修回日期: 2013-11-11
接受日期: 2013-11-19
在线出版日期: 2014-01-08
目的: 观察四氯化碳(CCl4)经腹腔注射法制备肝硬化动物模型(cirrhosis model, CM)实验中机体其他主要脏器的病理变化情况.
方法: 30只SD大鼠, 随机分为对照组和模型组. 模型组: 22只, 使用橄榄油配备浓度为600 mL/L的CCl4按3 μL/g体质量经腹腔注射, 3次/wk, 共计12 wk, 制备肝硬化大鼠模型(假小叶形成为判定CM成功的标准); 对照组: 8只, 按3 μL/g体质量给予橄榄油溶液腹腔注射, 3次/wk, 共计12 wk. 两组大鼠均给予普通饲料加清水喂养, 并监测实验期间大鼠体质量变化、活动及对外界的反应情况, 同时记录大鼠的死亡情况及死亡时间. 至12周末, 模型组死亡6只, 对照组全部存活. 处死存活下来的模型组大鼠做肝脏病理, 均符合肝硬化标准. 造模成功的同时收集食管、结肠、脾、肺等组织行HE染色, 肾脏组织行PAS染色、并在光镜下观察各器官的组织形态学改变.
结果: 与对照组相比, 模型组的大鼠肝组织呈典型的假小叶改变; 食管、结肠可见少量的炎症细胞浸润, 肾脏可见肾小管细胞轻度水肿. 肺泡结构破坏, 部分区域内有肺泡间隔绷紧, 有多个肺泡的融合, 肺泡腔内见急性炎细胞及红细胞. 脾的组织形态基本正常.
结论: 本文首次对CCl4腹腔注射法制备肝硬化动物模型的重要脏器进行病理学观察, 证明该方法制备的肝硬化大鼠模型对主要脏器的影响小, 与人类发生肝硬化时重要脏器的病理改变基本一致, 因此, 再次说明该方法是一种理想的实验性肝硬化动物模型的制备方法.
核心提示: 四氯化碳腹腔注射法制备肝硬化动物模型中除肝脏以外的多个重要脏器的病理改变轻微, 与人类发生肝硬化时这些脏器的病理改变基本保持一致, 是一种良好的肝硬化动物模型制备方法, 值得在临床及实验研究中推广应用.
引文著录: 张云巍, 胡亚卓, 徐丽娟, 潘美妍, 阎丽. 四氯化碳法制备肝硬化大鼠模型中重要脏器的病理改变. 世界华人消化杂志 2014; 22(1): 74-79
Revised: November 11, 2013
Accepted: November 19, 2013
Published online: January 8, 2014
AIM: To observe the pathologic changes in major organs of rats with liver cirrhosis induced by injecting carbon tetrachloride (CCL4) intraperitoneally.
METHODS: Thirty SD rats were randomly divided into two groups, a model group (n = 22) and a control group (n = 8). Rats in the model group were intraperitoneally injected with CCl4 (3 μL/g) three times a week for 12 wk to induce hepatic cirrhosis (The formation of pseudolobuli as the criterion for successful induction of cirrhosis). The control group was injected intraperitoneally with the vehicle. All rats were fed ordinary forage and water, and weight changes, activities and the reaction to the surroundings were monitored during the experiment. Animal deaths and death dates were also recorded. At the end of the 12th week, 6 rats in the model group were dead and all rats in the control group survived. The rest rats in the model group were executed, and the samples of the liver, esophagus, colon, spleen, and lung were collected and subjected to HE staining. PAS staining for the renal samples was performed. The pathological changes in the above organs were observed by light microscopy.
RESULTS: Compared with the controls, typical pseudolobes were formed in the animal model of liver cirrhosis. Gastrointestinal tissues were infiltrated by few inflammatory cells. The renal tubular cells showed mild edema. The structure of pulmonary alveolus was damaged or fused with acute inflammatory cells and blood cells, accompanied with alveolar septum tightening in some regions. Other major organs were not affected.
CONCLUSION: It is the first time to observe the pathological changes in major organs of rats with liver cirrhosis induced by intraperitoneally administration of CCl4. Mild pathological changes occurred in the animal models, which are consistent with pathological changes observed in human liver cirrhosis.
- Citation: Zhang YW, Hu YZ, Xu LJ, Pan MY, Yan L. Pathological changes in major organs of rats with carbon tetrachloride-induced liver cirrhosis. Shijie Huaren Xiaohua Zazhi 2014; 22(1): 74-79
- URL: https://www.wjgnet.com/1009-3079/full/v22/i1/74.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v22.i1.74
肝硬化(liver cirrhosis)是临床常见的一种慢性进行性肝病, 由一种或多种病因长期或反复作用形成的弥漫性肝损害[1], 严重威胁患者的生命[2]. 病理组织学上可见广泛的肝细胞坏死、残存肝细胞结节性再生、结缔组织增生与纤维隔形成, 导致肝小叶结构破坏和假小叶形成, 肝脏逐渐变形、变硬而发展为肝硬化. 然而, 肝硬化发病机制至今尚不明确. 近年来, 在实验性肝硬化动物模型(cirrhosis model, CM)建立的基础[3]上, 该病的发病机制及治疗方面的研究均取得了可喜的成就[4-6]. 然而, 目前报道的CM还仅局限于肝脏的病理学改变情况, 缺乏其他重要脏器的病理组织学研究[7]. 本文在以往研究的基础上, 首次就四氯化碳(CCl4)制备肝硬化模型实验中除肝脏外其他主要脏器的病理变化进行了研究.
♂SD大鼠, 30只, 体质量180-220 g, 由北京维通利华实验动物技术有限公司提供. 按清洁级标准饲养, 普通颗粒饲料喂养. CCL4(分析纯)购自宁波市江东化学试剂厂(批号: 9406661). 橄榄油购自上海卫辉化学试剂厂(批号: 930112). TD型电子天平(余姚市金诺天平有限公司)由解放军总医院动物实验中心提供. 显微镜(Olympus)由解放军总医院老年医学研究所病理科提供.
大鼠自购进后, 于实验室中适应性饲养7 d, 不限食水. 按体质量随机分为正常对照组8只和肝硬化模型组22只. 对照组: 按3 L/g体质量给予橄榄油溶液腹腔注射, 3次/wk, 普通饲料加清水喂养; 模型组大鼠按3 L/g体质量给予600 mL/L CCl4橄榄油溶液经腹腔注射, 3次/wk. 监测实验期间大鼠体质量变化、活动及对外界的反应情况, 同时记录大鼠的死亡情况及死亡时间. 12 wk处死大鼠取材, 取肝、食管、结肠、脾、肺组织, 行HE染色, 肾脏组织行PAS染色, 光镜观察各组织形态改变.
对照组的大鼠毛发光泽, 精神状态好, 活动多, 食欲佳, 对外界刺激反应敏感. 模型组大鼠毛发凌乱, 无光泽, 食欲差, 精神萎靡, 活动少, 对外界刺激反应迟钝. 对照组大鼠体质量增长迅速, 模型组大鼠体质量增长缓慢, 尤以实验前期明显. 模型组大鼠6只死亡, 对照组全部存活.
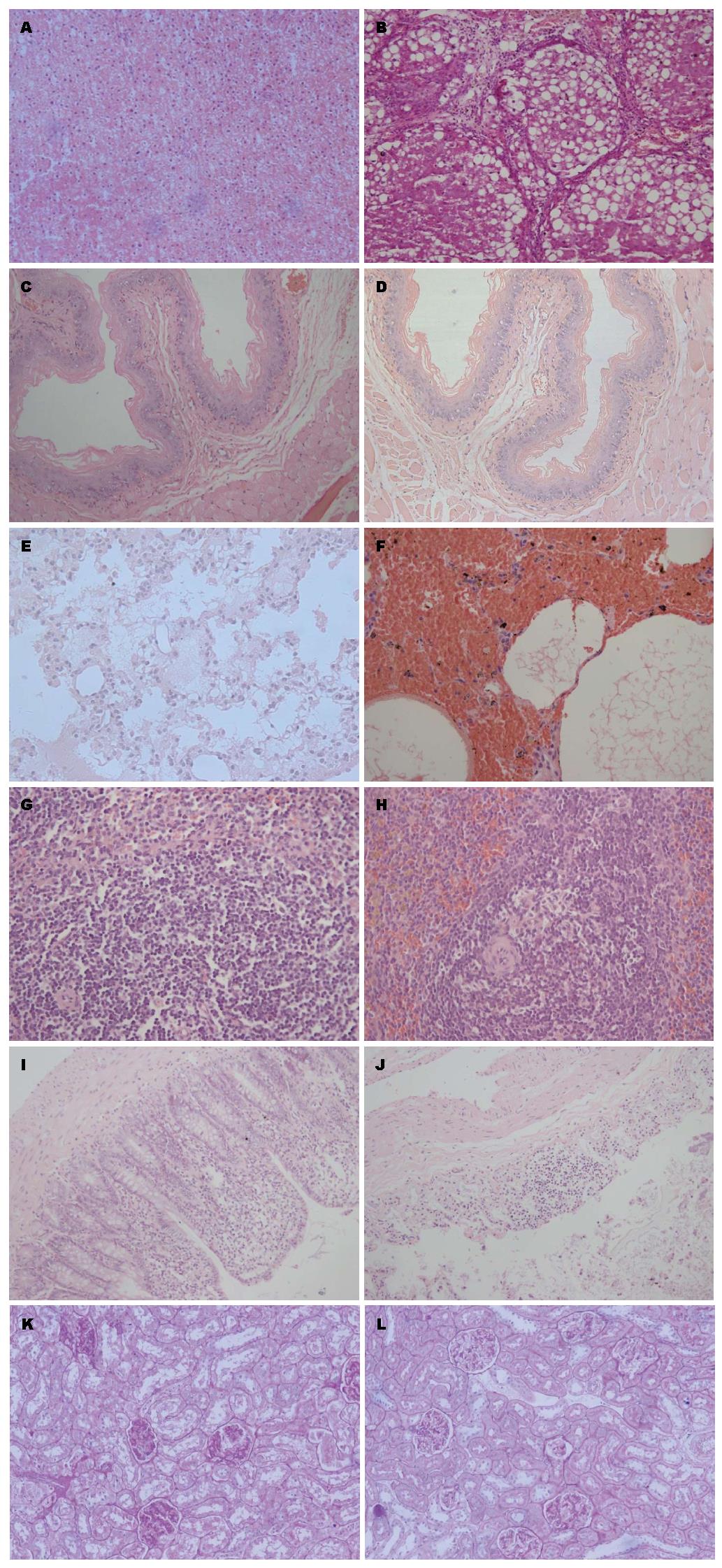
至造模第12周末取对照组及模型组的大鼠肝、食管、肺、脾、结肠组织行HE染色及肾脏行PAS染色(图1).
肝脏组织学改变: 对照组: 肝小叶结构正常(图1A), 中央静脉有放射状排列的肝细胞窦, 肝窦明显, 肝细胞体积较大, 核圆居中, 可见核仁, 胞浆丰富, 汇管区清晰可辨, 无明显的炎细胞浸润. 模型组: 肝组织内可见纤维增粗紊乱, 肝组织(图1B)的正常结构被破坏, 变得杂乱, 纤维束分割肝小叶, 破坏的肝小叶和部分增生的肝细胞团被较粗的纤维分割包绕, 有假小叶形成, 且汇管区出现典型的病变, 证明肝硬化造模成功.
食管组织学改变: 对照组食管壁(图1C)四层结构清晰, 上皮为复层扁平上皮, 结构正常. 模型组食管腔(图1D)可见角化不全的鳞状上皮, 黏膜下层见少许炎细胞浸润, 黏膜下层见扩张的脉管.
肺脏组织学改变: 对照组肺泡腔(图1E)内见轻度水肿, 间隔不宽, 上皮可见; 模型组肺泡(图1F)结构破坏, 部分区域内有肺泡间隔绷紧, 有多个肺泡的融合, 肺泡腔内见急性炎细胞及红细胞.
脾脏组织学改变: 模型组(图1H)相对于对照组(图1G), 血管内皮增厚明显, 血管内皮增生, 脾髓境界清楚, 可见含铁血黄素沉积.
结肠组织学改变: 模型组(图1J)与对照组(图1I)相比, 结肠黏膜层崩解, 肌层断裂, 有炎细胞浸润.
肾脏组织学改变: 对照组肾小球(图1K)结构正常, 无系膜增生及毛细血管纤维化, 肾小管为单层上皮性小管. 模型组肾小球(图1L)结构较为清晰, 无出现明显纤维化, 肾小管内皮细胞轮廓分明, 上皮细胞核浆比大体正常, 未见明显管型.
绝大部分肝硬化形成机制的研究及治疗药物的开发是建立在肝硬化动物模型的基础上, 制造CM的主要方法包括CCl4诱导性肝硬化模型、CCl4联合苯巴比妥肝硬化动物模型[8-10]、CCl4联合乙醇肝硬化动物模型[11,12]、化学致癌物诱导性肝硬化模型[13]、二甲基亚硝胺建立肝硬化模型[14-16]、酒精性肝硬化模型[17]、免疫性肝硬化动物模型[18]、胆总管结扎诱导性肝硬化模型[19]、病毒性肝硬化动物模型[20]、复合多因素诱导性肝硬化模型[21,22]等.
CCl4是CM常用的动物肝硬化造模药物, 具有成模率高、重复性好、方法简便易行等特点[23]. 其致病机制主要是被肝细胞微粒体细胞色素P450激活, 损害线粒体, 从而抑制脂肪酸氧化和三羧酸循环; 此外, 由于内质网受损可导致脂蛋白合成障碍, 使三酰甘油和脂肪酸在肝细胞内蓄积, 形成脂肪变性. 因此, 低浓度的CCl4反复应用, 可使大鼠的肝组织受到损害-修复-损害的循环破坏作用, 最终导致肝纤维化及肝硬化的发生[24-27]. 目前, CCl4制备CM的方法包括单因素CCl4法和多因素CCl4法. 单因素CCl4法诱导制备CM的主要途径包括皮下注射、腹腔注射、口服灌胃和蒸汽吸入等. 研究表明CCl4经皮下注射后, 迅速被机体吸收, 对脑、肾毒性较大, 注射位置易发生浸润性脓肿和溃疡, 死亡率可高达30%-40%. CCl4通过腹腔内注射, 经门静脉系统吸收, 使肝硬化形成时间短, 而较少的不良反应使死亡率降至20%-35%[28]; CCl4灌胃的吸收和代谢途径类似于腹腔注射, 且二者在生存曲线上并无特异差别, 但操作复杂, 肠道反应大; 蒸汽吸入呼吸道刺激大, 动物不耐受, 中枢毒性大, 易造成环境污染和实验人员伤害[29,30]. 多因素CCl4法: (1)CCl4加苯巴比妥. Krahenbuhl等[31]分别给鼠口服CCl4辅以苯巴比妥为唯一饮用水的方法建立肝纤维化动物模型, 结果显示肝脏表面可见结节形成, 实质变致密, 镜检可见在结节周围有少量纤维组织沉积, 但同时伴有脾大、慢性门脉高压表现; 死亡率高达40%-52.9%[32]; (2)CCl4加乙醇. 吴孟超等[33]报道在小鼠使用该方法60 d即可制成肝硬化模型, 肝硬化稳定, 分期明显, 动物死亡率低, 但停止刺激恢复较快.
本文首次对CCl4腹腔注射法制备肝硬化动物模型中除肝脏以外的多个重要脏器的病理改变进行探讨. 我们通过HE染色及PAS染色法, 观察了食管、胃、结肠以及肾脏等多个重要脏器在肝硬化发生过程中的病理改变. 结果提示上述重要器官在肝硬化建模过程中病理改变轻微, 与人类发生肝硬化时这些脏器的病理改变基本保持一致. 我们的研究结果再次表明采用CCl4经腹腔注射法制备大鼠肝硬化动物模型是一种良好的肝硬化动物模型制备方法, 值得在临床及实验研究中推广应用.
肝硬化是终末期肝病, 严重威胁患者的生命, 现已成为消化学界的研究热点, 而肝硬化动物模型是肝硬化的研究基础, 研究肝硬化的发生机制及其防治就必须建立良好的肝纤维化动物模型. 目前还没有与人类肝硬化完全相似的动物模型.
杨家和, 教授, 中国人民解放军第二军医大学附属东方肝胆外科医院综合治疗三科
目前制备肝硬化动物模型的方法较多, 从单因素发展到多因素, 甚至复合因素, 但是与理想的动物肝硬化模型(与人类肝硬化、门脉高压的特征相同、造模成功率高、病死率低、周期短、方法简便易行、所用造模因素对人体无害或较少危害)有较大的差距.
Wang等就CCl4致病机制做了详尽的报道, 主要是被肝细胞微粒体细胞色素P450激活; Krahenbuhl和吴孟超分别成功的用CCl4加苯巴比妥和CCl4加乙醇建造肝硬化动物模型, 造模时间比单独应用CCl4短, 但死亡率高, 操作复杂.
本文首次对CCl4腹腔注射法制备肝硬化动物模型的重要脏器进行病理学观察, 证明该方法制备的肝硬化大鼠模型对主要脏器的影响小, 与人类发生肝硬化时重要脏器的病理改变基本一致, 因此, 再次说明该方法是一种理想的实验性肝硬化动物模型的制备方法.
通过对CCl4腹腔注射法制备肝硬化动物模型的重要脏器进行病理学观察, 该法与理想肝硬化动物模型中的肝脏外脏器损伤小较接近, 成功率高, 操作简单易行, 重复性好, 较适合肝硬化研究的动物造模.
本文立意新颖, 另辟蹊径证明了传统方法的优越性.
编辑: 田滢 电编:鲁亚静
| 1. | Goldacre MJ, Wotton CJ, Yeates D, Seagroatt V, Collier J. Liver cirrhosis, other liver diseases, pancreatitis and subsequent cancer: record linkage study. Eur J Gastroenterol Hepatol. 2008;20:384-392. [PubMed] [DOI] |
| 2. | Durand F, Valla D. Assessment of prognosis of cirrhosis. Semin Liver Dis. 2008;28:110-122. [PubMed] [DOI] |
| 3. | Steib CJ, Hennenberg M, Beitinger F, Hartmann AC, Bystron M, De Toni EN, Gerbes AL. Amiloride reduces portal hypertension in rat liver cirrhosis. Gut. 2010;59:827-836. [PubMed] [DOI] |
| 4. | Singh M, Kumar V. Transgenic mouse models of hepatitis B virus-associated hepatocellular carcinoma. Rev Med Virol. 2003;13:243-253. [PubMed] |
| 5. | Rall GF, Lawrence DM, Patterson CE. The application of transgenic and knockout mouse technology for the study of viral pathogenesis. Virology. 2000;271:220-226. [PubMed] |
| 7. | Chang ML, Yeh CT, Chang PY, Chen JC. Comparison of murine cirrhosis models induced by hepatotoxin administration and common bile duct ligation. World J Gastroenterol. 2005;11:4167-4172. [PubMed] |
| 10. | Zhang JJ, Meng XK, Dong C, Qiao JL, Zhang RF, Yue GQ, Zhong HY. Development of a new animal model of liver cirrhosis in swine. Eur Surg Res. 2009;42:35-39. [PubMed] [DOI] |
| 13. | Kitamura K, Nakamoto Y, Akiyama M, Fujii C, Kondo T, Kobayashi K, Kaneko S, Mukaida N. Pathogenic roles of tumor necrosis factor receptor p55-mediated signals in dimethylnitrosamine-induced murine liver fibrosis. Lab Invest. 2002;82:571-583. [PubMed] |
| 14. | Vendemiale G, Grattagliano I, Caruso ML, Serviddio G, Valentini AM, Pirrelli M, Altomare E. Increased oxidative stress in dimethylnitrosamine-induced liver fibrosis in the rat: effect of N-acetylcysteine and interferon-alpha. Toxicol Appl Pharmacol. 2001;175:130-139. [PubMed] |
| 15. | Spahr L, Burkhard PR, Grötzsch H, Hadengue A. Clinical significance of basal ganglia alterations at brain MRI and 1H MRS in cirrhosis and role in the pathogenesis of hepatic encephalopathy. Metab Brain Dis. 2002;17:399-413. [PubMed] |
| 16. | George J, Rao KR, Stern R, Chandrakasan G. Dimethylnitrosamine-induced liver injury in rats: the early deposition of collagen. Toxicology. 2001;156:129-138. [PubMed] |
| 17. | Lieber CS. Relationships between nutrition, alcohol use, and liver disease. Alcohol Res Health. 2003;27:220-231. [PubMed] |
| 18. | Herkel J, Schuchmann M, Tiegs G, Lohse AW. Immune-mediated liver injury. J Hepatol. 2005;42:920-923. [PubMed] |
| 19. | García-Ayllón MS, Silveyra MX, Candela A, Compañ A, Clària J, Jover R, Pérez-Mateo M, Felipo V, Martínez S, Galcerán J. Changes in liver and plasma acetylcholinesterase in rats with cirrhosis induced by bile duct ligation. Hepatology. 2006;43:444-453. [PubMed] |
| 20. | Cote PJ, Korba BE, Miller RH, Jacob JR, Baldwin BH, Hornbuckle WE, Purcell RH, Tennant BC, Gerin JL. Effects of age and viral determinants on chronicity as an outcome of experimental woodchuck hepatitis virus infection. Hepatology. 2000;31:190-200. [PubMed] |
| 23. | Laviña B, Gracia-Sancho J, Rodríguez-Vilarrupla A, Chu Y, Heistad DD, Bosch J, García-Pagán JC. Superoxide dismutase gene transfer reduces portal pressure in CCl4 cirrhotic rats with portal hypertension. Gut. 2009;58:118-125. [PubMed] [DOI] |
| 24. | Wang L, Potter JJ, Rennie-Tankersley L, Novitskiy G, Sipes J, Mezey E. Effects of retinoic acid on the development of liver fibrosis produced by carbon tetrachloride in mice. Biochim Biophys Acta. 2007;1772:66-71. [PubMed] |
| 25. | Chae HB, Jang LC, Park SM, Son BR, Sung R, Choi JW. [An experimental model of hepatic fibrosis induced by alcohol and CCl4: can the lipopolysaccharide prevent liver injury induced by alcohol and CCl4?]. Taehan Kan Hakhoe Chi. 2002;8:173-178. [PubMed] |
| 26. | Boll M, Weber LW, Becker E, Stampfl A. Pathogenesis of carbon tetrachloride-induced hepatocyte injury bioactivation of CCI4 by cytochrome P450 and effects on lipid homeostasis. Z Naturforsch C. 2001;56:111-121. [PubMed] |
| 27. | Jeong WI, Lee CS, Park SJ, Chung JY, Jeong KS. Kinetics of macrophages, myofibroblasts and mast cells in carbon tetrachloride-induced rat liver cirrhosis. Anticancer Res. 2002;22:869-877. [PubMed] |
| 28. | Henkel C, Roderfeld M, Weiskirchen R, Berres ML, Hillebrandt S, Lammert F, Meyer HE, Stühler K, Graf J, Roeb E. Changes of the hepatic proteome in murine models for toxically induced fibrogenesis and sclerosing cholangitis. Proteomics. 2006;6:6538-6548. [PubMed] |
| 30. | Ugazio G, Bosia S, Cornaglia E. Experimental model of cirrhosis in rabbits exposed to carbon tetrachloride by inhalation. Res Commun Mol Pathol Pharmacol. 1995;88:63-77. [PubMed] |
| 31. | Krahenbuhl S, Weber FL, Brass EP. Decreased hepatic glycogen content and accelerated response to starvation in rats with carbon tetrachloride-induced cirrhosis. Hepatology. 1991;14:1189-1195. [PubMed] |
| 32. | Proctor E, Chatamra K. High yield micronodular cirrhosis in the rat. Gastroenterology. 1982;83:1183-1190. [PubMed] |