修回日期: 2013-12-24
接受日期: 2013-12-27
在线出版日期: 2014-02-18
目的: 探讨新药多肽P162通过抑制Hedgehog(Hh)信号转录因子Gli-1对人食管癌细胞株Eca109起放射增敏作用.
方法: 反复多次X线(剂量累计60 Gy)照射Eca109诱导食管癌细胞Eca109R, 采用CCK8法测增殖抑制率, 免疫细胞化学法、免疫荧光技术测Gli-1的表达, HE染色观察细胞形态学改变, Western blot测核内Gli-1表达及动态监测Eca109放疗后核内Gli-1变化, 流式细胞仪测细胞凋亡率. 实验分以下4组: 未照射加药组、照射加药组、未照射不加药组和照射不加药组, 每组中含Eca109、Eca109R两种细胞.
结果: Eca109R增殖抑制率明显低于Eca109, 具有一定放射抗拒性; Eca109R较Eca109高表达Gli-1(0.45±0.01, 0.32±0.01, P<0.0001); Eca109放疗后Gli-1于2 d, 14 d表达与对照相比(0.0882±0.011, 0.3560±0.015 vs 0.2552±0.0103), 差异有统计学意义(P<0.05); 20 µmol/L P162干预Eca109R、Eca109细胞中, 与0 µmol/L P162干预比较Gli-1表达下调, 分别为: 0.2553±0.011, 0.2578±0.014(未照射), 0.1324±0.012, 0.0595±0.011(照射2 d), 0.1741±0.013, 0.2397±0.112(照射14 d), 差异均有统计学意义(P<0.0001), P162联合放疗促细胞凋亡.
结论: Hh信号转录因子Gli-1与食管癌放射抗拒相关. P162放射增敏作用可能与抑制转录因子Gli-1、促细胞凋亡相关.
核心提示: Hedgehog(Hh)信号转录因子Gli-1与食管癌放射抗拒相关. P162放射增敏作用可能与抑制转录因子Gli-1、促细胞凋亡相关.
引文著录: 陈洁, 吴清明, 龙辉, 张红, 陈建华. P162抑制Hedgehog信号转录因子Gli-1增强食管癌Eca109细胞的放射敏感性. 世界华人消化杂志 2014; 22(5): 615-623
Revised: December 24, 2013
Accepted: December 27, 2013
Published online: February 18, 2014
AIM: To investigate whether P162 enhances radiosensitivity of esophageal carcinoma cell line Eca109 by inhibiting Hedgehog signaling transcription factor Gli-1.
METHODS: Eca109 cells (a total dose of 60 Gy) to induce radioresistant esophageal carcinoma cell line Eca109R. The inhibition of cell proliferation was determined by Cell Counting Kit assay. The expression of Gli-1 was detected by immunohistochemistry and immunofluorescence. HE staining was employed to observe the changes in cell morphology. Western blot was employed to determine the nuclear expression of Gli-1 and dynamic changes of Gli-1 in irradiated Eca109 cells. Apoptosis was determined by flow cytometry. The following four groups were included in the experiments: untreated cells, P162-treated cells, irradiated cells, and P162-treated irradiated cells. Eca109 and Eca109R cells were included in each group.
RESULTS: Eca109R possessing certain radiation resistance displayed lower ability of growth inhibition than Eca109 cells. Nuclear Gli-1 expression was significantly higher in Eca109R cells than in Eca109 cells (0.45 ± 0.01 vs 0.32 ± 0.01, P < 0.0001). On days 2 and 14 after irradiation, the nuclear expression of Gli-1 in Eca109 cells was higher than that in control cells (0.0882 ± 0.011, 0.3560 ± 0.015 vs 0.2552 ± 0.0103, P < 0.05). In both Eca109R and Eca109 cells, the nuclear expression of Gli-1 was reduced after treatment with 20 µmol/L P162 [0.2553 ± 0.011, 0.2578 ± 0.014 (non-irradiation); 0.1324 ± 0.012, 0.0595 ± 0.011(2 d after irradiation); 0.1741 ± 0.013, 0.2397 ± 0.112 (14 d after irradiation), P < 0.0001]. P162 combined with radiotherapy facilitated cells apoptosis.
CONCLUSION: Nuclear Gli-1 expression is related to radioresistance of esophageal cancer cells. P162 enhances radiosensitivity of Eca109 cell possibly by inhibiting Gli-1 expression and promoting apoptosis.
- Citation: Chen J, Wu QM, Long H, Zhang H, Chen JH. P162 enhances radiosensitivity of esophageal carcinoma Eca109 cells by inhibiting Hedgehog signaling transcription factor Gli-1. Shijie Huaren Xiaohua Zazhi 2014; 22(5): 615-623
- URL: https://www.wjgnet.com/1009-3079/full/v22/i5/615.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v22.i5.615
食管癌是常见的消化系恶性肿瘤, 放疗是晚期治疗重要手段, 但易形成放疗抵抗而复发[1,2], 靶向G3BP多肽162-P162(中国专利2010101400323, PCT WO2011/124063 A1), 其结构能与G3BP蛋白的NTF2结构域发生特异性结合, 干预G3BP蛋白磷酸化. 已证实P162对高表达G3BP的食管癌Eca109细胞有放射增敏作用, 但具体增敏机制尚不完全清楚[3], 研究显示Hedgehog(Hh)通路在食管癌异常激活[4-6], 且与放化疗抵抗相关[7,8]. 本实验以食管癌细胞株Eca109及其诱导的具有一定放射抗拒性细胞Eca109R为对象, 探讨食管癌放射抗拒及P162的放射增敏作用与Hh信号通路转录因子Gli-1的关系.
人食管癌细胞株Eca109(由太和医院馈赠); 多肽P162(武汉凯泰新生物技术有限公司); Gli-1抗体(Santa Cruz公司); 新生胎牛血清(杭州四季青有限公司); RPMI 1640培养粉(Gibco公司); 胰蛋白酶粉(Amresco公司), CCK-8试剂(Sigma公司); 苏木素(Gibco公司)、伊红(Gibco公司); 丙烯酰胺(Amresco); 甲叉双丙烯酰胺(Amresco); BCA蛋白浓度测定试剂盒(碧云天); 核蛋白和胞浆蛋白提取试剂盒(凯基生物); Annexin V-FITC凋亡试剂盒(Roche). CO2恒温细胞培养箱(Heal Force公司); 酶联免疫检测仪(美国宝特ELX800); 倒置显微镜(Olympus公司); 超速低温离心机(Sigma公司); 流式细胞仪(美国贝克曼公司FC 500 cytometers CXP分析软件); 37 ℃恒温水浴箱(华普达公司); 垂直电泳槽(北京六一仪器厂); Nikon ECLIPSE 80i荧光显微镜(日本Nikon 公司).
1.2.1 细胞培养: 细胞置37 ℃、5%CO2培养箱中培养, 其培养基为含有10%胎牛血清、青霉素100 U/L、链霉素100 U/L的RPMI 1640, 细胞处于对数生长期时进行实验.
1.2.2 诱导Eca109R细胞: 取亲本细胞Eca109采用Varian2300直线加速器6MV-X线照射8 Gy, 表面加1.5 cm标准等效填充物, 放射源至标本距离100 cm, 照射野10 cm×10 cm, 吸收剂量率为1.5 Gy/min, 照射后置培养箱继续培养, 待细胞接近长满瓶底时, 0.25%胰蛋白酶消化后重新接种培养. 细胞处于对数期时, 再次X射线照射8 Gy. 重复以上过程, 累计照射剂量至60 Gy[9].
1.2.3 CCK-8法测Eca109、Eca109R细胞增殖抑制率: 0、2、4、6、8 Gy剂量照射Eca109、Eca109R细胞后, 将其消化成悬液, 以5000 cells/well接种于96孔板, 重复6孔, 设空白调零组. 孔中培养24、48、72 h后加10 µL CCK-8, 培养箱孵育1 h, 选择450 nm处酶标仪测各孔吸光度(A), 计算增殖抑制率, 抑制率(inhibition rate, IR) =(1-实验组平均A值/对照组平均A值)×100%.
1.2.4 细胞免疫化学: 将对数生长期细胞Eca109、 Eca109R消化成悬液, 以5×104 cells/mL接种于6孔板中盖玻片上, 每孔2 mL, 孵育4 h后, 4%多聚甲醛固定, 0.5%Triton X-100室温通透, 3%H2O2-甲醇除内源性过氧化酶活性, 10%正常山羊血清封闭, 敷Ⅰ抗兔抗人Gli-1(1:100), 4 ℃湿盒过夜, 加入相应的Ⅱ抗, 显微镜下控制DAB显色, 待显色充分后, 水冲洗终止反应. 苏木素复染, 盐酸乙醇分化, 二甲苯透明, 树胶封片, 镜下观察, 拍照. Gli-1蛋白以胞质或胞核出现浅黄色至深棕色颗粒判断为阳性细胞. 在显微镜下随机计数6个高倍视野的细胞. 综合阳性细胞及染色强度所占百分比2个方面进行半定量分析. 染色强度评分: 0分: 无或染色极淡, 阴性(-); 1分: 浅棕黄色, 弱阳性(+); 2分: 棕黄色, 阳性(++); 3分: 棕褐色, 强阳性(+++). 阳性细胞百分比(A、B、C、D)分别是-、+、++、+++各种染色强度细胞的百分比. 染色强度指数 = A×0+B×1+C×2+D×3.
1.2.5 免疫荧光: 细胞爬片处理同细胞免疫化学, 4%的多聚甲醛固定, 0.5%Triton X-100室温通透20 min, 正常山羊血清封闭, 敷Gli-1Ⅰ抗(1:150), 4 ℃湿盒过夜; 敷Ⅱ抗荧光(Cy3)标记羊抗兔IgG(1:100), 湿盒中20 ℃-37 ℃孵育1 h, 复染核DAPI避光孵育5 min, 抗荧光淬灭剂封片液封片, 显微镜下随机计数6个高倍视野的细胞, 计算阳性细胞率.
1.2.6 HE染色观察P162对Eca109、 Eca109R细胞形态学影响: Eca109、 Eca109R细胞加入终浓度为20 µmol/L P162, 同时对照细胞加入等量RPMI 1640培养液, 作用48 h后做爬片处理, 苏木素染色10 min, 盐酸乙醇分化, 伊红染色2 min, 二甲苯透明, 树胶封片, 镜下观察, 拍照.
1.2.7 Western blot测放疗后Eca109核内Gli-1表达变化: 6 Gy X射线照射Eca109细胞后, 分别于1、2、4、24、48、72 h、7、10、14、18 d后提取核蛋白, 未照射Eca109细胞作空白对照. 测蛋白浓度, 取50 μg总蛋白上样电泳, 蛋白分离后停止电泳, 电转移至硝酸纤维素膜, 5%脱脂奶粉封闭2 h, 加入封闭液稀释兔抗Gli-1Ⅰ抗(1:300), 4 ℃孵育过夜, TBST洗膜, 加入相应Ⅱ抗室温孵育2 h, TBST洗膜, ECL试剂盒暗室曝光显影.
1.2.8 Western blot测处理组细胞核内Gli-1表达: 处理组给予两种干预方法, 先20 µmol/L P162干预48 h, 后6 Gy X线照射, 可分为4组, 即: 未照射不加药组、未照射加药组、照射加药组和照射不加药组, 每组均含Eca109、Eca109R两种细胞. 加药不加药干预48 h后, 不照射细胞立即提取核蛋白-80 ℃保存, 照射处理细胞于照射后48 h、14 d提取核蛋白, 待核蛋白提取完毕统一进行Western blot.
1.2.9 流式细胞术测细胞凋亡率: 上述4组细胞加药及不加干预理48 h后, 未照射及照射处理24 h后用不含EDTA胰酶消化收集5×105细胞, PBS洗涤2次(2000 r/min, 离心5 min), 悬浮于500 µL Binding Buffer中, 加入5 µL Annexin V-FITC混匀后, 再加入5 µL Propidium Iodide混匀, 避光孵育15 min, 过滤后上流式细胞仪检测.
统计学处理 采用SPSS17.0统计软件进行统计分析. 计量资料用mean±SD表示, 组间比较采用配对t检验, P162对Eca109、Eca109R细胞核内Gli-1影响采用2×2析因设计, 正态Shapiro-Wilk检验, P<0.05为差异有统计学意义.
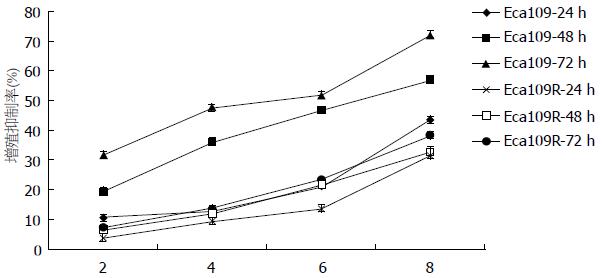
Eca109、Eca109R细胞经X线照射后24、48、72 h行CCK8检测, 发现相同时间各个照射剂量下Eca109R增殖抑制率明显低于Eca109(表1, 图1)(组间两两比较采用配对t检验, P<0.05). 与亲本Eca109细胞相比, Eca109R细胞放射敏感性较低, 具有一定放射抗拒性.
| 照射剂量(Gy) | IR | |||||
| 24 h | 48 h | 72 h | ||||
| Eca109 | Eca109R | Eca109 | Eca109R | Eca109 | Eca109R | |
| 2 | 10.70±1.08 | 3.91±1.44 | 19.35±1.45 | 6.65±1.19 | 31.79±1.25 | 7.51±1.00 |
| 4 | 12.65±1.09 | 9.53±1.17 | 36.11±1.47 | 11.81±1.14 | 47.94±0.93 | 13.64±1.17 |
| 6 | 21.23±1.55 | 13.73±1.41 | 46.98±1.32 | 21.73±1.34 | 51.72±1.32 | 23.68±1.12 |
| 8 | 43.52±1.07 | 31.64±1.41 | 56.93±1.20 | 32.84±1.52 | 71.95±1.36 | 38.35±1.11 |
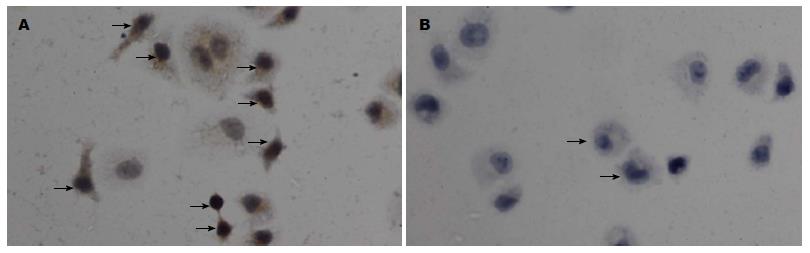
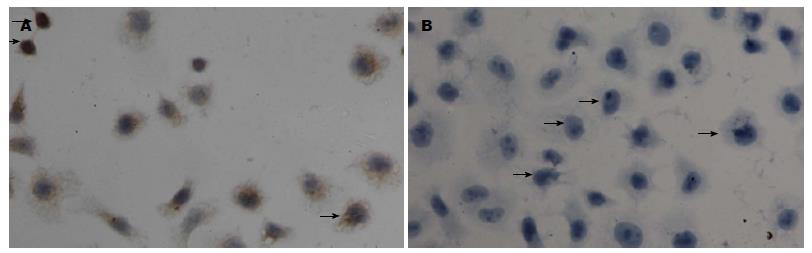
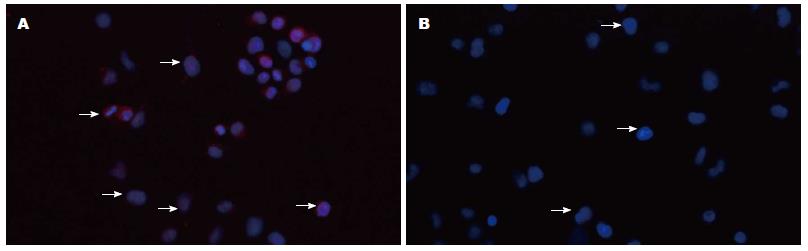
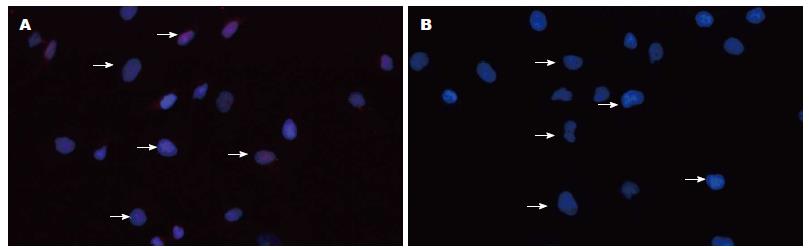
免疫细胞化学法、免疫荧光技术显示Gli-1蛋白表达位于胞核及胞浆, Eca109、Eca109R细胞均有表达, 免疫组织化学染色强度分别为: 1.05629±0.098, 1.703975±0.055(P<0.05)(图2, 3). 免疫荧光阳性细胞率分别为: 39.9567%± 0.0097%, 66.4589%±0.0251%(P<0.05)(图4, 5), 差异有统计学意义.
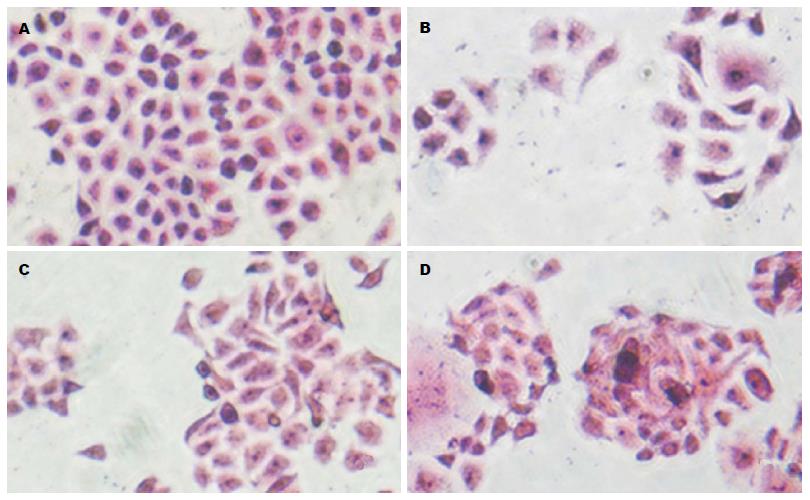
HE观察显示: Eca109细胞呈圆形或不规则多边形, 如铺路石排列(图6A). Eca109R细胞呈不规则梭性, 不规则排列(图6B). 20 µmol/L P162处理48 h后, Eca109细胞皱缩聚集, 变为不规则条形, 可见细胞裂解相互融合(图6C). Eca109R细胞亦表现为皱缩聚集, 可见核缩小、多核, 细胞裂解融合(图6D).
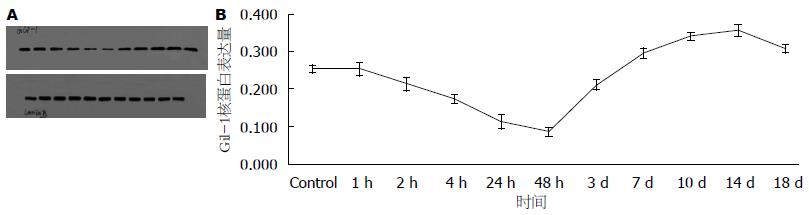
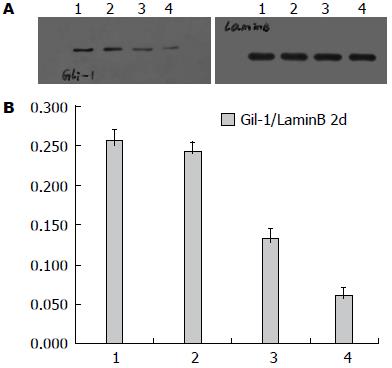
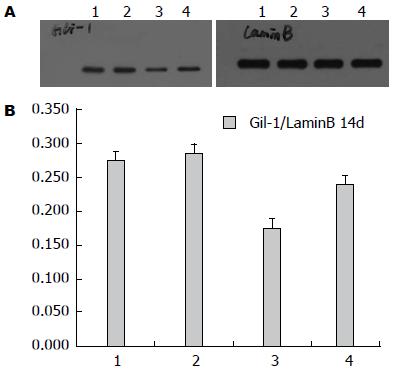
Western blot测X线照射后Eca109细胞1、2、4、24、48、72 h、7、10、14 d时间点核内Gli-1表达, 结果显示: 照射后Gli-1逐渐下调, 与未照射细胞相比于第48小时表达最低(P<0.05), 此后逐渐上调, 于第14天表达最高(P<0.05), 18 d趋于下降趋势(图7), 差异有统计学意义.
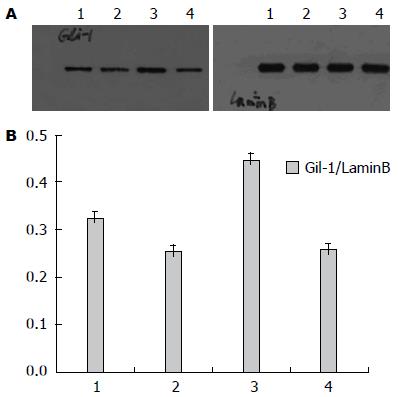
Western blot测射线照射及P162干预后Eca109、Eca109R细胞核内Gli-1变化, 结果显示: 未照射加药组较未照射不加药组低表达Gli-1, 其中未照射不加药组中, Eca109R较Eca109高表达Gli-1(F = 135.73, P<0.0001)(图8); 照射加药组较照射不药组(照射后2、14 d)低表达Gli-1, 差异均有统计学意义(P<0.05), 其中照射不加药组(照射后2、14 d)中, Eca109与Eca109R的 Gli-1表达无统计学差异[分别为F = 1.89, P = 0.2064(2 d), F = 0.45, P = 0.5191(4 d)](图9, 10).
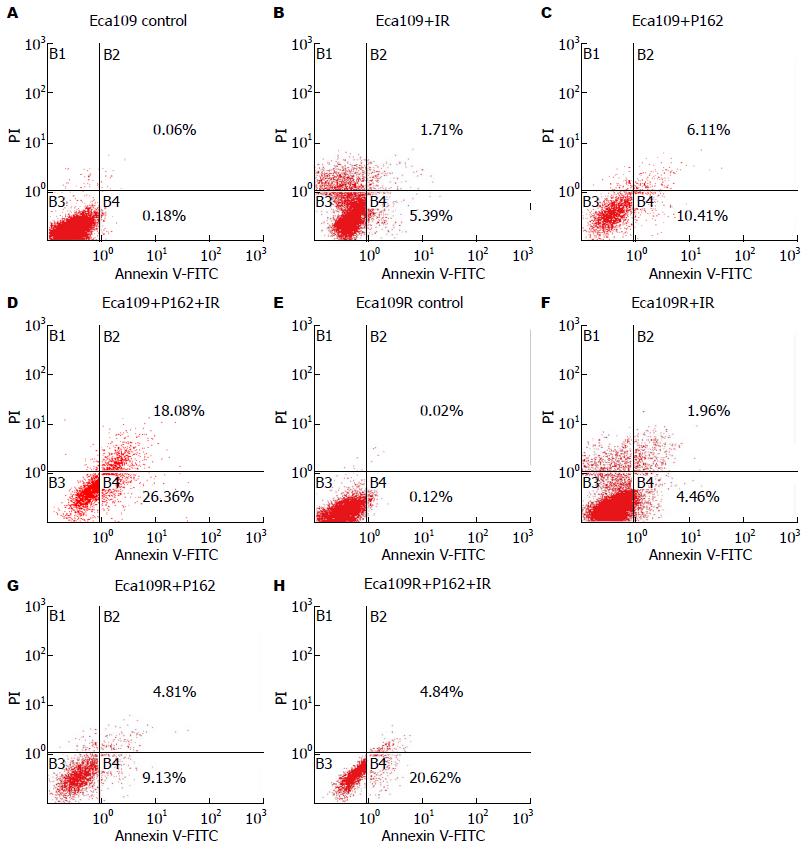
由表2、图11可知P162联合放疗促进Eca109R、Eca109细胞凋亡.
| 照射剂量(Gy) | 分组 | 早期 | 晚期 |
| 0 | Eca109 | 0.18 | 0.06 |
| Eca109R | 0.12 | 0.02 | |
| Eca109+P162 | 10.41 | 6.11 | |
| Eca109R+P162 | 9.13 | 4.81 | |
| 6 | Eca109 | 5.39 | 1.71 |
| Eca109R | 4.46 | 1.96 | |
| Eca109+P162 | 26.36 | 18.08 | |
| Eca109R+P162 | 20.62 | 4.84 |
Hh经典信号通路主要由Hh配体(Dhh、Ihh、Shh)、PTCH和Smo组成的受体复合物以及下游转录因子Gli等组成, 其中Gli-1是Hh下游靶基因的强激活子, 核内Gli-1蛋白水平与Gli-1的转录活性成正比, 核蛋白Gli-1的表达水平是反映Hh信号通路活性[10]. Yoshikaw等[11]报道核蛋白Gli-1的表达与食管鳞癌放疗抵抗及复发有显著联系. Zhu等[7]也表明Gli-1表达与食管鳞癌放疗抵抗相关. Chen等[12]研究显示放疗诱使肝癌细胞自分泌Shh(sonic hedgehog)配体激活Hh信号通路产生放疗抵抗. 本实验发现亲本食管癌Eca109细胞经放疗后Gli-1下调后上调, 放射抗拒性食管癌细胞较亲本食管癌细胞上调核蛋白Gli-1, 而两种细胞经放疗后2及4 d时Gli-1变化没有差异, 提示Hh信号通路异常激活可能与食管癌放射抗拒相关.
Hh信号诱导放疗抗拒的机制目前尚不清楚, 推测其可能的机制是因Hh为调控肿瘤干细胞的重要通路之一, 在肿瘤干细胞自我更新、细胞持续分化以及肿瘤细胞侵袭转移等方面均扮演重要角色, 维持肿瘤的生存和促进肿瘤细胞的再增殖[13,14], 而肿瘤干细胞是导致肿瘤复发、转移、放疗抵抗的主要根源[15-18].
已证实食管癌放射抗拒细胞富集大量食管癌干细胞, 具有无限增殖、自我更新、细胞球形成力、高致瘤力等干细胞特性[19,20], 前期我们发现食管癌Eca109细胞随着放疗剂量的增加SP细胞、干细胞标志物β-catenin、食管癌干细胞标志物P75NTR的表达逐渐上调, 而P162抑制P75NTR表达[3]、干扰Ras-PI3K-Akt的增殖信号通路、抑制AKT、ERK、NF-κB的表达, 上调凋亡信号通路[21]. 研究发现抑制Hh信号增敏放疗[22], Gu等[23]表明阻滞Hh通过干扰肿瘤干细胞增敏放疗并阻止远处癌肿转移, Chen等[24]表明抑制Gli-1 mRNA表达可促进肝癌细胞凋亡. 本实验发现单独P162及P162联合放疗下调Eca109、Eca109R核内蛋白Gli-1, 并促使其凋亡. 提示多肽P162抑制Hh信号激活, 联合放疗促进食管癌细胞凋亡.
然而P162具体通过什么信号分子抑制Hh通路亦尚未清楚, 已发现非经典Hh通路是由信号通路RAS、AKT、TGFβ不依赖配体蛋白激活转录因子Gli[25], 其中RAS-MEK信号通路是最主要的非经典Hh信号通路[26,27]. NF-κB靶向激活Shh mRNA及蛋白的表达抵抗TNF诱导凋亡[28], 据此可推测P162可通过NF-κB直接抑制经典Hh通路, 还可经 RAS-MEK、PI3K-Akt抑制非经典Hh通路.
总之, Hh信号通路在人食管癌Eca109细胞系中存在异常激活, 并与放射抗拒相关. P162对食管癌的放疗增敏作用可能涉及促进细胞凋亡, 抑制Hh信号激活及食管癌干细胞表达, 还可能涉及其他影响因素, 如DNA损伤修复机制、细胞周期再分布、肿瘤细胞再增殖等. Hh信号激活导致放射抗拒的机制及其与食管癌干细胞之间的关系、P162增敏放疗的具体机制尚需进一步探讨.
食管癌是消化系常见的恶性肿瘤之一, 放疗是其晚期主要治疗手段. 据统计发现临床上放疗后大约有68%的食管癌会复发, 研究证实放射治疗并不能完全杀死肿瘤细胞. 进一步研究发现其与放疗抵抗相关. 目前研究表明食管癌放疗抵抗涉及多种基因、多条信号通路调控细胞的DNA损伤修复、细胞周期重分布、细胞凋亡、细胞再增殖、细胞再氧合等. 因此研究食管癌放疗抵抗主要机制并使用药物增敏放射敏感性具有重要的临床价值.
张志坚, 教授, 福州总医院消化内科
Hedgehog(Hh)是肿瘤干细胞信号通路之一, 发现其信号分子在食管鳞癌中异常表达, 近年的研究显示肿瘤干细胞在肿瘤放疗抵抗中起极其重要的作用, 那么, Hedgehog是否导致食管癌的放疗抵抗.
Yoshikaw报道核蛋白Gli-1的表达与食管鳞癌放疗抵抗及复发有显著联系. Zhu等也表明Gli-1表达与食管鳞癌放疗抵抗相关. Chen等研究显示放疗诱使肝癌细胞自分泌Shh配体激活Hh信号通路产生放疗抵抗.
Hh信号转录因子Gli-1在食管癌Eca109细胞中表达, 并与其放射抗拒相关, 新药多肽P162能够抑制Gli-1的表达来增加食管癌放射敏感性.
靶向作用于G3BP的新型多肽P162通过抑制Hh信号通路逆转食管癌放射抗拒, 阐明食管癌放疗抗拒和P162增敏放疗的最新机制, 为食管癌防治提供新的理论依据和手段.
本文设计合理可靠, 有系统的理论分析和有价值的结论.
编辑: 田滢 电编:鲁亚静
| 1. | Wong R, Malthaner R. Combined chemotherapy and radiotherapy (without surgery) compared with radiotherapy alone in localized carcinoma of the esophagus. Cochrane Database Syst Rev. 2006;25:CD002092. [PubMed] [DOI] |
| 2. | Chen Y, Lu Y, Wang Y, Yang H, Xia Y, Chen M, Song H, Li T, Li D, Wang J. Comparison of salvage chemoradiation versus salvage surgery for recurrent esophageal squamous cell carcinoma after definitive radiochemotherapy or radiotherapy alone. Dis Esophagus. 2014;27:134-140. [PubMed] [DOI] |
| 4. | Wei L, Xu Z. Cross-signaling among phosphinositide-3 kinase, mitogen-activated protein kinase and sonic hedgehog pathways exists in esophageal cancer. Int J Cancer. 2011;129:275-284. [PubMed] [DOI] |
| 5. | Ma X, Sheng T, Zhang Y, Zhang X, He J, Huang S, Chen K, Sultz J, Adegboyega PA, Zhang H. Hedgehog signaling is activated in subsets of esophageal cancers. Int J Cancer. 2006;118:139-148. [PubMed] |
| 6. | Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846-851. [PubMed] [DOI] |
| 7. | Zhu W, You Z, Li T, Yu C, Tao G, Hu M, Chen X. Correlation of hedgehog signal activation with chemoradiotherapy sensitivity and survival in esophageal squamous cell carcinomas. Jpn J Clin Oncol. 2011;41:386-393. [PubMed] [DOI] |
| 8. | Sims-Mourtada J, Izzo JG, Apisarnthanarax S, Wu TT, Malhotra U, Luthra R, Liao Z, Komaki R, van der Kogel A, Ajani J. Hedgehog: an attribute to tumor regrowth after chemoradiotherapy and a target to improve radiation response. Clin Cancer Res. 2006;12:6565-6572. [PubMed] [DOI] |
| 10. | Mori Y, Okumura T, Tsunoda S, Sakai Y, Shimada Y. Gli-1 expression is associated with lymph node metastasis and tumor progression in esophageal squamous cell carcinoma. Oncology. 2006;70:378-389. [PubMed] |
| 11. | Yoshikawa R, Tsujimura T, Tao L, Kamikonya N, Fujiwara Y. The oncoprotein and stem cell renewal factor BMI1 associates with poor clinical outcome in oesophageal cancer patients undergoing preoperative chemoradiotherapy. BMC Cancer. 2012;12:461. [PubMed] [DOI] |
| 12. | Chen YJ, Lin CP, Hsu ML, Shieh HR, Chao NK, Chao KS. Sonic hedgehog signaling protects human hepatocellular carcinoma cells against ionizing radiation in an autocrine manner. Int J Radiat Oncol Biol Phys. 2011;80:851-859. [PubMed] [DOI] |
| 13. | Merchant AA, Matsui W. Targeting Hedgehog--a cancer stem cell pathway. Clin Cancer Res. 2010;16:3130-3140. [PubMed] [DOI] |
| 14. | Song Z, Yue W, Wei B, Wang N, Li T, Guan L, Shi S, Zeng Q, Pei X, Chen L. Sonic hedgehog pathway is essential for maintenance of cancer stem-like cells in human gastric cancer. PLoS One. 2011;6:e17687. [PubMed] [DOI] |
| 15. | Ogawa K, Yoshioka Y, Isohashi F, Seo Y, Yoshida K, Yamazaki H. Radiotherapy targeting cancer stem cells: current views and future perspectives. Anticancer Res. 2013;33:747-754. [PubMed] |
| 16. | Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer stem cells. Int J Biochem Cell Biol. 2012;44:2144-2151. [PubMed] [DOI] |
| 17. | Bohl SR, Pircher A, Hilbe W. Cancer stem cells: characteristics and their potential role for new therapeutic strategies. Onkologie. 2011;34:269-274. [PubMed] [DOI] |
| 18. | Moncharmont C, Levy A, Gilormini M, Bertrand G, Chargari C, Alphonse G, Ardail D, Rodriguez-Lafrasse C, Magné N. Targeting a cornerstone of radiation resistance: cancer stem cell. Cancer Lett. 2012;322:139-147. [PubMed] [DOI] |
| 19. | Che SM, Zhang XZ, Liu XL, Chen X, Hou L. The radiosensitization effect of NS398 on esophageal cancer stem cell-like radioresistant cells. Dis Esophagus. 2011;24:265-273. [PubMed] [DOI] |
| 20. | Li JC, Liu D, Yang Y, Wang XY, Pan DL, Qiu ZD, Su Y, Pan JJ. Growth, clonability, and radiation resistance of esophageal carcinoma-derived stem-like cells. Asian Pac J Cancer Prev. 2013;14:4891-4896. [PubMed] |
| 21. | Zhang H, Zhang S, He H, Zhao W, Ren K, Chen J, Shao RG. RasGAP-derived peptide 38GAP potentiates the cytotoxicity of cisplatin through inhibitions of Akt, ERK and NF-κB in colon carcinoma HCT116 cells. Cancer Lett. 2011;308:62-70. [PubMed] [DOI] |
| 22. | Zeng J, Aziz K, Chettiar ST, Aftab BT, Armour M, Gajula R, Gandhi N, Salih T, Herman JM, Wong J. Hedgehog pathway inhibition radiosensitizes non-small cell lung cancers. Int J Radiat Oncol Biol Phys. 2013;86:143-149. [PubMed] [DOI] |
| 23. | Gu D, Liu H, Su GH, Zhang X, Chin-Sinex H, Hanenberg H, Mendonca MS, Shannon HE, Chiorean EG, Xie J. Combining hedgehog signaling inhibition with focal irradiation on reduction of pancreatic cancer metastasis. Mol Cancer Ther. 2013;12:1038-1048. [PubMed] [DOI] |
| 24. | Chen XL, Cao LQ, She MR, Wang Q, Huang XH, Fu XH. Gli-1 siRNA induced apoptosis in Huh7 cells. World J Gastroenterol. 2008;14:582-589. [PubMed] [DOI] |
| 25. | Lauth M, Toftgård R. Non-canonical activation of GLI transcription factors: implications for targeted anti-cancer therapy. Cell Cycle. 2007;6:2458-2463. [PubMed] [DOI] |
| 26. | Goel HL, Pursell B, Chang C, Shaw LM, Mao J, Simin K, Kumar P, Vander Kooi CW, Shultz LD, Greiner DL. GLI1 regulates a novel neuropilin-2/α6β1 integrin based autocrine pathway that contributes to breast cancer initiation. EMBO Mol Med. 2013;5:488-508. [PubMed] [DOI] |
| 27. | Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, Beermann F, Ruiz I Altaba A. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci U S A. 2007;104:5895-5900. [PubMed] [DOI] |