修回日期: 2013-09-18
接受日期: 2013-09-30
在线出版日期: 2013-11-08
目的: 探讨银杏叶提取物(ginkgo biloba extract, EGb761)对顺铂或足叶乙甙诱导的胃癌细胞SGC-7901的增殖和凋亡的影响及其机制.
方法: EGb761、顺铂和足叶乙甙单用或者顺铂、足叶乙甙联合应用EGb761处理人胃癌细胞株SGC-7901, 采用四甲基偶氮唑蓝(methyl thiazolyl tetrazolium, MTT)法检测细胞的增殖活性, 流式细胞仪检测细胞的凋亡, 化学比色法检测细胞中过氧化物歧化酶(superoxide dismutase, SOD)、谷胱甘肽过氧化物酶(glutathione peroxidase, GSH-Px)和过氧化氢酶(catalase, CAT)的活性及丙二醛(malondialdehyde, MDA)的含量, 免疫印迹法检测细胞外信号调节激酶(extracellular signal regulated kinase, ERK)、磷酸化细胞外信号调节激酶(phosphorylation of ERK, p-ERK)和核因子-κB(nuclear factor kappa B, NF-κB)p65的表达.
结果: EGb761、顺铂、足叶乙甙对胃癌细胞的增殖均具有抑制作用, 呈时间剂量依赖性, 并明显诱导细胞的凋亡. EGb761可明显增强顺铂或足叶乙甙的细胞生长抑制作用并提高细胞的凋亡水平. EGb761能显著提高细胞中SOD、CAT和GSH-Px的活性[对照组和EGB761组SOD活性: 16.57 U/mg prot±3.20 U/mg prot vs 25.96 U/mg prot±3.57 U/mg prot; CAT的活性: 2.51 U/mg prot±0.32 U/mg prot vs 3.79 U/mg prot±0.55 U/mg prot; GSH-Px的活性: 22.18 µmol/(min·mg) prot±4.36 µmol/(min·mg) prot vs 33.49 µmol/(min·mg) prot±5.64 µmol/(min·mg) prot; 均P<0.05], 降低MDA的含量(对照组和EGB761组MDA的含量2.46 nmol/mg prot±0.38 nmol/mg prot vs 1.42 nmol/mg prot±0.26 nmol/mg prot, P<0.05), 同时能明显抑制由顺铂和足叶乙甙诱导的ERK、p-ERK和NF-κBp65的表达(对照组、顺铂组、EGB761+顺铂组、足叶乙甙组和EGB761+足叶乙甙组ERK的表达: 0.496±0.078, 0.831±0.091, 0.521±0.082, 0.816±0.101, 0.489±0.072; p-ERK的表达: 0.289±0.032, 0.521±0.068, 0.276±0.049, 0.486±0.087, 0.298±0.053; NF-κBp65的表达: 0.268±0.038, 0.456±0.08, 0.276±0.052, 0.446±0.076, 0.229±0.056).
结论: EGb761可增强顺铂或足叶乙甙对胃癌细胞生长抑制作用并提高细胞的凋亡水平. EGb761可能是通过增强细胞抗氧化能力, 下调ERK、p-ERK和NF-κBp65的表达而发挥作用.
核心提示: 银杏叶提取物(Ginkgo biloba extract, EGb761)可增强顺铂或足叶乙甙对胃癌细胞生长抑制作用并提高细胞的凋亡水平. EGb761可能是通过增强细胞抗氧化能力, 下调细胞外信号调节激酶(extracellular signal regulated kinase)、磷酸化细胞外信号调节激酶(phosphorylation of ERK)和核因子-κB(nuclear factor kappa B)p65的表达而发挥作用.
引文著录: 毛业波, 刘诗权, 谭林, 周巧, 黄杰安. EGb761对顺铂和足叶乙甙诱导的胃癌SGC-7901细胞凋亡的增敏作用. 世界华人消化杂志 2013; 21(31): 3330-3337
Revised: September 18, 2013
Accepted: September 30, 2013
Published online: November 8, 2013
AIM: To assess the effect of Ginkgo biloba extract (EGb761) combined with cisplatin or etoposide on cell proliferation and apoptosis in human gastric cancer cell line SGC-7901 and to explore the possible mechanisms involved.
METHODS: SGC-7901 cells were treated with EGb761, cisplatin, etoposide, or EGb761 combined with cisplatin or etoposide. Cell viability was measured by MTT assay, and apoptosis was measured by flow cytometry. The colorimetric method was used to detect the activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT) and the content of malondialdehyde (MDA) in cells. The protein expression of extracellular signal-regulated kinase1/2 (ERK1/2), p-ERK1/2 and nuclear transcription factor-kappa B (NF-κB) p65 was determined by Western blot.
RESULTS: Monotherapy with each of EGb761, cisplatin and etoposide significantly inhibited the growth of SGC-790l cells in a dose- and time-dependent manner. EGb761 significantly enhanced the inhibitory effect of cisplatin and etoposide on cell growth. Cells treated with EGb761 combined either cisplatin or EGb761 showed a significantly higher level of apoptosis than those treated with cisplatin or etoposide alone. Compared to the control group, the activities of SOD, GSH-Px and CAT were notably elevated (SOD: 16.57 U/mg prot ± 3.20 U/mg prot vs 25.96 U/mg prot ± 3.57 U/mg prot; CAT: 2.51 U/mg prot ± 0.32 U/mg prot vs 3.79 U/mg prot ± 0.55 U/mg prot; GSH-Px: 22.18 µmol/(min•mg) prot ± 4.36 µmol/(min•mg) prot vs 33.49 µmol/(min•mg) prot ± 5.64 µmol/(min•mg) prot; all P < 0.05) and the content of MDA was significantly decreased (2.46 nmol/mg prot ± 0.38 nmol/mg prot vs 1.42 nmol/mg prot ± 0.26 nmol/mg prot, P < 0.05) in cells treated with EGb761. The expression of ERK1/2, p-ERK1/2 and NF-κBp65 was significantly induced by cisplatin or etoposide, while EGb761 suppressed the expression of ERK1/2, p-ERK1/2 and NF-κBp65 induced by cisplatin or etoposide. The expression levels of ERK1/2, p-ERK1/2 and NF-κBp65 in the control group, cisplatin group, EGB761 + cisplatin group, etoposide group and EGB761 + etoposide group were as follows: ERK1/2: 0.496 ± 0.078, 0.831 ± 0.091, 0.521 ± 0.082, 0.816 ± 0.101, 0.489 ± 0.072; p-ERK1/2: 0.289 ± 0.032, 0.521 ± 0.068, 0.276 ± 0.049, 0.486 ± 0.087, 0.298 ± 0.053; NF-κBp65: 0.268 ± 0.038, 0.456 ± 0.08, 0.276 ± 0.052, 0.446 ± 0.076, 0.229 ± 0.056).
CONCLUSION: EGb761 enhances cisplatin- and etoposide-induced apoptosis of SGC-7901 cells possibly by enhancing cellular antioxidant capacity and suppressing the up-regulation of ERK, p-ERK and NF-κBp65 protein expression.
- Citation: Mao YB, Liu SQ, Tan L, Zhou Q, Huang JA. EGb761 enhances cisplatin- and etoposide-induced apoptosis of human gastric cancer SGC-7901 cells. Shijie Huaren Xiaohua Zazhi 2013; 21(31): 3330-3337
- URL: https://www.wjgnet.com/1009-3079/full/v21/i31/3330.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v21.i31.3330
化疗作为治疗肿瘤的手段之一在胃癌的综合治疗中有重要的地位, 但多药耐药现象的普遍存在使化疗治疗面临着极大的困难. 所以降低化疗耐药发生率, 提高化疗效果成为了胃癌治疗中急需解决的问题. 最近有研究显示银杏叶提取物(extract of Ginkgo biloba, EGb)可增强大鼠的抗氧化能力, 并阻止胃癌癌前病变的进展[1], 而且银杏叶类黄酮能明显抑制人胃癌细胞的增殖, 并诱导细胞凋亡[2]. 然而, EGb对胃癌化疗敏感性的影响还不明了. 本研究在体外观察EGb761对顺铂和足叶乙甙诱导的胃癌细胞增殖和凋亡的影响, 同时检测EGb761对胃癌细胞抗氧化能力以及细胞外信号调节激酶(extracellular signal-regulated kinase, ERK)、磷酸化细胞外信号调节激酶(phosphorylation of ERK, p-ERK)和核因子-κB(nuclear factor kappa B, NF-κB)p65表达的影响, 探讨EGb761对胃癌细胞化疗敏感性的影响及其机制.
EGb761购自威玛舒培博士药厂; 人胃癌细胞株SGC-7901购自中国科学院上海细胞生物所; 胎牛血清、DMEM(高糖型)均购自Hyclone公司; 四氮唑蓝(MTT)购自北京索莱宝科技有限公司; 膜联蛋白V-异硫氰酸荧光素(Annexin V-fluorescein isothiocyanate, Annexin V-FITC)细胞凋亡检测试剂盒购自Roche公司; 兔抗人ERK1/2、p-ERK多克隆抗体购自Cell Signaling Technology公司; 兔抗人NF-κBp65多克隆抗体、辣根过氧化酶标记山羊抗兔均购自Santa Cruz公司; 氧化物歧化酶(superoxide dismutase, SOD)、谷胱甘肽过氧化物酶(glutathione peroxidase, GSH-Px)和过氧化氢酶(catalase, CAT)的活性及丙二醛(malondialdehyde, MDA)检测试剂盒购自南京建成公司.
1.2.1 MTT法检测细胞的增殖: 取对数生长期的SGC-7901细胞, 以5×104个/mL接种于96孔板, 每孔200 μL, 每孔设5个复孔, 培养12 h后, 单药处理: 分别单独加入不同终浓度的EGb761、顺铂或足叶乙甙, EGb761浓度为80、160、320、640和1280 μg/mL, 顺铂浓度为0.5、1、2、4和8 μg/mL, 足叶乙甙浓度为5、10、20、40和80 μg/mL, 处理12、24和48 h, 并设不加药物的空白对照. 联合处理: 经0.5、1、2、4、8 μg/mL顺铂或者 5、10、20、40、80 μg/mL足叶乙甙联合320 μg/mL的EGb761处理24 h. 处理后加入20 μL浓度为5 mg/mL的MTT, 继续培养4 h, 弃上清. 每孔加入二甲基亚砜150 μL, 振荡10 min. 在酶标仪490 nm波长处检测各孔的吸光值(A值), 计算细胞生存率. 细胞生存率 = 加药孔A值/对照空A值×100%.
1.2.2 细胞凋亡的检测: 将SGC-7901细胞分为对照组、EGb761组(320 μg/mL)、顺铂组(2 μg/mL)、足叶乙甙组(10 μg/mL)、EGb761+顺铂组(320 μg/mL EGb761+2 μg/mL顺铂)和EGb761+足叶乙甙组(320 μg/mL EGb761+10 μg/mL足叶乙甙), 处理24 h, 收集各组细胞调整细胞数为1×106个/mL, 用冷PBS洗涤细胞2次. 加入结合缓冲液100 μL、FITC 2 μL和Annexin-V 2 μL, 室温避光15 min, 进行流式细胞仪分析.
1.2.3 检测细胞中SOD、CAT、GSH-Px的活性及MDA的含量: 收集细胞, 将细胞悬浮于PBS中, 用玻璃匀浆管在冰水浴条件, 手动匀浆, 取破碎好的匀浆液进行测定并按照说明书进行操作.
1.2.4 Western blot法检测细胞中ERK、p-ERK和NF-kBp65蛋白的表达: 收集细胞, 提取总蛋白, 行聚丙烯酰胺凝胶电泳并转膜, 脱脂奶粉进行封闭, 加一抗4 ℃振荡孵育过夜, 再用辣根过氧化物酶标记的二抗37 ℃孵育1 h, 采用增强化学发光盒检测杂交信号, X线医学胶片上曝光显影, 成像系统拍照, 图像分析软件测量A值, 以磷酸甘油醛脱氢酶(glyceraldehyde phosphate dehydrogenase, GAPDH)为内参, 蛋白相对表达强度 = 目的蛋白A值/GAPDH A值.
统计学处理 采用SPSS16.0统计软件进行数据分析. 所有实验均重复3次, 数据以mean±SD差表示. 两个均数比较采用ANOVA检验, P<0.05为差异具有统计学意义.
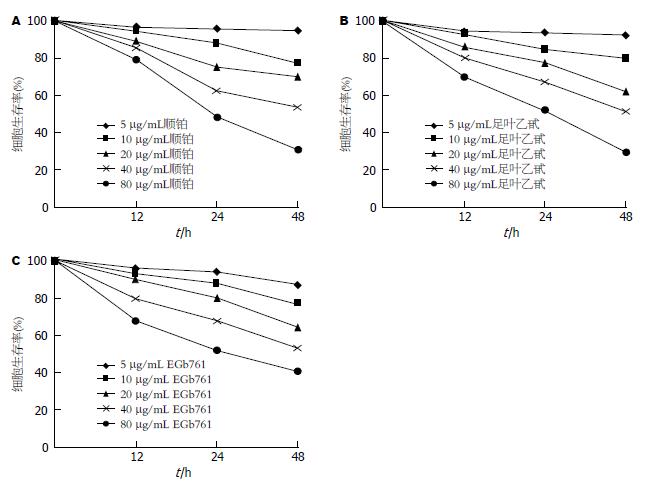
EGb761、顺铂或足叶乙甙对胃癌SGC-7901细胞均具有抑制作用, 并呈时间剂量依赖性(图1).
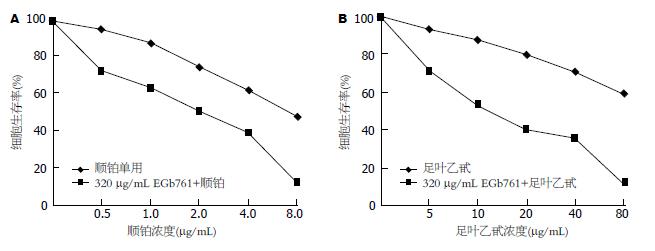
采用320 μg/mL EGb761与不同浓度的顺铂或足叶乙甙联合处理细胞24 h后, 细胞生长抑制作用较顺铂或足叶乙甙单用时明显增强, 与单独作用时差异具有统计学意义(P<0.01, 图2). 由此可见, 顺铂或者足叶乙甙联合应用EGb761能进一步抑制胃癌细胞的生长.
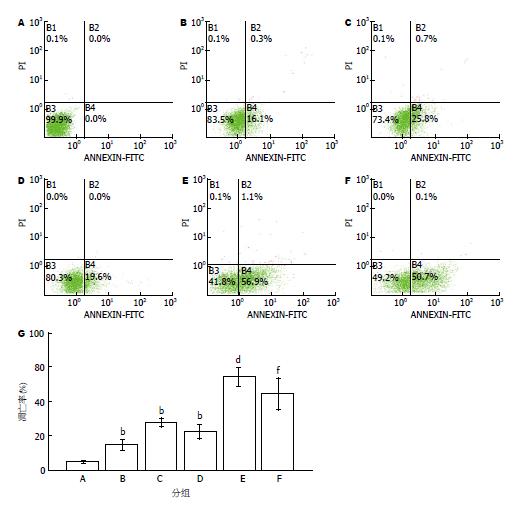
与对照组比较, EGb761组、顺铂组和足叶乙甙组明显抑制细胞凋亡(P<0.01), 而EGb761联合顺铂组和EGb761联合足叶乙甙组凋亡率均显著高于单用药组(P<0.01, 图3).
与对照组比较, 顺铂组和足叶乙甙组SOD、GSH-Px、CAT的活性和MDA含量改变无显著性, 差异无统计学意义(P>0.05), 而EGb761组、EGb761+顺铂组、EGb761+足叶乙甙组SOD、GSH-Px和CAT的活性均明显升高, 而MDA的含量则显著减少, 差异有统计学意义(P<0.05). EGb761+顺铂组与单用顺铂组比较, EGb761+足叶乙甙组与单用足叶乙甙组比较, SOD、 GSH-Px和CAT的活性均明显升高而MDA的含量显著减少, 差异具有统计学意义(P<0.05, 表1).
| 分组 | SOD(U/mg prot) | CAT(U/mg prot) | GSH-Px[mmol/(min•mg) prot] | MDA(nmol/mg prot) |
| 对照组 | 16.57±3.20 | 2.51±0.32 | 22.18±4.36 | 2.46±0.38 |
| EGb761组 | 25.96±3.57a | 3.79±0.55a | 33.49±5.64a | 1.42±0.26a |
| 顺铂组 | 17.36±3.13 | 2.56±0.37 | 23.98±3.35 | 2.27±0.39 |
| 足叶乙甙组 | 16.23±2.79 | 2.61±0.48 | 22.87±4.34 | 2.33±0.45 |
| EGb761+顺铂组 | 27.35±4.84ac | 3.91±0.59ac | 34.68±6.56ac | 1.39±0.25ac |
| EGb761+足叶乙甙组 | 26.40±4.27ae | 3.84±0.62ae | 35.33±5.90ae | 1.40±0.23ae |
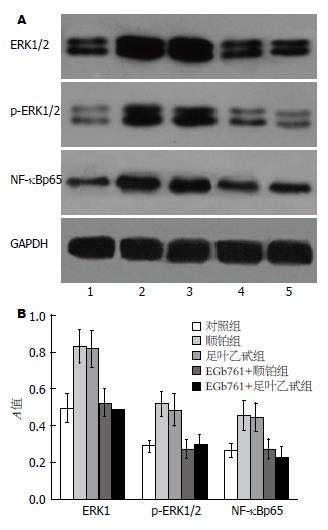
SGC-7901细胞中存在一定基础的ERK和磷酸化ERK和NF-κBp65蛋白表达, 顺铂或足叶乙甙作用后, ERK、p-ERK和NF-κBp65表达较对照组显著增强, 而EGb761联合顺铂或者足叶乙甙, 能够显著抑制ERK和磷酸化ERK以及NF-κBp65的表达(P<0.05, 图4).
我国是胃癌高发区, 早期胃癌诊断困难, 大部分患者就诊时已是届期, 需采取化疗为主的综合治疗, 然而胃癌细胞易对化疗药产生耐药性, 严重制约了化疗药物的应用. 因此, 寻找一种高效低毒的化疗方案是目前研究的焦点[3]. 银杏叶在我国用于入药已有数千年历史, EGb的主要成分是黄酮类化合物和萜烯. 目前国际上将银杏叶提取物标准品命名为EGb761, 其中银杏黄酮≥24%, 槲皮素与山奈酚峰比在0.8-1.5之间, 银杏总内酯≥6%, 银杏酸<5 ppm. EGb多用于治疗脑供血不足[4]、老年人轻度认知障碍[5]、Parkinson's病[6]以及痴呆[7]等多因素所致的疾病. 近年来的许多基础及临床研究发现EGb对多种肿瘤具有抑制作用, EGb761可以有效抑制白血病细胞的增殖活性[8], 诱导口腔癌细胞[9]、人乳腺癌细胞[10,11]、神经胶质瘤和肝癌细胞的凋亡[12,13], 能够诱导结肠癌周期G0/G1的停滞和细胞凋亡[14], EGb可降低阿霉素的心脏不良反应, 可用于辅助化疗[15]. 本研究结果提示, EGb761和顺铂或足叶乙甙联合应用时, 细胞生存率明显降低且凋亡率显著增高, 表明EGb761能增强胃癌细胞的化疗敏感性.
研究表明EGb761能清除氧自由基, 减轻脂质过氧化, 增加抗氧化酶活性, 是较强的自由基清除剂[16], 而由自由基引起的组织损伤和细胞结构的破坏在肿瘤的发生进程中起着重要作用[17]. 氧自由基的活化能够引起胃非黏液性瘤癌变, 而极低的抗氧化能力可导致胃黏液腺瘤癌变[18]. 实验结果显示EGb761能明显提高SOD、GSH-Px 和CAT的活性并降低MDA的含量, 显示EGb761可能是通过抗氧化应激增强胃癌化疗敏感性.
活性氧(reactive oxygen species, ROS)是机体正常代谢不断产生的产物, 正常情况下由于自由基清除酶的存在, 不会造成细胞损伤, 但许多癌细胞比正常细胞新陈代谢快, 这通常导致ROS数量显著增加, 可诱导DNA损伤[19]. 氧自由基造成的DNA损伤被认为是肿瘤发生和发展的重要原因[17]. ROS的产生超过细胞的防护系统, 一些信号蛋白激酶和转录调节因子被激活, 包括ERK[20,21]和NF-κB[22,23]. NF-κB和ERK虽然是不同的信号转导通路, 但Kefaloyianni等[24]证实氧化应激是两条途径的交叉应答, ERK信号级联反应激活后由胞质转位入胞核, 作用于核转录因子NF-κB等调控基因表达. 我们以前研究表明NF-κB和MAPK通路参与调节细胞的增殖、分化以及维持细胞形态、凋亡等多种生理功能, 并且MAPK/ERK和NF-κB通路的活化与肿瘤的发生发展、肿瘤细胞的侵袭转移密切相关[25,26]. 此外, 我们以前的研究也发现EGb不但可以阻断氧化应急, 还可以抑制NF-κB通路的活化[27,28], 而化疗药可诱导胃癌细胞MAPK/ERK和NF-κB信号通路的异常激活而降低胃癌细胞的化疗敏感性[29,30]. 本实验显示EGb761能增强胃癌细胞的抗氧化能力, 有效抑制ERK、p-ERK和NF-κB的表达. 表明EGb761可能是通过增强胃癌细胞的抗氧化能力, 进而抑制ERK和NF-κB通路的活化, 从而提高胃癌细胞化疗敏感性.
我国是胃癌高发区, 早期胃癌诊断困难, 大部分患者就诊时已是届期, 需采取化疗为主的综合治疗, 然而胃癌细胞易对化疗药产生耐药性, 严重制约了化疗药物的应用. 因此, 寻找一种高效低毒的化疗方案是目前研究焦点.
程英升, 教授, 上海交通大学附属第六人民医院放射科
银杏叶提取物能清除氧自由基, 减轻脂质过氧化, 增加抗氧化酶活性, 是较强的自由基清除剂, 研究表明, 银杏叶提取物对胃癌、口腔癌、乳腺癌、 神经胶质瘤、肝癌和结肠癌等多种癌症具有抑制作用.
NF-κB和MAPK通路参与调节细胞的增殖、分化以及维持细胞形态、凋亡等多种生理功能, 化疗药可诱导胃癌细胞MAPK/ERK和NF-κB信号通路的异常激活而降低胃癌细胞的化疗敏感性.
本文从MAPK信号通路中ERK和p-ERK以及NF-κBp65的表达变化, 来观察EGB761对顺铂和足叶乙甙诱导的胃癌细胞增殖及凋亡的作用, 并探讨其可能的分子机制.
增强细胞抗氧化能力, 下调ERK、p-ERK和NF-κBp65的表达, 可能是EGb761对顺铂和足叶乙甙诱导的胃癌细胞凋亡的增敏作用的机制之一, 本研究有助于了解EGB761对胃癌化疗效果的影响, 为提高胃癌化疗敏感性提供新思路.
银杏叶提取物EGb可增强大鼠的抗氧化能力, 并阻止胃癌癌前病变的进展, 而且银杏叶类黄酮能明显抑制人胃癌细胞的增殖, 并诱导细胞凋亡. EGb761能增强胃癌细胞的抗氧化能力, 有效抑制ERK、p-ERK和NF-κB的表达. 表明EGb761可能是通过增强胃癌细胞的抗氧化能力, 进而抑制ERK和NF-κB通路的活化, 从而提高胃癌细胞化疗敏感性, 具有一定的指导意义.
编辑: 郭鹏 电编:鲁亚静
| 1. | Jiang XY, Qian LP, Zheng XJ, Xia YY, Jiang YB, Sun da Y. Interventional effect of Ginkgo biloba extract on the progression of gastric precancerous lesions in rats. J Dig Dis. 2009;10:293-299. [PubMed] [DOI] |
| 3. | Kasibhatla S, Tseng B. Why target apoptosis in cancer treatment? Mol Cancer Ther. 2003;2:573-580. [PubMed] |
| 4. | Vilar JB, Leite KR, Chen Chen L. Antimutagenicity protection of Ginkgo biloba extract (Egb 761) against mitomycin C and cyclophosphamide in mouse bone marrow. Genet Mol Res. 2009;8:328-333. [PubMed] |
| 5. | Maclennan KM, Darlington CL, Smith PF. The CNS effects of Ginkgo biloba extracts and ginkgolide B. Prog Neurobiol. 2002;67:235-257. [PubMed] [DOI] |
| 6. | El-Ghazaly MA, Sadik NA, Rashed ER, Abd El-Fattah AA. Neuroprotective effect of EGb761(R) and low-dose whole-body γ-irradiationin a rat model of Parkinson's disease. Toxicol Ind Health. 2013; May 21. [Epub ahead of print]. [PubMed] [DOI] |
| 7. | Rainer M, Mucke H, Schlaefke S. Ginkgo biloba extract EGb 761 in the treatment of dementia: a pharmacoeconomic analysis of the Austrian setting. Wien Klin Wochenschr. 2013;125:8-15. [PubMed] [DOI] |
| 8. | Feng X, Zhang L, Zhu H. Comparative anticancer and antioxidant activities of different ingredients of Ginkgo biloba extract (EGb 761). Planta Med. 2009;75:792-796. [PubMed] [DOI] |
| 9. | Kang JW, Kim JH, Song K, Kim SH, Yoon JH, Kim KS. Kaempferol and quercetin, components of Ginkgo biloba extract (EGb 761), induce caspase-3-dependent apoptosis in oral cavity cancer cells. Phytother Res. 2010;24 Suppl 1:S77-S82. [PubMed] [DOI] |
| 10. | Yi SY, Nan KJ, Chen SJ. [Effect of extract of Ginkgo biloba on doxorubicin-associated cardiotoxicity in patients with breast cancer]. Zhongguo Zhongxi yi Jiehe Zazhi. 2008;28:68-70. [PubMed] |
| 11. | Park YJ, Kim MJ, Kim HR, Yi MS, Chung KH, Oh SM. Chemopreventive effects of Ginkgo biloba extract in estrogen-negative human breast cancer cells. Arch Pharm Res. 2013;36:102-108. [PubMed] [DOI] |
| 12. | Pretner E, Amri H, Li W, Brown R, Lin CS, Makariou E, Defeudis FV, Drieu K, Papadopoulos V. Cancer-related overexpression of the peripheral-type benzodiazepine receptor and cytostatic anticancer effects of Ginkgo biloba extract (EGb 761). Anticancer Res. 2006;26:9-22. [PubMed] |
| 13. | Li W, Pretner E, Shen L, Drieu K, Papadopoulos V. Common gene targets of Ginkgo biloba extract (EGb 761) in human tumor cells: relation to cell growth. Cell Mol Biol (Noisy-le-grand). 2002;48:655-662. [PubMed] |
| 14. | Chen XH, Miao YX, Wang XJ, Yu Z, Geng MY, Han YT, Wang LX. Effects of Ginkgo biloba extract EGb761 on human colon adenocarcinoma cells. Cell. Physiol Biochem. 2011;27:227-232. [PubMed] [DOI] |
| 15. | Liu TJ, Yeh YC, Ting CT, Lee WL, Wang LC, Lee HW, Wang KY, Lai HC, Lai HC. Ginkgo biloba extract 761 reduces doxorubicin-induced apoptotic damage in rat hearts and neonatal cardiomyocytes. Cardiovasc Res. 2008;80:227-235. [PubMed] [DOI] |
| 16. | Mahadevan S, Park Y. Multifaceted therapeutic benefits of Ginkgo biloba L.: chemistry, efficacy, safety, and uses. J Food Sci. 2008;73:R14-R19. [PubMed] [DOI] |
| 17. | Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37-56. [PubMed] |
| 18. | Wang SH, Wang YZ, Zhang KY, Shen JH, Zhou HQ, Qiu XY. Effect of superoxide dismutase and malondialdehyde metabolic changes on carcinogenesis of gastric carcinoma. World J Gastroenterol. 2005;11:4305-4310. [PubMed] |
| 19. | Scott TL, Rangaswamy S, Wicker CA, Izumi T. Repair of oxidative DNA damage and cancer: recent progress in DNA base excision repair. Antioxid Redox Signal. 2013; Oct 15. [Epub ahead of print]. [PubMed] [DOI] |
| 20. | Xiao H, Wang J, Yuan L, Xiao C, Wang Y, Liu X. Chicoric acid induces apoptosis in 3T3-L1 preadipocytes through ROS-mediated PI3K/Akt and MAPK signaling pathways. J Agric Food Chem. 2013;61:1509-1520. [PubMed] [DOI] |
| 21. | Conde de la Rosa L, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PL, Moshage H. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. J Hepatol. 2006;44:918-929. [PubMed] [DOI] |
| 22. | Sfikas A, Batsi C, Tselikou E, Vartholomatos G, Monokrousos N, Pappas P, Christoforidis S, Tzavaras T, Kanavaros P, Gorgoulis VG. The canonical NF-κB pathway differentially protects normal and human tumor cells from ROS-induced DNA damage. Cell Signal. 2012;24:2007-2023. [PubMed] [DOI] |
| 23. | Ji LL, Gomez-Cabrera MC, Vina J. Role of nuclear factor kappaB and mitogen-activated protein kinase signaling in exercise-induced antioxidant enzyme adaptation. Appl Physiol Nutr Metab. 2007;32:930-935. [PubMed] [DOI] |
| 24. | Kefaloyianni E, Gaitanaki C, Beis I. ERK1/2 and p38-MAPK signalling pathways, through MSK1, are involved in NF-kappaB transactivation during oxidative stress in skeletal myoblasts. Cell Signal. 2006;18:2238-2251. [PubMed] [DOI] |
| 25. | Liu SQ, Huang JA, Qin MB, Su YJ, Lai MY, Jiang HX, Tang GD. Sphingosine kinase 1 enhances colon cancer cell proliferation and invasion by upregulating the production of MMP-2/9 and uPA via MAPK pathways. Int J Colorectal Dis. 2012;27:1569-1578. [PubMed] [DOI] |
| 26. | 刘 诗权, 覃 蒙斌, 钟 月圆, 黄 杰安, 唐 国都, 姜 海行. 鞘氨醇激酶-1调控ERK和NF-κB通路促进HT-29细胞的增殖和侵袭. 中国现代医学杂志. 2011;21:1849-1853. |
| 27. | Liu SQ, Yu JP, Chen HL, Luo HS, Chen SM, Yu HG. Therapeutic effects and molecular mechanisms of Ginkgo biloba extract on liver fibrosis in rats. Am. J Chin Med. 2006;34:99-114. [PubMed] [DOI] |
| 29. | Liu SQ, Yu JP, Yu HG, Lv P, Chen HL. Activation of Akt and ERK signalling pathways induced by etoposide confer chemoresistance in gastric cancer cells. Dig Liver Dis. 2006;38:310-318. [PubMed] [DOI] |
| 30. | Manu KA, Shanmugam MK, Ramachandran L, Li F, Fong CW, Kumar AP, Tan P, Sethi G. First evidence that γ-tocotrienol inhibits the growth of human gastric cancer and chemosensitizes it to capecitabine in a xenograft mouse model through the modulation of NF-κB pathway. Clin Cancer Res. 2012;18:2220-2229. [PubMed] [DOI] |