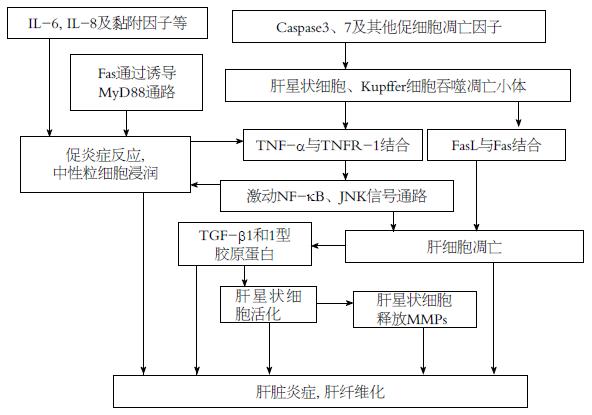
肝细胞凋亡是包括NAFLD在内的多种肝脏疾病发生、发展的重要环节, 并与其严重程度相关[18]. 肝星状细胞也在肝纤维化的过程中发挥了重要作用(图1), 是肝脏炎症及肝纤维化发生的重要环节. 肝星状细胞和Kupffer细胞可吞噬肝脏中的凋亡小体(apoptotic bodies), 促进Fas配体(Fas ligand, FasL)、肿瘤坏死因子α(tumor necrosis factor α, TNF-α)等死亡配体的产生, 促进肝细胞凋亡. 肝细胞凋亡可募集中性粒细胞至肝实质, TNF受体1(TNF receptor 1, TNFR-1)与TNF-α结合后, 可激动下游核因子κB(nuclear factor κB, NF-κB)及c-Jun氨基末端激酶(c-Jun N-terminal kinase, JNK)激酶信号传导通路, 与白介素(interleukin, IL)-6、IL-8及黏附分子等形成炎症瀑布反应, 活化中性粒细胞引起肝脏炎症. 另外, Fas可通过MyD88通路诱导巨噬细胞表达促炎症反应趋化因子, 促进中性粒细胞浸润及炎症反应[19]. 同时, 肝细胞凋亡产生转化生长因子β1(transforming growth factor β1, TGF-β1)和1型胶原蛋白(type 1 collagen)等促进纤维蛋白产生的因子(profibrogenic factors), 并释放与巨噬细胞和肝星状细胞上嘌呤受体相结合的核酸, 以进一步激活上述细胞致肝脏纤维化的作用[20]. 另外, 活化的肝星状细胞还分泌基质金属蛋白酶(matrix metalloproteinase, MMPs). 研究[21]表明, 在小鼠造模的肝脏疾病中, 抑制MMP可减少肝损伤、肝细胞凋亡和肝纤维化. NASH患者Caspase3、7和其他细胞凋亡的因子增加, 促进Kupffer细胞和肝星状细胞的肝细胞凋亡作用, 加重肝脏炎症和肝纤维化[22].
NAFLD常和胰岛素抵抗、2型糖尿病、肥胖、脂代谢紊乱、高血压、炎症等合并存在. 胰岛素抵抗是NAFLD的基本特征. 研究[23]表明, 胰岛素抵抗能降低NAFLD患者肝脏、骨骼肌和脂肪组织的胰岛素敏感性. 最终导致肌肉和肝脏摄取血浆中升高的甘油三酯和游离脂肪酸, 异位沉积在心肌细胞和肝脏中[24]. Alkhouri等[25]研究表明, 脂肪细胞凋亡是脂肪组织巨噬细胞浸润、胰岛素抵抗的初始事件, 脂肪组织巨噬细胞浸润在胰岛素抵抗的发展中也起到至关重要的作用. Wueest等[26]研究表明, 脂肪特异性的Fas敲除小鼠(选择地敲除脂肪组织中的Fas), 可免受高脂饮食诱导的胰岛素抵抗和肝脏脂肪变性. 此外, 无论患糖尿病与否, 肥胖患者脂肪组织中Fas和FasL上调, 表明Fas在肥胖诱导的胰岛素抵抗中起作用.
目前已有多项研究表明, NAFLD中存在miRNA的差异表达(表1). Cheung等[27]研究发现, 与控制组相比, NASH患者的肝组织中, 46种miRNA表达增加或降低. Li等[28]研究发现, 与正常对照组相比, 患有NAFLD的ob/ob小鼠, 有11种miRNA数量发生变化, 其中miR-34a、miR-31等8种升高, miR-29c、miR-451和miR-21降低. Dolganiuc等[29]发现NAFLD的小鼠模型中, miR-705、miR-1224等5种miRNA表达增加. Pogribny等[30]发现在NAFLD的小鼠模型中, miR-34a、miR-155等4种miRNA表达增加, miR-29c、miR-122等4种miRNA表达降低, 且miRNA表达的变化决定了NASH的严重程度和易感性. Wang等[31]发现, 缺乏胆碱及氨基酸饮食喂养的C57/BL6小鼠中, miR-181b、miR-181d两种miRNA升高, 且其升高可能与HCC的发病相关. Alisi等[32]发现, 与正常对照组相比, 患有NAFLD的大鼠模型中, 3种miRNA升高(miR-200a、miR-200b、miR-429); 3种miRNA降低(miR-122、miR-451、miR-27). Feng等[33]研究表明, NAFLD大鼠中, 44种miRNA表达升高, 12种miRNA表达降低. Celikbilek等[34]发现, 经活检证实的NAFLD患者, 与正常人相比, 其血清中miR-181d、miR-99a、miR-197和miR-146b的水平降低. 以上研究均表明miRNA参与了NAFLD的发病, 但并未阐明其影响NAFLD发病的具体机制.
| 模型 | 上调的miRNAs | 下调的miRNAs | 参考文献 |
| NASH患者 | miR-126、-28、-26b、-30d、 -122、-361、-574、-92b、 -768-5p、-375、-203、-223、-145、 -671、-139、-191*、-563、-188、 -601、-765、-198、-641、-617 | miR-125b、-23a、-23b、 -16、-100、-27b、-24、 -181b、-99b、-214、 -127、-128a、-21、 -128b、-455、-199a*、 -221、-199a、-222、 -146b、-200a、-34a、-224 | Cheung等[27], 2008 |
| ob/ob小鼠 | miR-34a、-31、-103、-107、-194、 -221、-335-5p、-200a | miR-29c、-451、-21 | Li等[28], 2009 |
| 小鼠造模 | miR-705、-1224、-182、-183、 -199a-3p | Dolganiuc等[29], 2009 | |
| 小鼠造模 | miR-34a、-155、-200b、-221 | miR-29c、-122、-192、-203 | Pogribny等[30], 2010 |
| 小鼠造模 | miR-181b、-181d | Wang等[31], 2010 | |
| 大鼠造模 | miR-200a、-200b、-429 | miR-122、-451、-27 | Alisi等[32], 2011 |
| 大鼠造模 | miR-880、-881、-741-3p、-200b*、 -200b、-200a、-141、-429、 -455、-200c、-146a、-499、-150、 -29b-1*、-221*、-9、-152、-30c、 -146b、-322*、-503、-128、-342-3p、 -181c、-30c-2*、-30b-5p、-142-5p、 -103、-199a-5p、-148b-3p、-320、 -181a-1*、-339-5p、-30d、-503*、 -185、-let-7f、-338、-34c、 -142-3p、-221、-186、-374、-210 | miR-671、-184、-451、 -501*、-183、-33、-182、 -206、-144、-143*、-1、 -96 | Feng等[33], 2014 |
| NAFLD患者 | miR-181d、-99a、-197、 -146b | Celikbilek等[34], 2014 |
2.2.1 miR-296-5p通过调节受p53基因上调表达的凋亡调控蛋白(p53-upregulated modulator of apoptosis, PUMA)参与NAFLD的脂肪细胞凋亡过程: Jabbour等[35]与Cazanave等[36]发现, PUMA的过表达可以促进细胞死亡, 而抑制PUMA蛋白表达的Huh-7细胞或先天缺乏puma基因的小鼠的初级肝细胞可以部分免除脂毒性的创伤. 研究[37]表明NAFLD患者的内脏脂肪组织中, miR-296-5p的表达降低、PUMA mRNA和蛋白的水平升高[36], 这说明miR-296-5p可能负向调控PUMA表达. 经过Cazanave等[38]的进一步研究发现: miR-296-5p表达增加可抑制PUMA表达, 减少Huh细胞因棕榈酸酯作用而发生的细胞凋亡; 反之, miR-296-5p表达减少可使PUMA表达增加, 进而使细胞凋亡增加, 其机制在于miR-296-5p"种子区域"可分别与PUMA 3'UTR的1299/1306(BS1)、1465/1471(BS2)位点结合, 进而降解PUMA转录物或阻断PUMA蛋白翻译以调节PUMA的表达[38]. 而PUMA蛋白的升高可直接促进线粒体功能障碍, 导致Caspase3/7的激活, 最终导致细胞死亡[39]. 另外, miR-221、miR-222和miR-483-3p也可以负向调控PUMA表达[40,41].
2.2.2 miR-34a通过miR-34a/SIRT/p53途径参与肝细胞凋亡: 与脂肪样变相比, 轻中度NASH患者的miR-34a表达增加了约2倍, 重度NASH患者miR-34a增加了3倍以上[42], 进一步的研究表明NAFLD患者肝脏miR-34a的表达与SIRT1(sirtuin 1)、乙酰化的P53蛋白水平, 以及肝细胞凋亡有关. Castro等[42]发现: miR-34a/SIRT1/p53途径参与调控NAFLD的肝细胞凋亡, 即miR-34a通过下调靶点SIRT1, 使p53乙酰化、转录及促凋亡基因puma增加, 从而参与肝细胞凋亡的调控过程, 而熊去氧胆酸(ursodeoxycholic acid, UDCA)可作用于该通路调控细胞凋亡. 在原代大鼠肝细胞中, p53依赖的细胞凋亡, 部分通过miR-34a/SIRT1/p53途径发挥作用, 而p53可正向调节miR-34a表达[43].
2.2.3 Mmu-miR-615-3p通过抑制C/EBP同源蛋白(C/EBP homologous protein, CHOP)调控脂肪细胞凋亡: Miyamoto等[44]使用棕榈酸酯诱导内质网应激, 发现受试细胞系中有5种miRNA(Mmu-miR-92b-3p、Mmu-miR-574-5p、Mmu-miR-615-3p、Mmu-miR-484、Mmu-miR-382-3p)表达降低. 通过观察使用miR-615-3p类似物及拮抗剂后的CHOP表达情况, 猜测CHOP表达可能受miR-615-3p的负性调控. 而后该实验也证实, 小鼠及人类CHOP 3'UTR均有一个潜在的miR-615-3p结合位点, Mmu-miR-615-3p可与该位点结合负向调控CHOP表达. 所以NAFLD患者miR-615-3p表达降低, 引起CHOP表达增加, 最终导致脂肪细胞凋亡增加.
2.2.4 其他可能的调控机制: NASH进一步发展可导致肝纤维化或HCC. 研究表明, NAFLD和HCC中均存在miRNA-122表达的明显下降, Lin等[45]发现: miRNA-122的过表达可抑制B细胞淋巴瘤/白血病-w(B-cell lymphoma/Leukemia-w, Bcl-w)、无翅有关的鼠乳腺肿瘤病毒整合位点1(wing-less-related MMTV integration site 1, wnt-1) mRNA及其蛋白的表达, 导致肝细胞凋亡增加; 反之, 在HCC肝癌细胞中, miRNA-122表达降低可使Bcl-w、wnt-1 mRNA及其蛋白的表达增加, 从而减少肝细胞凋亡的发生, 这可能是NAFLD向HCC发展的机制. Ma等[46]用腺病毒载体介导miRNA-122的表达, 发现miRNA-122表达可诱导肝癌细胞株发生细胞凋亡、使细胞周期停滞. 这些研究均表明miRNA-122通过负向调控Bcl-w、wnt-1的表达参与NAFLD的致病过程. Gramantieri等[47]发现HCC细胞的miRNA-221表达增加, 其通过抑制Bcl-2蛋白修饰因子(Bcl-2 modifying factor, Bmf)表达, 减少肝细胞凋亡发生. 也有miR221/222促进细胞凋亡的报道, 如Dai等[48]发现: 在HCC发生内质网应激的条件下, miR-221/222表达降低, 通过调节视网膜母细胞瘤蛋白(retinoblastoma protein, p27 Kip1)和丝裂原激活/细胞外信号调节激酶(mitogen-activated/extracellular signal-regulated kinase, MEK)/细胞外调节蛋白激酶(extracellar regulated protein kinase, ERK)通路使细胞周期G1停滞, 从而抑制肝细胞凋亡. 另外, Li等[49]研究发现, HCC细胞中, miRNA-183表达增加, miRNA-183通过抑制程序性细胞死亡因子4(programmed cell death 4, PDCD4)调控肝细胞凋亡. NAFLD发展到肝纤维化或HCC时miR-21的表达增加, Liu等[50]研究发现: 在HCC中, miR-21通过调控第10号染色体同源丢失磷酸酶张力蛋白(phosphatase and tensin homolog detected on chromosome ten, PTEN)、PDCD4及回复引导半胱氨酸丰富蛋白Kazal基元(reversion inducing cysteine rich protein with Kazal motifs, RECK)抑制肝细胞凋亡, 参与HCC的发病. 虽然这些调控机制不是在NAFLD模型中阐明, 但鉴于NAFLD和HCC存在共同表达的差异miRNA(表2), 提示这些miRNA可能在NAFLD的发病及NAFLD向HCC发展的过程中起作用, 其作用的靶位点也有可能是一致的.
miRNA种类复杂, 广泛参与NAFLD中胰岛素抵抗、内质网损伤和细胞凋亡等过程, 是近年来的研究热点. 但目前关于研究miRNA在NAFLD发病机制中的作用主要集中在miRNA调控代谢综合症这方面, 而关于miRNA与细胞凋亡关系的研究又主要集中在肿瘤方面, 所以在NAFLD的背景下研究miRNA与细胞凋亡的关系是非常必要的. 虽然有研究表明, miRNA参与NAFLD中肝细胞及脂肪细胞凋亡的调控, 但其具体机制还有待进一步的研究和阐明. 随着NAFLD中miRNA调控细胞凋亡研究的深入, 用分子学的方式干预NAFLD的发展, 将成为NAFLD的诊断、治疗的新途径.
随着生活水平的提高及饮食结构的改变, 非酒精性脂肪性肝病(non-alcoholic fatty liver disease, NAFLD)的发病率逐年增加, 给人类健康及经济带来严重负担, 但其发病机制不清. 微小RNA(microRNA, miRNA)是近年的研究热点, 不但参与调控细胞代谢、分化、增殖、肿瘤形成等过程, 也参与了NAFLD脂质代谢、胰岛素抵抗、细胞凋亡等环节.
张锦生, 教授, 复旦大学上海医学院病理学系; 陈茂伟, 教授, 广西医科大学第一附属医院质量管理办公室
多项试验证实NAFLD患者或动物模型中存在miRNA的差异性表达, miRNA可通过调控肝细胞或脂肪细胞的凋亡过程参与NAFLD的发病与进展, 虽涉及其具体调控机制的资料尚少, 但为未来用分子学的方式诊断、治疗NAFLD提供了新途径, 是以后的研究热点.
Cheung等是第一个研究miRNA在NAFLD中异常表达的人: 与控制组相比, 非酒精性脂肪性肝炎患者的肝组织中, 46种miRNA表达增加或降低. 经过进一步的研究发现: miR-296-5p、miR-221、miR-222、miR-483-3p通过调节受p53基因上调表达的凋亡调控蛋白(p53-upregulated modulator of apoptosis, PUMA)调控NAFLD脂肪细胞凋亡; Mmu-miR-615-3p通过抑制C/EBP同源蛋白(C/EBP homologous protein, CHOP)调控脂肪细胞凋亡.
miRNA是近年来的研究热点, 虽然miRNA在NAFLD发病中的作用及机制已有不少研究, 但其主要集中在调控代谢综合症这方面, 且相关综述不多, 国内外仅个别报道. 但NAFLD时, miRNA调控相关细胞凋亡的内容尚未见有综述. 本文就miRNA调控NAFLD细胞凋亡的机制作一综述.
本综述主要阐述miRNA调控肝细胞及脂肪细胞凋亡的机制, 小结了2014年前国内外发表的相关文章, 对相关临床医师和研究人员有一定参考价值, 为进一步阐明NAFLD发病机制、用分子学方式干预NAFLD进展提供理论依据.
受p53基因上调表达的凋亡调控蛋白(PUMA): p53基因属于抑癌基因, 可单独或与其他蛋白协同作用, 发挥促细胞凋亡的作用; B细胞淋巴瘤基因-2(B cell lymphoma 2, Bcl-2)基因: 属于癌基因, 主要抑制细胞凋亡. puma是p53的下游靶向基因, 同时也是Bcl-2家族中编码BH3-only蛋白的成员. 他通过p53及Bcl-2家族成员的进一步作用发挥促细胞凋亡作用.
miRNAs在NAFLD发病时的作用及机制已有不少研究, 但有关综述尚不多, 国内外仅个别报道. NAFLD时, miRNAs调控相关细胞凋亡的内容尚未见有综述. 该综述小结了2014年前国内外发表的相关文章, 对相关临床医师和研究人员有一定参考价值.
编辑: 韦元涛 电编:都珍珍
| 1. | Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519-1523. [PubMed] [DOI] |
| 2. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [PubMed] [DOI] |
| 3. | Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44-52. [PubMed] [DOI] |
| 4. | Margariti E, Deutsch M, Manolakopoulos S, Papatheodoridis GV. Non-alcoholic fatty liver disease may develop in individuals with normal body mass index. Ann Gastroenterol. 2012;25:45-51. [PubMed] |
| 5. | Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol. 2009;50:204-210. [PubMed] [DOI] |
| 6. | Amarapurkar DN, Hashimoto E, Lesmana LA, Sollano JD, Chen PJ, Goh KL. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J Gastroenterol Hepatol. 2007;22:788-793. [PubMed] [DOI] |
| 7. | Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212-1218. [PubMed] [DOI] |
| 8. | Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC, Frier BM, Van Look LA, Johnston GI, Reynolds RM. Prevalence of and risk factors for hepatic steatosis and nonalcoholic Fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care. 2011;34:1139-1144. [PubMed] [DOI] |
| 9. | Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415-1428. [PubMed] [DOI] |
| 10. | Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-1645. [PubMed] [DOI] |
| 12. | Fernández-Hernando C, Ramírez CM, Goedeke L, Suárez Y. MicroRNAs in metabolic disease. Arterioscler Thromb Vasc Biol. 2013;33:178-185. [PubMed] [DOI] |
| 13. | Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843-854. [PubMed] [DOI] |
| 14. | Mathieu J, Ruohola-Baker H. Regulation of stem cell populations by microRNAs. Adv Exp Med Biol. 2013;786:329-351. [PubMed] [DOI] |
| 15. | Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev. 2011;91:827-887. [PubMed] [DOI] |
| 16. | Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642-655. [PubMed] [DOI] |
| 17. | Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215-233. [PubMed] [DOI] |
| 18. | Abu-Shanab A, Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7:691-701. [PubMed] [DOI] |
| 19. | Altemeier WA, Zhu X, Berrington WR, Harlan JM, Liles WC. Fas (CD95) induces macrophage proinflammatory chemokine production via a MyD88-dependent, caspase-independent pathway. J Leukoc Biol. 2007;82:721-728. [PubMed] [DOI] |
| 20. | Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282-286. [PubMed] [DOI] |
| 21. | Kahraman A, Bronk SF, Cazanave S, Werneburg NW, Mott JL, Contreras PC, Gores GJ. Matrix metalloproteinase inhibitor, CTS-1027, attenuates liver injury and fibrosis in the bile duct-ligated mouse. Hepatol Res. 2009;39:805-813. [PubMed] [DOI] |
| 22. | Ogawa W, Kasuga M. Cell signaling. Fat stress and liver resistance. Science. 2008;322:1483-1484. [PubMed] [DOI] |
| 23. | Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:1389-1397. [PubMed] [DOI] |
| 24. | Machado MV, Ferreira DM, Castro RE, Silvestre AR, Evangelista T, Coutinho J, Carepa F, Costa A, Rodrigues CM, Cortez-Pinto H. Liver and muscle in morbid obesity: the interplay of fatty liver and insulin resistance. PLoS One. 2012;7:e31738. [PubMed] [DOI] |
| 25. | Alkhouri N, Gornicka A, Berk MP, Thapaliya S, Dixon LJ, Kashyap S, Schauer PR, Feldstein AE. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem. 2010;285:3428-3438. [PubMed] [DOI] |
| 26. | Wueest S, Rapold RA, Schumann DM, Rytka JM, Schildknecht A, Nov O, Chervonsky AV, Rudich A, Schoenle EJ, Donath MY. Deletion of Fas in adipocytes relieves adipose tissue inflammation and hepatic manifestations of obesity in mice. J Clin Invest. 2010;120:191-202. [PubMed] [DOI] |
| 27. | Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, Kellum JM, Min H, Luketic VA, Sanyal AJ. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology. 2008;48:1810-1820. [PubMed] [DOI] |
| 28. | Li S, Chen X, Zhang H, Liang X, Xiang Y, Yu C, Zen K, Li Y, Zhang CY. Differential expression of microRNAs in mouse liver under aberrant energy metabolic status. J Lipid Res. 2009;50:1756-1765. [PubMed] [DOI] |
| 29. | Dolganiuc A, Petrasek J, Kodys K, Catalano D, Mandrekar P, Velayudham A, Szabo G. MicroRNA expression profile in Lieber-DeCarli diet-induced alcoholic and methionine choline deficient diet-induced nonalcoholic steatohepatitis models in mice. Alcohol Clin Exp Res. 2009;33:1704-1710. [PubMed] [DOI] |
| 30. | Pogribny IP, Starlard-Davenport A, Tryndyak VP, Han T, Ross SA, Rusyn I, Beland FA. Difference in expression of hepatic microRNAs miR-29c, miR-34a, miR-155, and miR-200b is associated with strain-specific susceptibility to dietary nonalcoholic steatohepatitis in mice. Lab Invest. 2010;90:1437-1446. [PubMed] [DOI] |
| 31. | Wang B, Hsu SH, Majumder S, Kutay H, Huang W, Jacob ST, Ghoshal K. TGFbeta-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. Oncogene. 2010;29:1787-1797. [PubMed] [DOI] |
| 32. | Alisi A, Da Sacco L, Bruscalupi G, Piemonte F, Panera N, De Vito R, Leoni S, Bottazzo GF, Masotti A, Nobili V. Mirnome analysis reveals novel molecular determinants in the pathogenesis of diet-induced nonalcoholic fatty liver disease. Lab Invest. 2011;91:283-293. [PubMed] [DOI] |
| 33. | Feng YY, Xu XQ, Ji CB, Shi CM, Guo XR, Fu JF. Aberrant hepatic microRNA expression in nonalcoholic fatty liver disease. Cell Physiol Biochem. 2014;34:1983-1997. [PubMed] [DOI] |
| 34. | Celikbilek M, Baskol M, Taheri S, Deniz K, Dogan S, Zararsiz G, Gursoy S, Guven K, Ozbakır O, Dundar M. Circulating microRNAs in patients with non-alcoholic fatty liver disease. World J Hepatol. 2014;6:613-620. [PubMed] [DOI] |
| 35. | Jabbour AM, Heraud JE, Daunt CP, Kaufmann T, Sandow J, O'Reilly LA, Callus BA, Lopez A, Strasser A, Vaux DL. Puma indirectly activates Bax to cause apoptosis in the absence of Bid or Bim. Cell Death Differ. 2009;16:555-563. [PubMed] [DOI] |
| 36. | Cazanave SC, Mott JL, Elmi NA, Bronk SF, Werneburg NW, Akazawa Y, Kahraman A, Garrison SP, Zambetti GP, Charlton MR. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J Biol Chem. 2009;284:26591-26602. [PubMed] [DOI] |
| 37. | Estep M, Armistead D, Hossain N, Elarainy H, Goodman Z, Baranova A, Chandhoke V, Younossi ZM. Differential expression of miRNAs in the visceral adipose tissue of patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2010;32:487-497. [PubMed] [DOI] |
| 38. | Cazanave SC, Mott JL, Elmi NA, Bronk SF, Masuoka HC, Charlton MR, Gores GJ. A role for miR-296 in the regulation of lipoapoptosis by targeting PUMA. J Lipid Res. 2011;52:1517-1525. [PubMed] [DOI] |
| 39. | Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36:487-499. [PubMed] [DOI] |
| 40. | Zhang CZ, Zhang JX, Zhang AL, Shi ZD, Han L, Jia ZF, Yang WD, Wang GX, Jiang T, You YP. MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol Cancer. 2010;9:229. [PubMed] [DOI] |
| 41. | Veronese A, Lupini L, Consiglio J, Visone R, Ferracin M, Fornari F, Zanesi N, Alder H, D'Elia G, Gramantieri L. Oncogenic role of miR-483-3p at the IGF2/483 locus. Cancer Res. 2010;70:3140-3149. [PubMed] [DOI] |
| 42. | Castro RE, Ferreira DM, Afonso MB, Borralho PM, Machado MV, Cortez-Pinto H, Rodrigues CM. miR-34a/SIRT1/p53 is suppressed by ursodeoxycholic acid in the rat liver and activated by disease severity in human non-alcoholic fatty liver disease. J Hepatol. 2013;58:119-125. [PubMed] [DOI] |
| 43. | Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745-752. [PubMed] [DOI] |
| 44. | Miyamoto Y, Mauer AS, Kumar S, Mott JL, Malhi H. Mmu-miR-615-3p regulates lipoapoptosis by inhibiting C/EBP homologous protein. PLoS One. 2014;9:e109637. [PubMed] [DOI] |
| 45. | Lin CJ, Gong HY, Tseng HC, Wang WL, Wu JL. miR-122 targets an anti-apoptotic gene, Bcl-w, in human hepatocellular carcinoma cell lines. Biochem Biophys Res Commun. 2008;375:315-320. [PubMed] [DOI] |
| 46. | Ma L, Liu J, Shen J, Liu L, Wu J, Li W, Luo J, Chen Q, Qian C. Expression of miR-122 mediated by adenoviral vector induces apoptosis and cell cycle arrest of cancer cells. Cancer Biol Ther. 2010;9:554-561. [PubMed] [DOI] |
| 47. | Gramantieri L, Fornari F, Ferracin M, Veronese A, Sabbioni S, Calin GA, Grazi GL, Croce CM, Bolondi L, Negrini M. MicroRNA-221 targets Bmf in hepatocellular carcinoma and correlates with tumor multifocality. Clin Cancer Res. 2009;15:5073-5081. [PubMed] [DOI] |
| 48. | Dai R, Li J, Liu Y, Yan D, Chen S, Duan C, Liu X, He T, Li H. miR-221/222 suppression protects against endoplasmic reticulum stress-induced apoptosis via p27(Kip1)- and MEK/ERK-mediated cell cycle regulation. Biol Chem. 2010;391:791-801. [PubMed] [DOI] |
| 49. | Li J, Fu H, Xu C, Tie Y, Xing R, Zhu J, Qin Y, Sun Z, Zheng X. miR-183 inhibits TGF-beta1-induced apoptosis by downregulation of PDCD4 expression in human hepatocellular carcinoma cells. BMC Cancer. 2010;10:354. [PubMed] [DOI] |