修回日期: 2012-11-12
接受日期: 2012-11-15
在线出版日期: 2012-12-18
目的: 探讨白介素-10(interleukin-10, IL-10)基因819T/C多态性与克罗恩病(Crohn's disease, CD)风险的相关性.
方法: 计算机检索CBM、CNKI、PubMed和EMbase数据库, 收集从建库至2012-08关于IL-10基因819T/C多态性与CD风险相关性的病例对照研究. 采用RevMan 5.1.4软件计算合并效用量OR及其95%CI, 运用Egger's回归法、Begg秩相关法检测论文发表偏倚.
结果: 共纳入11个研究, 1 670例患者和3 312例对照. 各遗传模型Meta分析结果显示IL-10基因819T/C多态性多态性与CD风险的相关性无统计学意义[共显性遗传模型T/T vs C/C: OR = 0.90, 95%CI(0.70, 1.17 ); T/C vs C/C: OR = 0.84, 95%CI(0.56, 1.27); 显性遗传模型: OR = 0.97, 95%CI(0.86, 1.10); 隐性遗传模型: OR = 0.90, 95%CI(0.71, 1.14 )].
结论: 基于目前研究结果显示, IL-10基因819T/C多态性与CD风险无明显相关性. 受纳入研究质量和数量限制, 上述结论尚待更多高质量的前瞻性研究进一步验证.
引文著录: 谭诗云, 吴鹏波, 张国, 罗和生, 叶会兰. 白介素-10基因819T/C多态性与克罗恩病相关性的Meta分析. 世界华人消化杂志 2012; 20(35): 3603-3608
Revised: November 12, 2012
Accepted: November 15, 2012
Published online: December 18, 2012
AIM: To explore the association between interleukin-10 (IL-10) 819T/C polymorphism and Crohn's disease susceptibility.
METHODS: A systematic search of electronic databases such as CBM, CNKI, PubMed, Elsevier and EMbase was performed to retrieve relevant studies. Pooled odds ratios (ORs) with 95% confidence intervals (95% CI) were calculated using the RevMan 5.1.4 software, and publication bias was tested by Egger's regression test and Begg's test.
RESULTS: A total of 11 studies involving 1670 patients with Crohn's disease and 3312 healthy controls were identified. The results of meta-analyses showed no significant association between IL-10 819T/C polymorphism and susceptibility to Crohn's disease (for T/T vs C/C: OR = 0.90, 95% CI: 0.70 to 1.17; T/C vs C/C: OR = 0.84, 95% CI: 0.56 to 1.27; for dominant inheritance model: OR = 0.97, 95% CI 0.86 to 1.10; for recessive inheritance model: OR = 0.90, 95% CI: 0.71 to 1.14).
CONCLUSION: Current evidence strongly suggests that there is no significant association between IL-10 819T/C polymorphism and susceptibility to Crohn's disease.
- Citation: Tan SY, Wu PB, Zhang G, Luo HS, Ye HL. Association between interleukin-10-819 promoter polymorphism and susceptibility to Crohn's disease: A meta-analysis. Shijie Huaren Xiaohua Zazhi 2012; 20(35): 3603-3608
- URL: https://www.wjgnet.com/1009-3079/full/v20/i35/3603.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v20.i35.3603
克罗恩病(Crohn's disease, CD)是一种病因尚不十分明确的非特异性慢性胃肠道炎性肉芽肿疾病, 目前认为其发病与环境、遗传、微生物及免疫等因素有关. CD的家族聚集现象和CD发病的种族差异性提示遗传因素在CD发病过程中起着至关重要的作用. 自从Hugot等[1]确定CARD15/NOD2为CD易感基因以来, 已发现多个基因的单核苷酸多态性(single nucleotide polymorphism, SNP)位点与CD发病相关, 表明CD是一种复杂的多基因病. 白介素-10(interleukin-10, IL-10)基因定位于染色体1q31-32上[2], 其启动子区域-819点为多态性位点. 相关研究表明IL-10启动子区的-819位点基因多态性与IL-10的表达高低相关性[3-5]. 到目前为止, 已有很多研究探讨了IL-10基因-819T/C多态性与CD风险的关系, 但由于单个研究样本量小和地区差异, 导致各研究结论不一. 为解决这些分歧, 我们对已发表的相关研究进行Meta分析, 以明确IL-10-819-T/C多态性与CD风险的相关性.
计算机检索EMbase(1966/2011-08)、PubMed(2000-04/2011-08)、CBM(1978/2011-08)和CNKI(1994/2011-08). 英文检索式:"interleukin 10 or interleukin-10 or IL10 or IL-10"and"mutation or variant or polymorphism"and"Crohn's disease or CD or inflammatory bowel disease or IBD"; 中文检索式: "白细胞介素10或IL-10"和"基因多态性或突变"和"克罗恩病或炎性肠疾病". 所有研究的研究对象限人类, 文种限中、英文, 同时追溯所获文献的参考文献以及手工检索相关杂志.
1.2.1 纳入标准: (1)涉及IL-10基因819 T/C多态性与CD相关性的文献; (2)公开发表的病例对照研究或巢式病例对照研究; (3)全文发表; (4)有足够的数据计算比值比(odds ratio, OR)和95%CI; (5)病例均为经确诊CD患者, 其诊断符合文献[6-9]诊断标准, 或者经病理联合内镜诊断; (6)对照组基因分布必须服从Hardy-Weinberg平衡(H-W平衡).
1.2.2 排除标准: (1)研究未设立对照组; (2)具体数据描述不清; (3)重复报道; (4)CD相关的综述以及动物实验.
1.2.3 资料提取: 由2位评价者独立根据纳入标准, 对文献进行筛选, 通过讨论达成一致. 对原始文献记录数据的收集也由两位评阅人独立完成. 对检出的文献提取以下信息: 第一作者姓名、出版年份、国家、病例组对照组基因型分布及其频率、对照组是否符合H-W平衡(P<0.05认为不符合H-W平衡).
1.2.4 质量评价: 采用Newcastle-Ottawa Scale(NOS)标准[10]评价纳入各个研究的质量. 该评分系统采取星级对3部分进行评价: (1)病例组和对照组研究对象选择; (2)病例组和对照组研究对象的可比性; (3)危险因素的暴露情况.得分>7分(含)的研究为高质量.
统计学处理 采用RevMan 5.1.4和Stata 11.0软件进行统计分析. 合并效应量选用OR及其95%CI. OR值的计算分别根据共显性遗传模型(T/T vs C/C; T/C vs C/C)、显性遗传模型(T/T+T/C vs C/C)和隐性遗传模型(T/T vs T/C+C/C). 对纳入研究进行异质性检验, 各研究结果间无异质性采用固定效应模型进行合并分析, 否则采用随机效应模型进行合并分析. 各研究结果间的异质性检验采用Q检验和I2统计量, P<0.1或I2>50%提示各研究间存在异质性. 敏感性分析为依次排除单个文献后重新进行Meta分析, 估计综合效应大小. 发表偏倚通过Egger回归法、Begg秩相关法进行量化检测(P<0.05认为存在发表).
根据检索策略, 初检出476篇文献, 其中中文4篇, 英文472篇. 通过初步阅读文题和摘要, 排除456篇不符合纳入标准的文献经初步筛查. 进一步阅读全文, 3篇文献缺少对照组, 1篇文献重复报道, 5篇文献涉及动物研究. 最终纳入11篇文献的11个病例-对照研究被纳入本研究. 研究时间分布1998-2011年, 研究人群分布亚洲、欧洲、大洋洲和北美洲. 各个纳入研究的基本特征见表1[11-21].
| 作者 | 地区 | 发表年份 | 克罗恩病 | 对照 | NOS评分 | H-W平衡(P值) | ||||
| TT | TC | CC | TT | TC | CC | |||||
| Crusius等[11] | Netherlands | 1998 | 6 | 24 | 58 | 3 | 35 | 71 | 5 | 0.591 |
| Wang等[12] | NewZealand | 2011 | 14 | 121 | 206 | 32 | 205 | 365 | 7 | 0.617 |
| Kim等[13] | Korea | 2003 | 31 | 30 | 1 | 96 | 85 | 18 | 6 | 0.895 |
| Fernandez等[14] | Spain | 2005 | 13 | 89 | 126 | 42 | 185 | 293 | 8 | 0.099 |
| Castro-Santos等[15] | Spain | 2006 | 15 | 57 | 74 | 31 | 122 | 190 | 8 | 0.081 |
| Andersen等[16] | Denmark | 2010 | 9 | 111 | 216 | 37 | 259 | 483 | 7 | 0.763 |
| Ahirwar等[17] | India | 2012 | 9 | 16 | 11 | 40 | 106 | 61 | 8 | 0.616 |
| Sanchez等[18] | Canada | 2009 | 14 | 39 | 64 | 7 | 37 | 50 | 6 | 0.966 |
| Koss等[19] | UK | 2000 | 3 | 9 | 16 | 7 | 18 | 27 | 8 | 0.833 |
| Amre等[20] | Canada | 2009 | 15 | 94 | 158 | 28 | 132 | 172 | 8 | 0.708 |
| Garza-González等[21] | Mexico | 2010 | 1 | 13 | 7 | 15 | 40 | 20 | 7 | 0.536 |
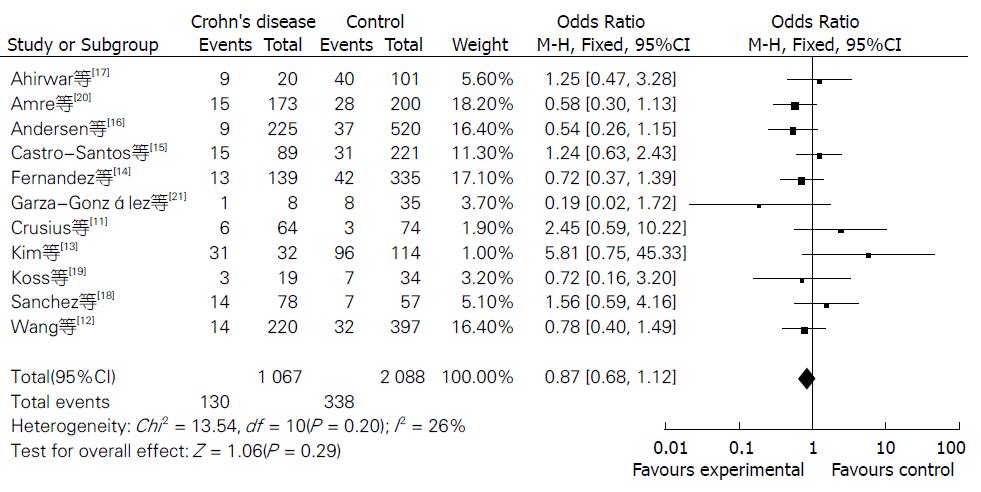
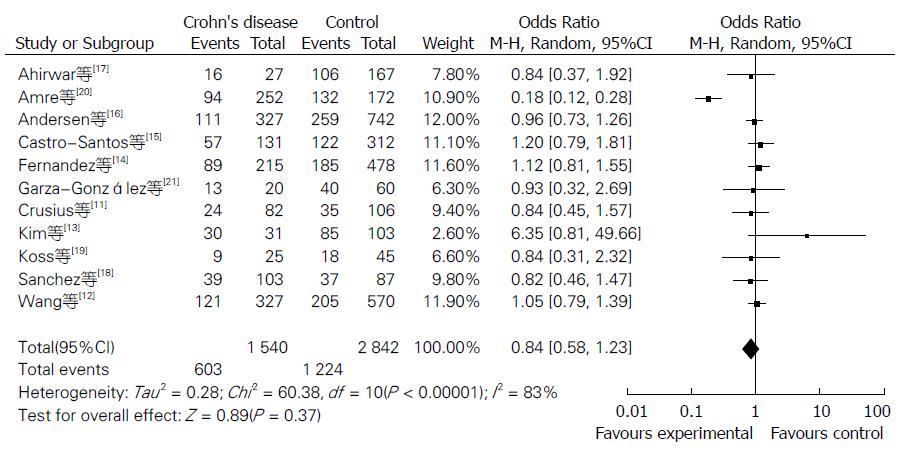
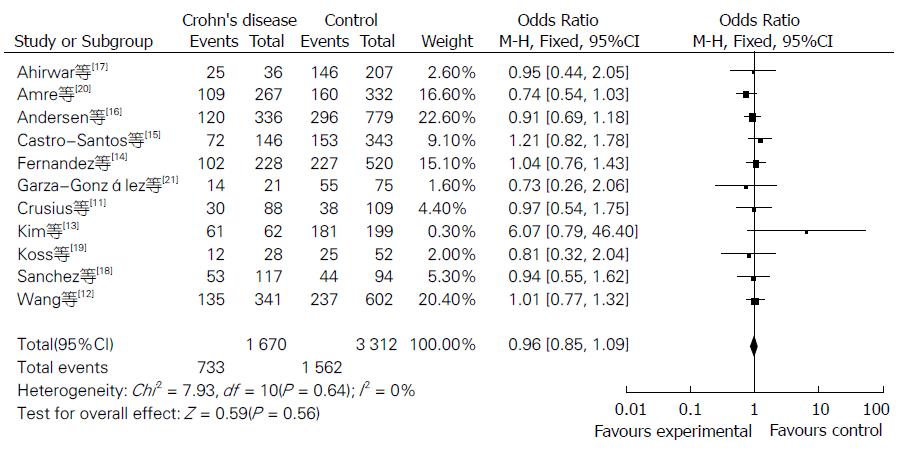
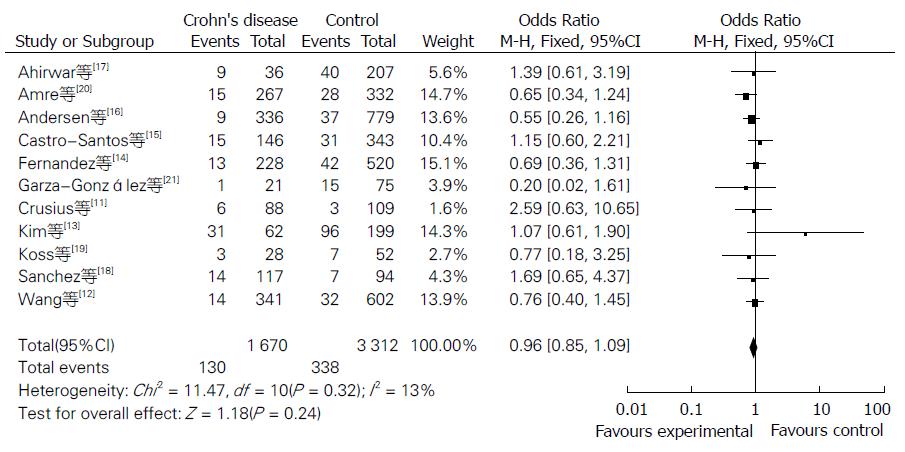
Meta分析的结果见表1. NOS评分结果显示平均得分为7.1(5-9分), 提示纳入的研究质量较好. 当11个研究合并分析时显示IL-10-8819T/C基因多态性与CD风险的相关性无统计学意义[共显性遗传模型T/T vs C/C: OR = 0.87, 95%CI(0.68, 1.12); T/C vs C/C: OR = 0.84, 95%CI(0.58, 1.23); 显性遗传模型: OR = 0.96, 95%CI(0.85, 1.09); 隐性遗传模型: OR = 0.87, 95%CI(0.69, 1.10)](图1, 图2, 图3和图4).
依次单独剔除1篇文献后, 各遗传模型合并效应量均无明显改变.
Egger回归法分析结果显示:共显性遗传模型(T/T vs C/C: P = 0.99; T/C vs C/C: P = 0.96)、显性遗传模型(P = 0.96)、隐性遗传模型(P = 0.97). Begg秩相关法分析结果显示: 共显性遗传模型(T/T vs C/C: P = 0.99; T/C vs C/C: P = 0.35)、显性遗传模型(P = 0.53)、隐性遗传模型(P = 0.76). Egger回归法、Begg秩相关法量化检测也未见发表偏倚.
L-10主要由辅助性T细胞亚群Th2细胞、单核细胞、巨噬细胞及基质细胞等在各种免疫激活状态下分泌的细胞因子, 他具有抑制Th1细胞功能的作用, 处于机体免疫调节的中心环节, 对机体免疫具有抑制和促进双重作用[22]. 国外学者Reuss等[23]发现不同个体的全血细胞产生IL-10的能力不同, 其可能原因是IL-10基因启动子的多态性导致其mRNA合成率不同[24].
众所周知, CD发病是由于肠道黏膜内环境紊乱导致促进和抑制炎症反应两者失衡造成的. 虽然CD具体的发病机制尚不明确, 但国内外学者一致认为CD的发生以及发展与CD4+ T细胞过度激活有关. 鉴于IL-10能抑制在CD患者肠道黏膜CD4+ T细胞活化[25,26], IL-10细胞因子在CD发生发展的作用得到前所未有的重视. 以往研究发现各IL-10-819T/C基因型CD患者组血清IL-10低于健康人群组, 这提示IL-10-819T/C基因多态性是通过降低机体内能够抑制CD发生以及发展的IL-10起作用的. 但是近来有学者发现CD患者组清IL-10浓度高于健康对照组人群[12,27].
本文结果分析显示IL-10-819T/C多态性与CD发病风险无明显相关性, 这一结果与Parkes等[28]结果相似. 得出上述结果的可能原因有以下几点: (1)IL-10基因819T/C多态性与CD的易感性确实无关; (2)IL-10-819位点与其他基因位点等位基因紧密连锁, 可能共同作用于CD的病变, 而不仅仅与IL-10基因的819T/C位点多态性有关; (3)CD患者的肠道黏膜炎症是众多细胞因子作用的结果, 而不仅仅是IL-10.
Meta分析能够将研究目的相同的多个病例一对照研究结果进行定量合并分析和综合评价, 以提高统计检验功效, 解决研究结果不一致的问题, 他克服了单个研究样本量小、地区局限的不足[29]. 异质性是影响Meta分析结果可靠性的主要因素, 4个遗传模型经异质性检验显示T/C vs C/C模型存在异质性, 我们试图通过敏感性分析寻找异质性所在. 但未能通过敏感性分析发现异质性来源. 另外, 本研究虽然设计了科学研究方案, 本次分析仍存在一定的局限性, 其原因如下: (1)本Meta分析只检索了国内外几个代表性数据库, 检索语言仅为中文和英文, 且只限于公开发表的文献, 影响了纳入研究的全面性; (2)纳入研究的人群分布限于亚洲、欧洲、大洋洲、北美洲; (3)未根据遗传背景做亚组分析.
总之, 可以认为IL-10基因819T/C多态性可能与CD易感性不相关. 然而, 受研究数量所限, 仍需开展更多高质量、大样本、包括不同种族群体的针对IL-10基因819T/C多态性和CD易感性之间关系的研究以进一步验证819T/C多态性与CD易感性的关系.
克罗恩病(CD)是常见的慢性胃肠道非特异性炎性疾病, 目前对于CD的病因和发病机制尚未完全明了, 其发生与可能与精神心理因素、感染、免疫异常、等多种因素有关. 白介素-10(IL-10)的功能基因多态性可以通过影响其表达高低调节宿主炎性反应强弱及炎症转归结果, 最终改变其罹患CD的风险.
白爱平, 副教授, 江西省南昌大学第一附属医院消化病研究所
IL-10作为体内免疫反应的重要调节因子, 在肠道炎症反应中起重要作用, 研究证明其与乙型肝炎发病密切相关.
Franke等1项研究纳入了1 851名CD患者, 结果显示CD发病机制中涉及到细胞因子IL-10的参与.
近年来, 有大量关于IL-10-819T/C基因多态性与CD患者易感性的关系的研究, 但结论不一致, 所以本文就此进行研究, 分析IL-10-819T/C基因多态性与CD易感性的关系.
相同的基因位点, 不同的研究结论可能与样本量大小、环境因素及种族遗传变异有关. 本文将IL-10-819位点与CD的功能基因多态性文章进行荟萃分析, 为以后科研和临床研究提供一定的思路.
本文对IL-10基因819T/C多态性与CD发病相关性进行了荟萃分析, 结果显示, 二者之间无明显相关性. 文章选题准确, 结论可靠.
编辑: 田滢 电编:闫晋利
| 1. | Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599-603. [PubMed] [DOI] |
| 2. | Eskdale J, Kube D, Tesch H, Gallagher G. Mapping of the human IL10 gene and further characterization of the 5' flanking sequence. Immunogenetics. 1997;46:120-128. [PubMed] [DOI] |
| 3. | Tesse R, Del Vecchio GC, De Mattia D, Sangerardi M, Valente F, Giordano P. Association of interleukin-(IL)10 haplotypes and serum IL-10 levels in the progression of childhood immune thrombocytopenic purpura. Gene. 2012;505:53-56. [PubMed] [DOI] |
| 4. | Zhang G, Manaca MN, McNamara-Smith M, Mayor A, Nhabomba A, Berthoud TK, Khoo SK, Wiertsema S, Aguilar R, Barbosa A. Interleukin-10 (IL-10) polymorphisms are associated with IL-10 production and clinical malaria in young children. Infect Immun. 2012;80:2316-2322. [PubMed] [DOI] |
| 5. | Holtzman S, Abbey SE, Chan C, Bargman JM, Stewart DE. A genetic predisposition to produce low levels of IL-10 is related to depressive symptoms: a pilot study of patients with end stage renal disease. Psychosomatics. 2012;53:155-161. [PubMed] [DOI] |
| 6. | Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2-6; discussion 16-19. [PubMed] [DOI] |
| 7. | Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439-444. [PubMed] |
| 8. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [PubMed] [DOI] |
| 9. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5-36. [PubMed] |
| 10. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [PubMed] [DOI] |
| 11. | Crusius JBA, Pérez Centeno CM, Keijsers V, Bouma G, Meuwissen SGM, Huizinga TWJ, Peña AS. Interleukin 10 gene polymorphisms in ulcerative colitis and Crohn's disease. Gastroenterology. 1998;114:A957. [DOI] |
| 12. | Wang AH, Lam WJ, Han DY, Ding Y, Hu R, Fraser AG, Ferguson LR, Morgan AR. The effect of IL-10 genetic variation and interleukin 10 serum levels on Crohn's disease susceptibility in a New Zealand population. Hum Immunol. 2011;72:431-435. [PubMed] [DOI] |
| 13. | Kim TH, Kim BG, Shin HD, Kim JW, Kim CG, Kim JS, Jung HC, Song IS. [Tumor necrosis factor-alpha and interleukin-10 gene polymorphisms in Korean patients with inflammatory bowel disease]. Korean J Gastroenterol. 2003;42:377-386. [PubMed] |
| 14. | Fernandez L, Martinez A, Mendoza JL, Urcelay E, Fernandez-Arquero M, Garcia-Paredes J, Diaz-Rubio M, de la Concha EG. Interleukin-10 polymorphisms in Spanish patients with IBD. Inflamm Bowel Dis. 2005;11:739-743. [PubMed] [DOI] |
| 15. | Castro-Santos P, Suarez A, López-Rivas L, Mozo L, Gutierrez C. TNFalpha and IL-10 gene polymorphisms in inflammatory bowel disease. Association of -1082 AA low producer IL-10 genotype with steroid dependency. Am J Gastroenterol. 2006;101:1039-1047. [PubMed] [DOI] |
| 16. | Andersen V, Ernst A, Christensen J, Østergaard M, Jacobsen BA, Tjønneland A, Krarup HB, Vogel U. The polymorphism rs3024505 proximal to IL-10 is associated with risk of ulcerative colitis and Crohns disease in a Danish case-control study. BMC Med Genet. 2010;11:82. [PubMed] [DOI] |
| 17. | Ahirwar DK, Kesarwani P, Singh R, Ghoshal UC, Mittal RD. Role of tumor necrosis factor-alpha (C-863A) polymorphism in pathogenesis of inflammatory bowel disease in Northern India. J Gastrointest Cancer. 2012;43:196-204. [PubMed] [DOI] |
| 18. | Sanchez R, Levy E, Costea F, Sinnett D. IL-10 and TNF-alpha promoter haplotypes are associated with childhood Crohn's disease location. World J Gastroenterol. 2009;15:3776-3782. [PubMed] [DOI] |
| 19. | Koss K, Satsangi J, Fanning GC, Welsh KI, Jewell DP. Cytokine (TNF alpha, LT alpha and IL-10) polymorphisms in inflammatory bowel diseases and normal controls: differential effects on production and allele frequencies. Genes Immun. 2000;1:185-190. [PubMed] [DOI] |
| 20. | Amre DK, Mack DR, Morgan K, Israel D, Lambrette P, Costea I, Krupoves A, Fegury H, Dong J, Grimard G. Interleukin 10 (IL-10) gene variants and susceptibility for paediatric onset Crohn's disease. Aliment Pharmacol Ther. 2009;29:1025-1031. [PubMed] [DOI] |
| 21. | Garza-González E, Pérez-Pérez GI, Mendoza-Ibarra SI, Flores-Gutiérrez JP, Bosques-Padilla FJ. Genetic risk factors for inflammatory bowel disease in a North-eastern Mexican population. Int J Immunogenet. 2010;37:355-359. [PubMed] [DOI] |
| 22. | Chen YX, Man K, Ling GS, Chen Y, Sun BS, Cheng Q, Wong OH, Lo CK, Ng IO, Chan LC. A crucial role for dendritic cell (DC) IL-10 in inhibiting successful DC-based immunotherapy: superior antitumor immunity against hepatocellular carcinoma evoked by DC devoid of IL-10. J Immunol. 2007;179:6009-6015. [PubMed] |
| 23. | Reuss E, Fimmers R, Kruger A, Becker C, Rittner C, Höhler T. Differential regulation of interleukin-10 production by genetic and environmental factors--a twin study. Genes Immun. 2002;3:407-413. [PubMed] [DOI] |
| 24. | Bialecka M, Klodowska-Duda G, Kurzawski M, Slawek J, Opala G, Bialecki P, Safranow K, Droździk M. Interleukin-10 gene polymorphism in Parkinson's disease patients. Arch Med Res. 2007;38:858-863. [PubMed] [DOI] |
| 25. | Ranatunga DC, Ramakrishnan A, Uprety P, Wang F, Zhang H, Margolick JB, Brayton C, Bream JH. A protective role for human IL-10-expressing CD4+ T cells in colitis. J Immunol. 2012;189:1243-1252. [PubMed] [DOI] |
| 26. | Mandaric S, Walton SM, Rülicke T, Richter K, Girard-Madoux MJ, Clausen BE, Zurunic A, Kamanaka M, Flavell RA, Jonjic S. IL-10 suppression of NK/DC crosstalk leads to poor priming of MCMV-specific CD4 T cells and prolonged MCMV persistence. PLoS Pathog. 2012;8:e1002846. [PubMed] [DOI] |
| 27. | Kucharzik T, Stoll R, Lügering N, Domschke W. Circulating antiinflammatory cytokine IL-10 in patients with inflammatory bowel disease (IBD). Clin Exp Immunol. 1995;100:452-456. [PubMed] [DOI] |
| 28. | Parkes M, Satsangi J, Jewell D. Contribution of the IL-2 and IL-10 genes to inflammatory bowel disease (IBD) susceptibility. Clin Exp Immunol. 1998;113:28-32. [PubMed] [DOI] |