修回日期: 2012-09-01
接受日期: 2012-10-29
在线出版日期: 2012-11-18
目的: 探讨术前放化疗(chemoradiotherapy followed by surgery, CRTS)与单纯手术治疗对可切除食管癌患者生存及手术的影响.
方法: 检索PubMed、中国知网和万方数据资源系统中所有CRTS与单纯手术(surgery alone, SA)治疗可切除食管癌的随机对照研究(randomized controlled trial, RCT), 应用RevMan 5.1软件进行Meta分析.
结果: 共21篇文献. CRTS组比SA组提高了1、3、5年生存率, 差异均有统计学意义(P<0.05); 提高了东方、西方、同步放化疗和鳞癌患者3、5年生存率, 差异均有统计学意义(P<0.05). 两组序贯放化疗、腺癌患者3、5年生存率相比差异均无统计学意义(P>0.05). SA组比CRTS组有较高的手术率, 差异有统计学意义(P<0.05); 而CRTS组比SA组有更显著的根治切除率、R0切除率和较低的术后局部复发率, 差异均有统计学意义(P<0.05). 两组术后并发症发生率、远处转移率及死亡率比较,差异均无统计学意义(P>0.05).
结论: CRTS比SA明显改善了可切除食管癌患者的生存预后及手术情况.
引文著录: 王亚梅, 王峰, 何炜, 樊青霞. 术前放化疗与单纯手术治疗可切除食管癌的Meta分析. 世界华人消化杂志 2012; 20(32): 3140-3153
Revised: September 1, 2012
Accepted: October 29, 2012
Published online: November 18, 2012
AIM: To assess the efficacy of preoperative chemoradiotherapy followed by surgery (CRTS) in the management of resectable esophageal carcinoma.
METHODS: Randomized controlled trials (RCTs) comparing the efficacy of preoperative chemoradiotherapy versus surgery alone (SA) in the treatment of resectable oesophageal carcinoma were searched in PubMed, China National Knowledge Infrastructure (CNKI) and Wan Fang databases. The RevMan 5.1 software was used for meta-analysis.
RESULTS: This meta-analysis included 21 RCTs. Compared to the SA group, the 1-, 3-, and 5-year survival rates were significantly higher in the CRTS group (all P < 0.05). The 3- and 5-year survival rates for the Eastern patients, Western patients, patients undergoing concurrent chemoradiotherapy, and patients with squamous cell carcinoma were significantly higher in the CRTS group (all P < 0.05). There were no statistical significances in the 3- and 5-year survival rates for patients undergoing sequential chemoradiotherapy or patients with adenocarcinoma between the two groups (all P > 0.05). Compared to the RCTS group, the surgery rate in the SA group was higher (P < 0.05), while the CRTS group had significantly higher radical resection rate, R0 resection rate and lower postoperative local recurrence rate (all P < 0.05). The differences in postoperative complication incidence, post-operative distant metastasis and postoperative mortality rate were not statistically significant between the two groups (all P > 0.05).
CONCLUSION: CRTS can significantly improve the survival and surgical conditions of patients with resectable esophageal carcinoma.
- Citation: Wang YM, Wang F, He W, Fan QX. Preoperative chemoradiotherapy followed by surgery versus surgery alone for resectable oesophageal carcinoma: A meta-analysis. Shijie Huaren Xiaohua Zazhi 2012; 20(32): 3140-3153
- URL: https://www.wjgnet.com/1009-3079/full/v20/i32/3140.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v20.i32.3140
食管癌是原发于食管黏膜上皮的恶性肿瘤, 全球年新发病约为482 300例, 每年年死亡约406 800例[1], 术后5年总生存率2001年和2005年间保持在30.5%[2]. 目前认为与单一外科手术相比较, 术前放疗对延长总体远期生存作用有限, 化疗增加患者的不良反应, 对提高切除率和完全性切除率不明显[3]. 因此, 近年来食管癌术前放化疗(chemoradiotherapy followed by surgery, CRTS)得到越来越多的关注, 已进行了大量临床研究, 但其是否能改善可切除食管癌患者生存预后及手术情况的研究结果不一致. 为此我们选择关于CRTS对比单纯手术(surgery alone, SA)治疗可切除食管癌的前瞻性临床随机对照研究(randomized controlled trial, RCT)进行Meta分析, 以期为临床决策提供有价值的循证依据.
检索PubMed、中国知网和万方数据资源系统, 检索文献起止时间均为1992-01/2012-04. 所有检索均采用主题词与自由词相结合的方式, 检索词主要包括"neoadjuvant therapy、chemoradiotherapy followed by surgery、chemoradiotherapy、surgery or operation or esophagectomy、esophagus or esophageal cancer or carcinoma"等及对应的中文检索词, 检索语种不限, 同时运用搜索引擎在互联网上查找相关文献, 以期扩大检索范围.
1.2.1 纳入标准: (1)研究设计: CRTS与SA治疗可切除食管癌的前瞻性RCT, 无论是否采用盲法; (2)研究对象: 经组织或细胞病理学确诊的未治疗过的可切除食管癌, 且对患者的血常规、肝肾心肺等进行评估, 确认患者能否耐受手术. 原始文献中有明确的随访截止日期的存活例数或有清晰的生存曲线, 随访率>95%.
1.2.2 排除标准: 研究对象为曾接受单独化疗或单独放疗的食管癌患者, 剔除重复报道、信息太少以及数据描述不详的文献.
1.2.3 结局指标: 主要指标为两组1、3和5年生存率, 次要指标为两组手术率、手术根治率、R0切除率、术后局部复发率、术后并发症、术后死亡率和术后远处转移率.
1.2.4 数据提取: 所有数据均由2位评价者提取, 分歧通过协商解决.
统计学处理 采用RevMan 5.1(从Cochrane图书馆下载)软件进行Meta分析. 其结局变量为接受CRTS和SA的患者生存率、术后根治率等发生率之比, 即治疗相对危险度(relative risk, RR). 各纳入研究结果之间的异质性采用χ2检验, 若纳入研究具有足够一致性(P>0.1)时, 采用固定效应模型计算合并RR及95%可信区间(95%CI); 否则采用随机效应模型. 以P<0.05为差异有统计学意义. 对主要结果进一步行亚组、漏斗图和/或敏感性分析.
初检314篇相关文献, 阅读文献标题和摘要后初步纳入RCT59篇, 进一步阅读全文后排除文献38篇(不符合纳入标准11篇, 非随机对照15篇, 回顾性研究10篇, 未发表全文2篇). 最终纳入RCT 21篇, 患者2 755例, 其中CRTS组1 366例, SA组1 389例(表1).
| 第一作者 | 发表年份 | 国家 | 放疗方案 | 化疗方案 | 放化疗顺序 | 病理类型 | 临床分期 | 分组 | 人数(n) | 男/女(n) | 中位年龄(yr) | 中位生存期(mo) | 中位随访期(mo) | Jadad评分[4] |
| Nygaard等[5] | 1992 | Norway | 35 Gy, 1.75 Gy per fraction over 4 wk | Two cycles: cisplatin 20 mg/m2 d1-5; bleomycin 5 mg/m2 1-5 d | Sequential | SCC | T1 or T2, Nx, M0 | CRTS | 47 | 33/14 | 60.1 | 7 | 18 | 2 |
| SA | 41 | 31/10 | 61.4 | 7 | ||||||||||
| Apinop等[6] | 1994 | Thailand | 40 Gy, 2 Gy per fraction over 4 wk | Two cycles: cisplatin 100 mg/m2 1 d; fluorouracil 1000 mg/m2 1-4 d | Concurrent | SCC | IIb(11); III(57) | CRTS | 35 | 28/7 | 59.6 | 9.7 | 12 | 1 |
| SA | 34 | 26/8 | 59.8 | 7.4 | ||||||||||
| Le Prise等[7] | 1994 | France | 40 Gy, 20 Gy in 10 fractions over 12 d | Two cycles: cisplatin 100 mg/m2 d1, 21; fluorouracil 600 mg/m2 2-5 d, 22-25 d | Sequential | SCC | I(23); II(63) | CRTS | 41 | 80/6 | 56 | 10.2 | 16 | 2 |
| SA | 45 | 59 | 11 | |||||||||||
| Walsh等[8] | 1996 | Ireland | 40 Gy in 15 fractions over 3 wk | Two cycles: cisplatin 75 mg/m2 1 d; fluorouracil 15 mg/kg 1-5 d | Concurrent | AC | not distant metastases | CRTS | 58 | 39/19 | 65 | 16 | 10 | 2 |
| SA | 55 | 44/11 | 65 | 11 | ||||||||||
| Bosset等[9] | 1997 | France | 37 Gy, 3.7 Gy per fraction over 2 wk | Two cycles: cisplatin 80 mg/m2 0-2 d | Sequential | SCC | T1N0(49); T2N0(92); T3N0(76); T1N1 or T2N1(65) | CRTS | 143 | 129/14 | 56.7 | 18.6 | 55.2 | 3 |
| SA | 139 | 134/5 | 56.6 | 18.6 | ||||||||||
| Urba等[10] | 2001 | USA | 45 Gy; 1.5 Gy per fraction over 3 wk | Two cycles: cisplatin 20 mg/m2 1-5 d; fluorouracil 300 mg/m2 1-21 d; vinblastine 1 mg/m2 1-4 d | Concurrent | SCC(25%); AC(75%) | not distant metastases | CRTS | 50 | 42/8 | 62 | 16.9 | 98.4 | 2 |
| SA | 50 | 43/7 | 64 | 17.6 | ||||||||||
| 安丰山等[11] | 2003 | China | 36 Gy, 1.2 Gy per fraction over 17 d | First cycle: 5-fluorouracil 1 mg/m2, 5-6 h, 1-5 d; cisplatin 25 mg/m2. | Sequential | SCC | II, III | CRTS | 48 | 31/17 | 58.4 | 42 | 34.12 | 3 |
| SA | 50 | 47/3 | 63 | 27.3 | ||||||||||
| 第一作者 | 发表 年份 | 国家 | 放疗方案 | 化疗方案 | 放化疗顺序 | 病理类型 | 临床分期 | 分组 | 人数(n) | 男/女 (n) | 中位年 龄(yr) | 中位生 存期(mo) | 中位随 访期(mo) | Jadad 评分[4] |
| Nygaard等[5] | 1992 | Norway | 35 Gy, 1.75 Gy per fraction over 4 wk | Two cycles: cisplatin 20 mg/m2 d1-5; bleomycin 5 mg/m2 1-5 d | Sequential | SCC | T1 or T2, Nx, M0 | CRTS | 47 | 33/14 | 60.1 | 7 | 18 | 2 |
| SA | 41 | 31/10 | 61.4 | 7 | ||||||||||
| Apinop等[6] | 1994 | Thailand | 40 Gy, 2 Gy per fraction over 4 wk | Two cycles: cisplatin 100 mg/m2 1 d; fluorouracil 1000 mg/m2 1-4 d | Concurrent | SCC | IIb(11); III(57) | CRTS | 35 | 28/7 | 59.6 | 9.7 | 12 | 1 |
| SA | 34 | 26/8 | 59.8 | 7.4 | ||||||||||
| Le Prise等[7] | 1994 | France | 40 Gy, 20 Gy in 10 fractions over 12 d | Two cycles: cisplatin 100 mg/m2 d1, 21; fluorouracil 600 mg/m2 2-5 d, 22-25 d | Sequential | SCC | I(23); II(63) | CRTS | 41 | 80/6 | 56 | 10.2 | 16 | 2 |
| SA | 45 | 59 | 11 | |||||||||||
| Walsh等[8] | 1996 | Ireland | 40 Gy in 15 fractions over 3 wk | Two cycles: cisplatin 75 mg/m2 1 d; fluorouracil 15 mg/kg 1-5 d | Concurrent | AC | not distant metastases | CRTS | 58 | 39/19 | 65 | 16 | 10 | 2 |
| SA | 55 | 44/11 | 65 | 11 | ||||||||||
| Bosset等[9] | 1997 | France | 37 Gy, 3.7 Gy per fraction over 2 wk | Two cycles: cisplatin 80 mg/m2 0-2 d | Sequential | SCC | T1N0(49); T2N0(92); T3N0(76); T1N1 or T2N1(65) | CRTS | 143 | 129/14 | 56.7 | 18.6 | 55.2 | 3 |
| SA | 139 | 134/5 | 56.6 | 18.6 | ||||||||||
| Urba等[10] | 2001 | USA | 45 Gy; 1.5 Gy per fraction over 3 wk | Two cycles: cisplatin 20 mg/m2 1-5 d; fluorouracil 300 mg/m2 1-21 d; vinblastine 1 mg/m2 1-4 d | Concurrent | SCC(25%); AC(75%) | not distant metastases | CRTS | 50 | 42/8 | 62 | 16.9 | 98.4 | 2 |
| SA | 50 | 43/7 | 64 | 17.6 | ||||||||||
| 安丰山等[11] | 2003 | China | 36 Gy, 1.2 Gy per fraction over 17 d | First cycle: 5-fluorouracil 1 mg/m2, 5-6 h, 1-5 d; cisplatin 25 mg/m2. | Sequential | SCC | II, III | CRTS | 48 | 31/17 | 58.4 | 42 | 34.12 | 3 |
| Second cycle: 5-fluoro- uracil 0.5 g/m2, 21-25 d; cisplatin 25 mg/m2, 22-25 d | SA | 49 | 30/19 | 57.6 | 28 | |||||||||
| Lee等[12] | 2004 | Korea | 45.6 Gy, 1.2 Gy per fraction over 28 d | Two cycles: cisplatin 60 mg/m2 d1; fluorouracil 1000 mg/m2 3-5 d | Concurrent | SCC | IIA(36); IIB(22); III(43); N0(36); N1(65) | CRTS | 51 | 46/5 | 63 | 28.2 | 25 | 2 |
| Burmeister 等[13] | 2005 | Australia | 35 Gy in 15 fractions over 3 wk | One cycles: cisplatin 80 mg/m2 d1; fluorouracil 800 mg/m2 1-4 d | Concurrent | SCC(37%); AC(62%) | T1-3, N0-1 | CRTS | 128 | 106/22 | 61 | 22.2 | 65 | 3 |
| SA | 128 | 100/28 | 62 | 19.3 | ||||||||||
| Natsugoe 等[14] | 2006 | Japan | 40 Gy, 2 Gy per fraction over 4 wk | cisplatin 7 mg over 2 h; 5-fluorouracil 350 mg over 24 h | Concurrent | SCC | II(9); III(24); IV(12) | CRTS | 22 | - | - | - | 24 | 2 |
| SA | 23 | - | - | - | ||||||||||
| Tepper等[15] | 2008 | USA | 50.4 Gy, 1.8 Gy per fraction over 5.6 wk | Two cycles: cisplatin 100 mg/m2 1 d; fluorouracil 1 000 mg/(m2·d ) 1-4 d | Concurrent | SCC(25%); AC(75%) | T1-3, Nx | CRTS | 30 | 28/2 | 59.9 | 53.76 | 72 | 2 |
| SA | 26 | 23/3 | 62.2 | 21.48 | ||||||||||
| 彭林等[16] | 2008 | China | 40 Gy, 2 Gy per fraction over 4 wk | Two cycles: cisplatin 75 mg/m2 1 d; 5-fluorouracil 500 mg/m2 over 5d | Concurrent | SCC | IIB; III | CRTS | 40 | 30/40 | 58.4 | 30 | 37.5 | 3 |
| SA | 40 | 32/8 | 57.5 | 24 | ||||||||||
| 金明根等[17] | 2008 | China | 38-44 Gy, 2 Gy per fraction, total 19-22 fractions | Paclitaxe 135 mg/m2, 1 d,22; cisplatin 20-30 mg/m2, dl-5, 22-26 d | Concurrent | SCC(92%); AC(8%) | II(26); III(36) | CRTS | 30 | 22/8 | 49 | - | - | 3 |
| SA | 30 | 20/10 | - | |||||||||||
| Cao等[18] | 2009 | China | 40 Gy, 2 Gy per fraction over 4 wk | cisplatin 20 mg/(m2·d) ds1-5; fluorouracil 500 mg/(m2·d) 1-5 d; mitomycin 10 mg/(m2·d) 1 d | Concurrent | SCC | II(15); III(211); IV(10) | CRTS | 118 | 60/58 | - | 59 | - | 2 |
| SA | 118 | 67/51 | - | 43 | ||||||||||
| 吕进等[19] | 2009 | China | 40 Gy, 2 Gy/d, 1-5 d, 8-12, 15-19, 22-26 | Two cycles: Paclitaxe 125 mg/m2, l d; | Concurrent | SCC | IIb(15); III(213); IV(10) | CRTS | 119 | 61/58 | - | 30.5 | - | 2 |
| cisplatin 20 mg/m2, 1-3 d | SA | 119 | 68/51 | - | 18.3 | |||||||||
| Lv等[20] | 2010 | China | 40 Gy, 2 Gy per fraction over 4 wk | Two cycles: cisplatin 20 mg/(m2·d), 1-3 d, 22-25; paclitaxel 135 mg/m2 starting on 1 d, 22 of radiotherapy | Concurrent | SCC | II(71); III(89) | CRTS | 80 | 52/28 | - | 53 | 45 | 2 |
| SA | 80 | 50/30 | - | 36 | ||||||||||
| 谢军等[21] | 2010 | China | 45-5l Gy, 2 Gy per faction, 1-5 d/wk, total 2l-25 fractions | 5-fluorouracil 2.4 g/m2 l-3 d, 22-24; cisplatin 75 mg/m2, 1 d, 22 | Concurrent | SCC | II(22); III(47); IVa(1) | CRTS | 35 | 27/8 | 50 | - | - | 3 |
| SA | 35 | 25/10 | - | |||||||||||
| 张文山等[22] | 2011 | China | 40 Gy, 1.8-2.0 Gy per fraction over 4 wk | Two cycles 5-fluorouracil 500 mg/m2, 1-5 d; cisplatin 30 mg/(m2·d) | Concurrent | - | III | CRTS | 29 | 18/11 | 50.4 | 40 | - | 3 |
| SA | 40 | 26/14 | 52.2 | 29 | ||||||||||
| 金芳玲等[23] | 2011 | China | 50 Gy, 2 Gy per fraction over 5 wk mg/(m2·d), 1-5 d | Two cycles: cisplatin 75 mg/(m2·d), d1; 5-fluorouracil 500 mg/(m2·d), 1-5 d | Concurrent | SCC(92%); AC(8%) | IIa(10); IIb(9); III(41) | CRTS | 30 | 18/12 | 54 | 34 | - | 2 |
| SA | 30 | 21/9 | 22 | |||||||||||
| van Hagen 等[24] | 2012 | Netherlands | 41.4 Gy, 1.8 Gy per fraction over 4.6 wk | 5 wk chemotherapy; carboplatin area under curve = 2 and paclitaxel 50 mg/m2 on 1 d weekly | Concurrent | SCC(23%); AC(75%) | T1N1 or T2-3N0-1M0 | CRTS | 178 | 134/44 | 60 | 49.4 | 45.4 | 2 |
| SA | 188 | 152/26 | 60 | 24 | ||||||||||
| 杨弘等[25] | 2012 | China | 40 Gy, 2 Gy per fraction over 4 wk | Two cycles: navelbine 25 mg/m2 1,8,22,29 d;cisplatin 75 mg/m2 1,22 d | Concurrent | SCC | IIB(20) III(113) | CRTS | 54 | 46/8 | 54 | - | - | 3 |
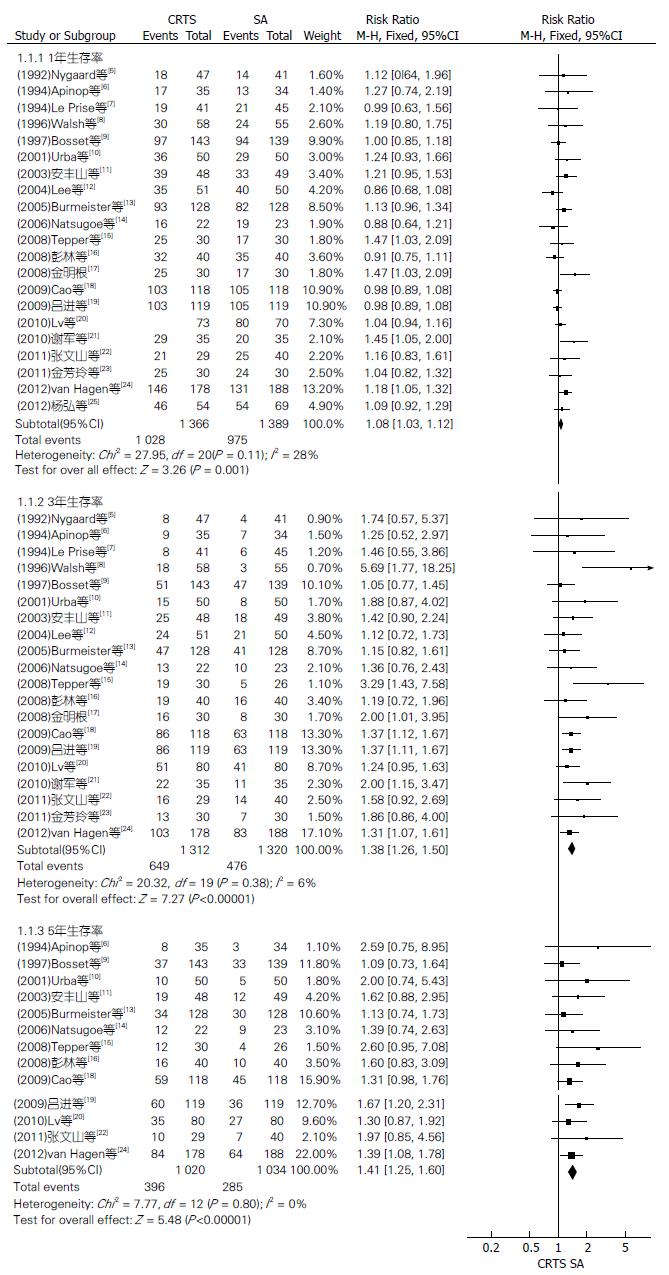
21篇RCT提供1年生存率、20篇提供3年生存率、13篇提供5年生存率, 异质性分析P值分别为0.11、0.38和0.80, 采用固定效应模型分析, 结果两组1、3、5年生存率相比RR(95%CI, P值)分别为1.08(1.03-1.12, P = 0.001)、1.38(1.26-1.50, P<0.00001)、1.41(1.25-1.60, P<0.00001), 差异均有统计学意义(图1).
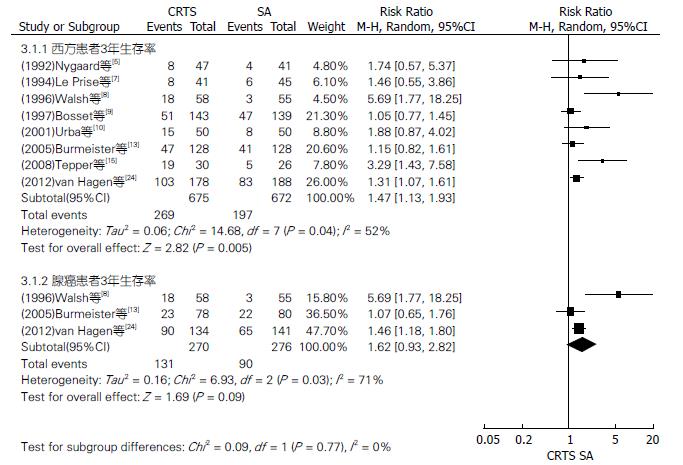
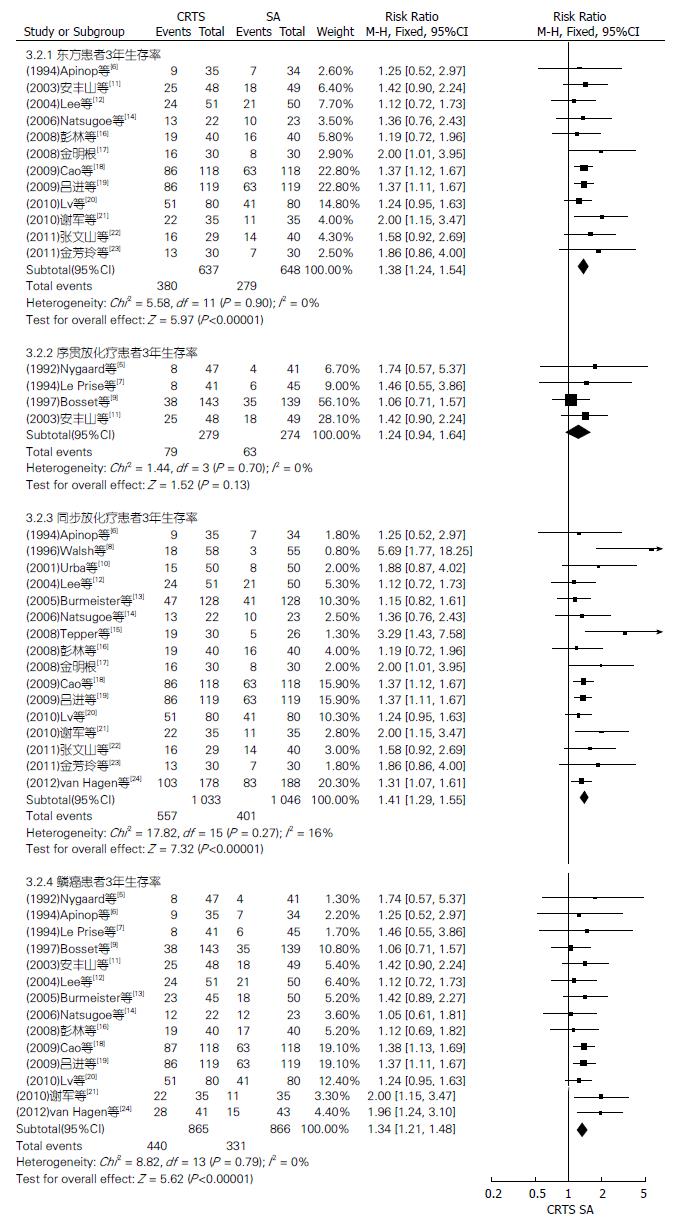
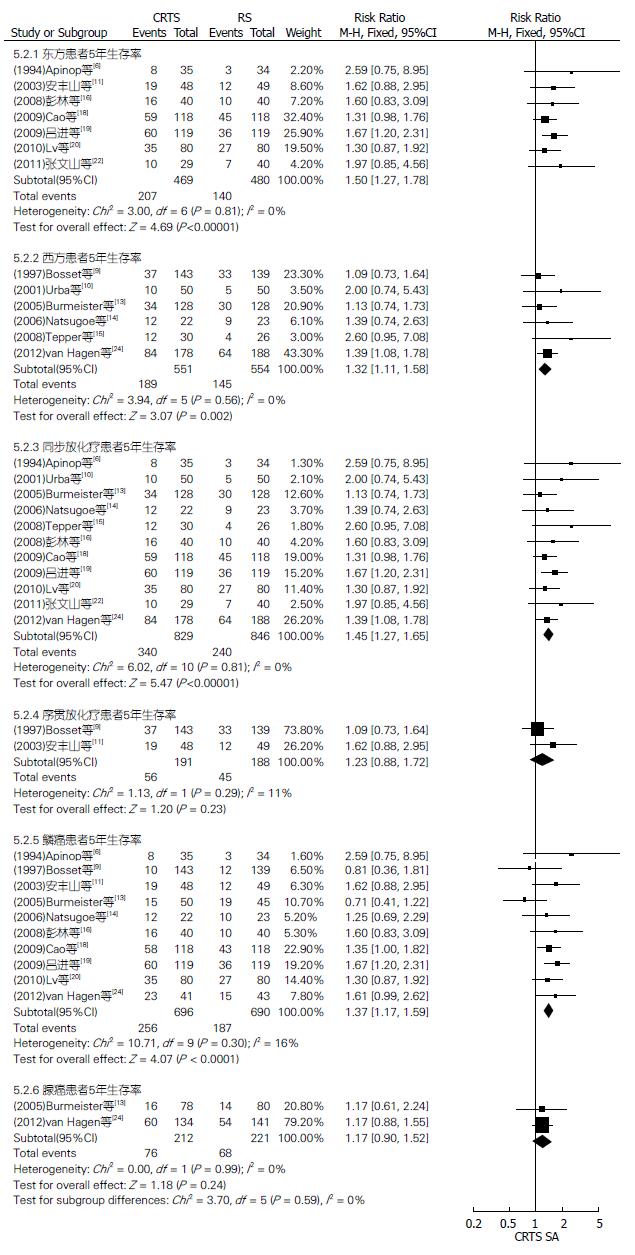
对两组种族、放化疗顺序及病理类型不同的患者3、5年生存率行亚组分析(图2-4). CRTS组比SA组提高了东方、西方、同步放化疗和鳞癌患者3、5年生存率, 其3年生存率相比RR(95%CI, P值)分别为1.38(1.24-1.54, P<0.00001)、1.47(1.13-1.93, P = 0.005)、1.41(1.29-1.55, P<0.00001)、1.34(1.21-1.48, P<0.00001); 其5年生存率相比RR(95%CI, P值)分别为1.50(1.27-1.78, P<0.00001)、1.32(1.11-1.58, P = 0.002)、1.45(1.27-1.65, P<0.00001)、1.37(1.17-1.59, P<0.0001), 差异均有统计学意义. 两组序贯放化疗、腺癌患者3年生存率相比RR(95%CI, P值)分别为1.24(0.94-1.64, P = 0.13)、1.62(0.93-2.82, P = 0.09); 5年生存率相比RR(95%CI, P值)分别为1.23(0.88-1.72, P = 0.23)、1.17(0.90-1.52, P = 0.24), 差异均无统计学意义.
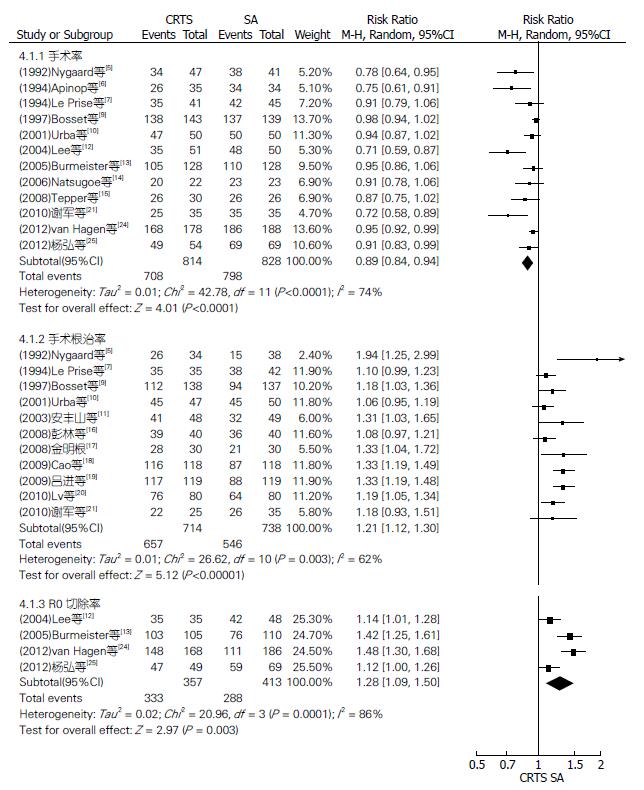
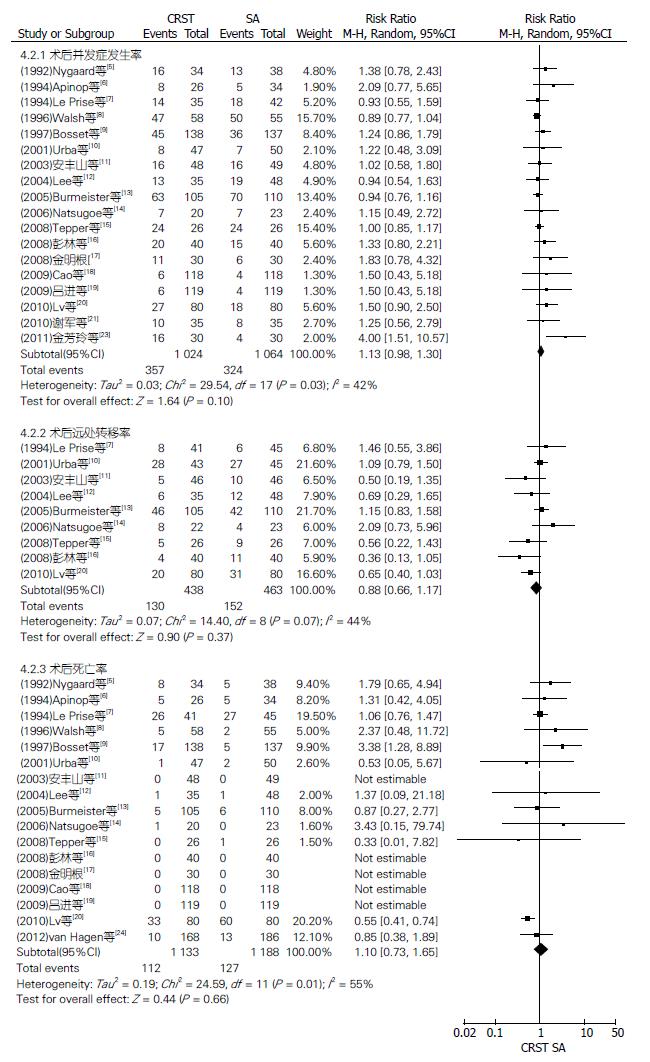
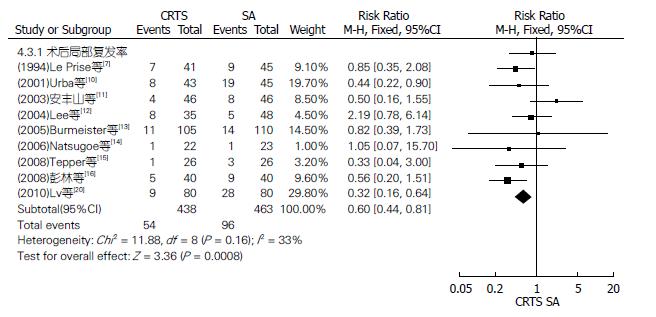
均以实际手术人数进行分析. 12篇RCT报道了手术率、11篇报道了手术根治率、4篇报道了R0切除率(图5), 18篇RCT报道了术后并发症发生率、9篇报道了术后远处转移率、17篇报道了术后死亡率(图6), 各RCT间均有异质性, 均采用随机效应模型进行Meta分析; 9篇RCT报道了术后局部复发率(图7), 各RCT间无异质性, 采用固定效应模型分析. 结果显示: SA组比CRTS组有较高的手术率, 差异有统计学意义, RR(95%CI, P值)为0.89(0.84-0.94, P<0.0001); 而CRTS组比SA组有更显著的治切除率、R0切除率和较低的术后局部复发率, 差异均有统计学意义, RR(95%CI, P值)分别为1.21(1.12-1.30, P<0.00001)、1.28(1.09-1.50, P = 0.003)和0.60(0.44-0.81, P = 0.0008). 两组术后并发症发生率、远处转移率及死亡率比较, RR(95%CI, P值)分别为1.13(0.98-1.30, P = 0.10)、0.88(0.66-1.17, P = 0.37)及1.10(0.73-1.65, P = 0.66), 差异均无统计学意义.
剔除任意一篇文献前后, 两组3年生存率、5年生存率、手术率、手术根治率、R0切除率、术后死亡率、术后局部复发率及术后远处转移率Meta分析均未发生明显变化, 结论的性质没有改变. 剔除Jadad质量记分为1和2的研究后, 两组1年生存率Meta分析未发生明显变化; 剔除Burmeister等[13]研究后, 两组术后并发症发生率差异有统计学意义(RR = 1.19, 95%CI = 1.001.41; P = 0.05), 结论的性质改变, 但处在统计学意义的边缘, 稳健性较低, 尚待更进一步验证. 以RR值为横坐标, SE[log (RR)]为纵坐标绘制漏斗图均基本对称, 纳入本研究的文献没有发表偏倚.
食管癌早期诊断较困难, 远处转移是食管癌远期生存的重要影响因素. 目前, 任何单一治疗方式都难以大幅度提高食管癌患者的生存率, 而研究显示多学科综合治疗能显著提高食管癌治疗效果[26]. 与传统术后辅助放化疗相比, 理论上术前放化疗具有一定优势[23]. 本文对21篇RCT行Meta分析, 收集文献较齐全, 行生存率和手术情况发生率详细分类比较, 并对远期生存率给予亚组、敏感性和漏斗图分析, 综合评估了CRTS与SA治疗可切除食管癌的优缺点. 本Meta分析结果显示: 与SA治疗可切除食管癌相比, CRTS降低了肿瘤术后局部复发率, 提高了患者生存率、肿瘤根治率、R0切除率, 提高了东西方、同步放化疗、鳞癌患者远期生存率, 且未增加术后并发症发生率、死亡率和远处转移率, 但术后并发症发生率稳健性较低. 有报道证明CRTS治疗食管癌可降低远处转移率, 部分不良反应增加, 但患者能够耐受, 可提高局部晚期食管癌的有效率、总生存率[27]. 一项更新的荟萃分析[28]显示: CRTS较SA改善了可切除食管癌患者的生存, 可能成为标准治疗措施; 这种治疗方案应用之所以在增加, 是因为其没有表现重大的不良并发症导致死亡率的增加, 可在患者中安全应用[29]; 且适当的营养支持可增加患者获得充分放化疗剂量和根治手术的可能性[30]. 本Meta分析显示CRTS对序贯放化疗及腺癌患者远期生存率未改善(P>0.05), 间接提示食管同步放化疗及鳞癌患者是CRTS的真正受益者, 也许术后放化疗能够使序贯放化疗及腺癌获益, 结果尚未明确. 同时本文还显示SA较CRTS有更高的手术率(P<0.05), 这可能跟有些术前放化疗的不良反应导致患者体质耐受较差有关. 目前CRTS的最佳方案与最佳用药剂量均未明确, 新药应用相对较少, 每项研究的化疗剂量也各不相同, 本项Meta所纳入研究中多采用"5-fluorouracil+cisplatin"方案, 因此下一步应研究术后放化疗及不同化疗方案、剂量联合术前放疗治疗可切除食管癌的差异, 期待进一步提高临床疗效.
食管癌综合治疗新辅助策略的管理是复杂的, 并和现有证据冲突, 试验设计已经取得初步建议并试图解决, 但这仍是开放的讨论和审议[31]. CRTS较术前化疗间尚未建立明显优势. 迫切需要允许个体化的多学科综合治疗局部晚期食管癌的预测指标[32]. 到目前为止, 治疗食管癌尚无公认的标准治疗方案, 但多数临床研究显示, 局部晚期食管癌CRTS并手术是一个可提高临床有效率和长期生存率较为现实可行的、有发展前景的、值得进一步研究的三联综合治疗模式, 可能会成为标准治疗方案.
总之, 与SA治疗可切除食管癌相比, CRTS降低了肿瘤术后局部复发率, 提高了患者生存率、肿瘤根治率、R0切除率, 提高了东西方、同步放化疗、鳞癌患者远期生存率, 且未增加患者术后并发症发生率、死亡率和远处转移率, 但其手术率较低. 然而, 序贯放化疗及腺癌患者的远期生存率未能从CRTS中受益. 本Meta分析来自不同种族人群, 病理类型不同; 各研究在放化疗剂量、方法及手术方式有差异, 时间跨度大等方面存在一定的局限性, 尚需更多更高质量的大型临床研究来进一步明确RCTS的作用, 并探索更为有效、安全的RCTS治疗方案.
中国食管癌粗发病率和死亡率均居世界第1位. CRTS治疗可切除食管癌得到越来越多的关注, 已进行大量的临床研究, 但研究结果不一致.
张力为, 副教授, 新疆医科大学第一附属医院胸外科
CRTS与SA相比, 能否明显改善可切除食管癌患者的生存预后及手术情况?迫切需要允许个体化的多学科综合治疗局部晚期食管癌的预测指标, 及更为有效、安全的CRTS方案.
本文提示与SA治疗可切除食管癌相比, CRTS降低了肿瘤术后局部复发率, 提高了患者的生存率、肿瘤根治率、R0切除率; 提高了东西方、同步放化疗、鳞癌患者3、5年生存率, 且不增加术后并发症发生率、死亡率和远处转移率.
CRTS比SA明显改善了可切除食管癌患者的生存预后及手术情况, 而对序贯放化疗和鳞癌患者的3、5年生存率无改善, 且最佳用药剂量尚未明确.
本文选题较好, 文章内容能从多方面进行比较, 相对全面, 具有一定的临床参考价值.
编辑: 田滢 电编:闫晋利
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] |
| 2. | Rutegård M, Charonis K, Lu Y, Lagergren P, Lagergren J, Rouvelas I. Population-based esophageal cancer survival after resection without neoadjuvant therapy: An update. Surgery. 2012;152:903-910. [PubMed] [DOI] |
| 4. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [PubMed] [DOI] |
| 5. | Nygaard K, Hagen S, Hansen HS, Hatlevoll R, Hultborn R, Jakobsen A, Mäntyla M, Modig H, Munck-Wikland E, Rosengren B. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg. 1992;16:1104-1109; discussion 1110. [PubMed] [DOI] |
| 6. | Apinop C, Puttisak P, Preecha N. A prospective study of combined therapy in esophageal cancer. Hepatogastroenterology. 1994;41:391-393. [PubMed] |
| 7. | Le Prise E, Etienne PL, Meunier B, Maddern G, Ben Hassel M, Gedouin D, Boutin D, Campion JP, Launois B. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer. 1994;73:1779-1784. [PubMed] [DOI] |
| 8. | Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462-467. [PubMed] [DOI] |
| 9. | Bosset JF, Gignoux M, Triboulet JP, Tiret E, Mantion G, Elias D, Lozach P, Ollier JC, Pavy JJ, Mercier M. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337:161-167. [PubMed] [DOI] |
| 10. | Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305-313. [PubMed] |
| 12. | Lee JL, Park SI, Kim SB, Jung HY, Lee GH, Kim JH, Song HY, Cho KJ, Kim WK, Lee JS. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol. 2004;15:947-954. [PubMed] [DOI] |
| 13. | Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, Ackland S, Gotley DC, Joseph D, Millar J. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659-668. [PubMed] [DOI] |
| 14. | Natsugoe S, Okumura H, Matsumoto M, Uchikado Y, Setoyama T, Yokomakura N, Ishigami S, Owaki T, Aikou T. Randomized controlled study on preoperative chemoradiotherapy followed by surgery versus surgery alone for esophageal squamous cell cancer in a single institution. Dis Esophagus. 2006;19:468-472. [PubMed] [DOI] |
| 15. | Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, Kiel K, Willett C, Sugarbaker D, Mayer R. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086-1092. [PubMed] [DOI] |
| 18. | Cao XF, He XT, Ji L, Xiao J, Lv J. Effects of neoadjuvant radiochemotherapy on pathological staging and prognosis for locally advanced esophageal squamous cell carcinoma. Dis Esophagus. 2009;22:477-481. [PubMed] [DOI] |
| 20. | Lv J, Cao XF, Zhu B, Ji L, Tao L, Wang DD. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol. 2010;16:1649-1654. [PubMed] [DOI] |
| 21. | 谢 军, 崔 继承, 王 根彩. 新辅助放化疗在局部晚期食管癌治疗中的价值. 南通大学学报(医学版). 2010;30:488, 490. |
| 24. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [PubMed] [DOI] |
| 25. | 杨 弘, 傅 剑华, 刘 孟忠, 方 文涛, 王 家明, 陈 于平, 陈 志坚, 相 加庆, 杨 焕军, 毛 伟敏. 术前放化疗并手术治疗局部晚期食管鳞癌的多中心随机对照临床研究. 中华医学杂志. 2012;92:1028-1032. |
| 26. | Kelly P, Appleyard V, Murray K, Paulin F, Lamont D, Baker L, Suttie S, Exon D, Thompson A. Detection of oesophageal cancer biomarkers by plasma proteomic profiling of human cell line xenografts in response to chemotherapy. Br J Cancer. 2010;103:232-238. [PubMed] [DOI] |
| 28. | Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, Gebski V. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681-692. [PubMed] [DOI] |
| 29. | Bagheri R, RajabiMashhadi MT, Ghazvini K, Asnaashari A, Zahediyan A, Sahebi MA. The effect of neoadjuvant chemoradiotherapy on airway colonization and postoperative respiratory complications in patients undergoing oesophagectomy for oesophageal cancer. Interact Cardiovasc Thorac Surg. 2012;14:725-728. [PubMed] [DOI] |
| 30. | Zemanova M, Novak F, Vitek P, Pazdro A, Smejkal M, Pazdrova G, Petruzelka L. Outcomes of patients with oesophageal cancer treated with preoperative chemoradiotherapy, followed by tumor resection: influence of nutritional factors. J BUON. 2012;17:310-316. [PubMed] |
| 31. | Hingorani M, Crosby T, Maraveyas A, Dixit S, Bateman A, Roy R. Neoadjuvant chemoradiotherapy for resectable oesophageal and gastro-oesophageal junction cancer--do we need another randomised trial? Clin Oncol (R Coll Radiol). 2011;23:696-705. [PubMed] [DOI] |
| 32. | Vallböhmer D, Schröder W, Brabender J, Hölscher AH. [Oesophageal cancer: current status of multimodality therapy]. Zentralbl Chir. 2011;136:312-316. [PubMed] [DOI] |