修回日期: 2011-12-31
接受日期: 2012-01-13
在线出版日期: 2012-01-28
目的: 观察小分子RNA(shRNA)沉默后HMG-A2基因在胃癌细胞株MKN-45的表达, 并探讨HMGA2基因对胃癌细胞的增殖与凋亡的影响.
方法: 构建针对人HMGA2基因的shRNA真核表达载体, 瞬时转染人胃癌细胞株MKN-45. 用细胞免疫组化的方法观察转染72 h后HMGA2的蛋白表达水平, 以MTT比色法、流式细胞术检测转染后MKN-45细胞的生长增殖、凋亡的情况.
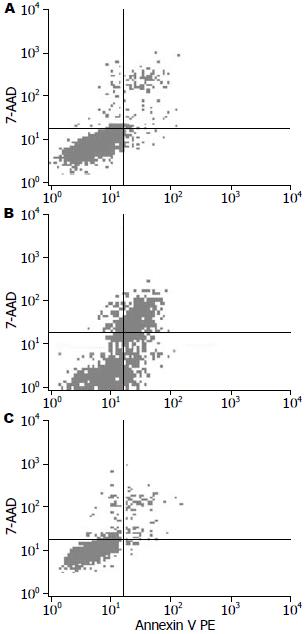
结果: 转染HMGA2-shRNA组的蛋白表达强度(171.34±19.61)明显弱于scrambled组(143.48±19.04)和空白对照组(141.79±18.09, P<0.05), 较之scrambled组(5.66%±0.63%)和空白对照组, HMGA2-shRNA组(39.32%±2.37%)能明显抑制细胞的增殖(P<0.05), HMGA2-shRNA组的凋亡率(39.67%±2.35%)与scrambled组(4.29%±1.33%)和空白对照组(5.05%±1.84%)相比明显增加(P<0.05).
结论: 靶向HMGA2基因的shRNA可以有效抑制人胃癌MKN-45细胞的生长, 并促进细胞的凋亡, HMGA2可能是胃癌治疗的一个潜在靶点.
引文著录: 陈家庄, 赖铭裕, 韦丽秀. shRNA沉默干扰HMGA2基因对胃癌细胞株MKN-45的增殖与凋亡的影响. 世界华人消化杂志 2012; 20(3): 187-192
Revised: December 31, 2011
Accepted: January 13, 2012
Published online: January 28, 2012
AIM: To investigate the effect of short hairpin RNA (shRNA)-mediated HMGA2 gene silencing on cell growth and apoptosis in human gastric cancer cell line MKN-45.
METHODS: A shRNA eukaryotic expression vector that expresses shRNA targeting the HMGA2 gene was constructed and transfected into MKN-45 cells. HMGA2 protein expression was measured by immunocytochemistry 72 hours after transfection. Cell growth and apoptosis were determined by MTT assay and flow cytometry, respectively.
RESULTS: Compared to the scrambled siRNA group and blank control group, the expression of HMGA2 protein was significantly decreased (171.34 ± 19.61 vs 143.48 ± 19.04, 141.79 ± 18.09, both P < 0.05); cell growth was significantly inhibited (39.32% ± 2.37% vs 5.66% ± 0.63%, P< 0.05); and cell apoptosis was significantly enhanced in the HMGA2-shRNA group (39.67% ± 2.35% vs 4.29% ± 1.33%, 5.05% ± 1.84%, both P < 0.05).
CONCLUSION: ShRNA-mediated HMGA2 gene silencing can effectively induce growth inhibition and apoptosis of MKN-45 cells. HMGA2 might be a potential target for the therapy of gastric cancer.
- Citation: Chen JZ, Lai MY, Wei LX. ShRNA-mediated HMGA2 gene silencing inhibits cell proliferation and promotes apoptosis in human gastric cancer cell line MKN-45. Shijie Huaren Xiaohua Zazhi 2012; 20(3): 187-192
- URL: https://www.wjgnet.com/1009-3079/full/v20/i3/187.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v20.i3.187
胃癌是人类常见的恶性肿瘤, 居全球肿瘤发病和癌症死亡率的第2位[1]. 尽管近年来对胃癌的研究取得了进展, 但大多数患者出现临床症状时都已处于晚期并出现远处转移. HMGA2是近年来研究的热点癌基因之一, 其属于高迁移率蛋白A家族中的一员, 含有3个AT-钩结构与1个酸性C末端. HMGA2蛋白是一种非组蛋白染色体蛋白, 本身缺乏转录活性, 但能通过与染色质结合而改变其结构, 或者直接与相关蛋白结合发生作用, 继而调节其他基因的转录[2]. 研究表明HMGA2蛋白的表达参与人类多种肿瘤的发生, 如大肠癌[3]、胰腺癌[4]、脂肪瘤[5]、肺癌[6]、卵巢癌[7]、输卵管癌[8]、膀胱癌[9]、垂体瘤[10]等. 这些肿瘤的发生、发展均与HMGA2基因表达异常有关, 并发现其表达增强与预后有关, 但目前人们对于肿瘤的发生机制并不清楚, 尤其是有关胃癌的发生机制研究并不多. 为探讨HMGA2基因的表达对胃癌细胞的生长、凋亡的影响, 本研究构建shRNA真核表达载体并瞬时转染MKN-45细胞, 观察沉默干扰HMGA2基因对细胞的生长、凋亡影响, 为胃癌的HMGA2靶向基因治疗提供依据.
人胃癌细胞株MKN-45购自ATCC细胞库; DMEM培养基购自Solarbio公司; 胎牛血清购自杭州四季青公司; LipofectamineTM2000购自美国Invitrogen公司; 质粒PLL2G-shHMGA2、pLL2G-scrambled均由赛业(广州)生物科技有限公司设计合成; 质粒小提试剂盒购自OMIGA公司; 人抗兔HMGA2多克隆抗体、SP试剂盒均购自北京博奥森生物技术有限公司; MTT购自Solarbio; Annexin v-FITC&PE凋亡试剂盒购自BD公司.
1.2.1 shRNA靶序列的构建与鉴定: 根据GeneBank中人HMGA2全长cDNA序列(NM.003483)设计并合成shRNA oligo: 正义链5'-TAGTCCCTCTAAAGCAGCTCAACTCGAGTTGAGCTGCTTTAGAGGGACTTTTTTC-3'; 反义链5'-TCGAGAAAAAAGTCCCTCTAAAGCAGCTCAACTCGAGTTGAGCTGCTTTAGAGGGACTA-3'. 另行设计一条与人的任何基因序列均无同源关系的随机乱码阴性(scrambled)序列: 正义链5'-TGCGCGCTTTGTAGGATTCGCTCGAGCGAATCCTACAAAGCGCGCTTTTTC-3'; 反义链5'-TCGAGAAAAAGCGCGCTTTGTAGGATTCGCTCGAGCGAATCCTACAAAGCGCGCA-3', 其形成的shRNA不对人源mRNA进行干扰. 上述质粒载体由赛业(广州)生物科技有限公司构建, 并进行测序鉴定.
1.2.2 细胞培养及瞬时转染: 人胃癌细胞MKN-45培养在含100 mL/L胎牛血清的DMEM培养基, 置于37 ℃、50 mL/L CO2培养箱中培养. 转染前1 d, 用0.25%胰酶+EDTA消化细胞并计数, 以4×105/孔的密度种在6孔板, 用含血清、无双抗的DMEM培养基放在CO2培养箱培养过夜. 使其在转染当天细胞密度达80%-90%. 分别用250 μL的DMEM稀释4 μg质粒、4 μL LipofectamineTM2000, 5 min后将两者混合, 室温放置20 min后将混合物加入到6孔板中, 用无血清的DMEM培养基继续培养, 6 h后换液. 实验分3组: 空白对照组(只加脂质体); scrambled组(转染阴性质粒); HMGA2-shRNA组(转染HMGA2的shRNA质粒).
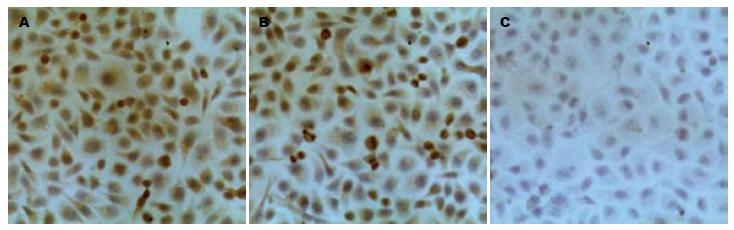
1.2.3 细胞免疫组织化学染色: 将转染72 h后的各组MKN-45细胞用40 g/L甲醛固定20 min, 纯甲醛加过氧化氢灭活内源性的过氧化物酶高压高温抗原修复10 min, SP法染色, DAB显色, 苏木素复染, 常规脱水、透明、封片, 观察结果. 采用PBS代替一抗做阴性对照. 阳性染色呈棕黄色颗粒, 位于细胞质和细胞核中. 应用全自动图像分析仪Image-Pro Plus6.0图像分析软件检测阳性细胞的平均灰度值, 灰度值越高, 表达强度越弱.
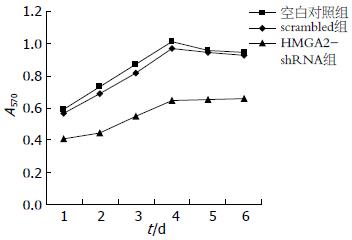
1.2.4 MTT法: 取指数生长期细胞, 以4 000个/孔的密度接种于96孔板, 每组各设3个复孔, 按照LipofectamineTM2000说明书的步骤转染, 6 h后换液. 于转染后24、48、72、96、120、144 h加20 μL MTT, 置于CO2培养箱继续培养, 4 h后小心吸取上清液, 加200 μL的DMSO, 避光振荡10 min后用酶标仪的570 nm波长检测吸光度(A)值绘制细胞生长曲线, 计算生长抑制率(%) = (1-A实验组/A对照组)×100%.
1.2.5 流式细胞术测细胞凋亡: 取转染后24、48、72 h的细胞, 用0.25%胰酶消化成单细胞悬液, 弃上清, 用冷PBS洗涤, 加Annexin v-FITC&PE凋亡试剂盒中的Buffer 100 μL, 各加5 μL ADD和5 μL PE, 室温避光放置15 min, 之后再加400 μL Buffer, 上机检测各组细胞的凋亡率.
统计学处理 应用SPSS13.0软件包进行统计学处理, 所有实验数据均以mean±SD表示, 对各指标进行单因素方差分析(one-way ANOVA), 两组均数间的比较用SNK检验, P<0.05为差异有统计学意义.
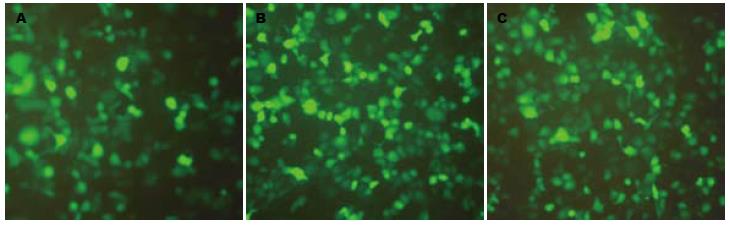
将质粒 PLL2G-shHMGA2、pLL2G-scrambled分别转染MKN-45细胞, 于转染后24、48、72 h后, 用荧光显微镜观察细胞GFP的表达情况. 结果显示48 h后的转染效率明显增高, 可达60%-70%, 72 h后转染效率相比48 h后转染效率没有明显变化(图1).
转染72 h后HMGA2蛋白在空白对照组、scrambled组有较强的表达, 二者无统计学差异(P>0.05). 而HMGA2-shRNA组较其他两组表达明显减弱, 有统计学意义(171.34±19.61 vs 143.48±19.04, 141.79±18.09, P<0.05, 图2, 表1)
空白对照组与scrambled组在各时间段细胞的生长未受影响(P>0.05), 而HMGA2-shRNA组在转染24 h后细胞生长明显受到抑制(39.32%±2.37% vs 5.66%±0.63%, P<0.05), 转染48 h后的抑制率最明显(图3, 表2), 之后该组的细胞增殖仍然被抑制, 证明沉默干扰后近、远期细胞的增殖能力减弱.
胃癌的发生、发展与原癌基因的激活, 抑癌基因的失活, 以及凋亡相关基因的异常表达等均有密切的关系. 研究表明, 凋亡障碍是胃癌发生、浸润和转移的基础. Lowed等[11]研究认为, 胃癌存在着细胞凋亡明显受抑制的现象, 且受抑制愈明显, 预后愈差, 故凋亡指数可以作为胃癌预后不良的重要指标.
HMGA2可以通过AT-hooks与DNA结合, 继而改变染色质的结构, 使之发生弯曲、拉伸、卷曲、成环或解链, 从而调节靶基因的转录, 因此又被称为构筑性转录因子. 作为一种结构转录因子, 可以对大量基因的转录和活化进行调节, 特别是那些与细胞增殖和凋亡相关的基因[12-15]. HMGA2在正常组织中低表达或不表达, 而在胚胎期以及恶性肿瘤组织中的表达明显上调[16-19]. HMGA2在肿瘤的发生、侵袭和转移过程中发挥着重要作用. Li等[20]研究认为HMGA2调节hTERT的转录从而促进肿瘤发生. Ohshima等[21]认为HMGA2与let-7家族负向调控, 与胃癌的侵袭性相关. Zha等[22]认为HMGA2的表达可能与胃癌临床转移和进展能力有关, 而其主要机制可能是HMGA2促进胃癌细胞EMT的发生, 使之具有更强的侵袭转移能力, 进而加快肿瘤进展. 在一些恶性肿瘤中, HMGA2可以作为诊断分子标志物或判断预后的独立因子. Hristov等[4]认为HMGA2在胰腺导管腺癌中表达量增加, 并与淋巴结转移和肿瘤分级呈正相关, 这可能是胰腺癌的一个重要生物标志. Wang等[3]研究发现HMGA2是大肠癌的重要生物标志物.
RNA干扰(RNA interference, RNAi)是由双链RNA(double stranded RNA, dsRNA)所引起的序列特异性基因沉默. 双链RNA经酶切后会形成很多小片段, 称为siRNA. siRNA是由21-23个核苷酸组成的小片段双链RNA, 可特异和互补靶基因mRNA序列结合, 诱导其降解, 产生强大的RNA干扰效应[23]. RNAi技术是一项高效且高特异性的基因封闭技术, 通过抑制病原微生物或肿瘤细胞的特定基因, 可导致病原微生物或肿瘤细胞死亡. RNAi技术在子宫颈癌、乳腺癌、肝癌等人类肿瘤细胞株体外抑制实验中获得了成功[24-26].
本研究结果显示, 转染HMGA2-shRNA组的HMGA2蛋白的表达量明显低于空白对照组和scrambled组, 说明沉默干扰效果明显. 转染HMGA2-shRNA组的细胞生长抑制率、细胞凋亡率明显高于空白对照组和scrambled组, 由此表明HMGA2基因表达对胃癌细胞的增殖和凋亡调控有重要的作用. 但其中的分子机制现在还未明确. De Martino等[27]研究发现HMGA2诱导转录因子E2F1活性增加并促进细胞异常增生, HMGA2还可通过与细胞周期素B2(cyclin B2, ccnb2)基因启动子结合, 上调ccnb2的活性, 增加ccnb2的表达, 从而促进细胞周期G2/M期的转化, 诱导肿瘤生长. HMGA2可以通过cAMP反应元件抑制p120E4F, 上调细胞周期素A的表达, 干扰细胞周期, 促进肿瘤发生[28]. Motoyama等[29]对110例胃癌患者的HMGA2 mRNA的表达研究发现, HMGA2 mRNA的高表达与胃癌患者的浆膜浸润淋巴结转移及静脉的浸润成正相关, 但与性别、肿块大小和组织病理的类型无明显相关性. Dicello等[6]研究发现用反义HMGA2质粒转染肺癌细胞, HMGA2的蛋白表达水平明显下降, 并对细胞的增殖有明显的抑制作用.
细胞凋亡与胃癌的发病关系已引起人们的广泛重视, 借助诱导胃癌细胞凋亡来治疗胃癌亦已成为研究的热点. 应用小分子干扰RNA技术, 剪切癌基因或凋亡抑制基因而诱导胃癌细胞的自发凋亡, 成为了胃癌治疗的一个新思路和研究[30]. 本研究表明利用RNAi干扰沉默HMGA2后, 可以通过抑制胃癌细胞的生长、促进胃癌细胞的凋亡而可能达到治疗胃癌的目的. 但是如何做到特异而持久有效的转染载体体系, 如何发现更多的基因和认识更多的基因功能, 还需在理论和技术上做出更多的努力. HMGA2基因对胃癌细胞的生长和凋亡作用的具体分子机制还有待进一步实验探索.
HMGA2是高迁移率蛋白A家族中的一员, 近年来研究的热点癌基因之一, HMGA2蛋白的表达参与人类多种肿瘤的发生, 但是发生肿瘤的机制并不是十分清楚, 特别是在胃癌的发生机制中研究并不多.
樊晓明, 主任医师, 复旦大学附属金山医院消化内科
HMGA2作为肿瘤转移和进展中的重要因子, 不仅可以作为判断预后的分子标志物, 而且还可能成为阻止胃癌进展和转移的潜在靶点.
Motoyama1等研究发现HMGA2 mRNA的高表达与胃癌患者的浆膜浸润淋巴结转移及静脉的浸润成正相关, 但与性别、肿块大小和组织病理的类型无明显相关性. OhshimaK等认为HMGA2与let-7家族负向调控以及与胃癌的侵袭性相关.
本研究通过构建shRNA真核表达载体瞬时转染MKN-45细胞, 观察沉默干扰HMGA2基因后对细胞的生长、凋亡影响, 为胃癌的HMGA2靶向基因治疗提供依据.
本文探讨了shRNA沉默干扰HMGA2基因对胃癌细胞株MKN-45的增殖与凋亡的影响. 研究设计较为合理,数据材料较为充分, 具有一定科研意义.
编辑: 张姗姗 电编:闫晋利
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] [DOI] |
| 2. | Cleynen I, Van de Ven WJ. The HMGA proteins: a myriad of functions (Review). Int J Oncol. 2008;32:289-305. [PubMed] |
| 3. | Wang X, Liu X, Li AY, Chen L, Lai L, Lin HH, Hu S, Yao L, Peng J, Loera S. Overexpression of HMGA2 promotes metastasis and impacts survival of colorectal cancers. Clin Cancer Res. 2011;17:2570-2580. [PubMed] [DOI] |
| 4. | Hristov AC, Cope L, Reyes MD, Singh M, Iacobuzio-Donahue C, Maitra A, Resar LM. HMGA2 protein expression correlates with lymph node metastasis and increased tumor grade in pancreatic ductal adenocarcinoma. Mod Pathol. 2009;22:43-49. [PubMed] [DOI] |
| 5. | Bartuma H, Panagopoulos I, Collin A, Trombetta D, Domanski HA, Mandahl N, Mertens F. Expression levels of HMGA2 in adipocytic tumors correlate with morphologic and cytogenetic subgroups. Mol Cancer. 2009;8:36. [PubMed] [DOI] |
| 6. | Di Cello F, Hillion J, Hristov A, Wood LJ, Mukherjee M, Schuldenfrei A, Kowalski J, Bhattacharya R, Ashfaq R, Resar LM. HMGA2 participates in transformation in human lung cancer. Mol Cancer Res. 2008;6:743-750. [PubMed] [DOI] |
| 7. | Malek A, Bakhidze E, Noske A, Sers C, Aigner A, Schäfer R, Tchernitsa O. HMGA2 gene is a promising target for ovarian cancer silencing therapy. Int J Cancer. 2008;123:348-356. [PubMed] [DOI] |
| 8. | Wei JJ, Wu J, Luan C, Yeldandi A, Lee P, Keh P, Liu J. HMGA2: a potential biomarker complement to P53 for detection of early-stage high-grade papillary serous carcinoma in fallopian tubes. Am J Surg Pathol. 2010;34:18-26. [PubMed] [DOI] |
| 9. | Yang GL, Zhang LH, Bo JJ, Hou KL, Cai X, Chen YY, Li H, Liu DM, Huang YR. Overexpression of HMGA2 in bladder cancer and its association with clinicopathologic features and prognosis HMGA2 as a prognostic marker of bladder cancer. Eur J Surg Oncol. 2011;37:265-271. [PubMed] [DOI] |
| 10. | Fedele M, Palmieri D, Fusco A. HMGA2: A pituitary tumour subtype-specific oncogene? Mol Cell Endocrinol. 2010;326:19-24. [PubMed] [DOI] |
| 11. | Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485-495. [PubMed] [DOI] |
| 12. | Ashar HR, Chouinard RA, Dokur M, Chada K. In vivo modulation of HMGA2 expression. Biochim Biophys Acta. 2010;1799:55-61. [PubMed] |
| 13. | Winter N, Nimzyk R, Bösche C, Meyer A, Bullerdiek J. Chromatin immunoprecipitation to analyze DNA binding sites of HMGA2. PLoS One. 2011;6:e18837. [PubMed] [DOI] |
| 14. | Thuault S, Tan EJ, Peinado H, Cano A, Heldin CH, Moustakas A. HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition. J Biol Chem. 2008;283:33437-33446. [PubMed] [DOI] |
| 15. | Summer H, Li O, Bao Q, Zhan L, Peter S, Sathiyanathan P, Henderson D, Klonisch T, Goodman SD, Dröge P. HMGA2 exhibits dRP/AP site cleavage activity and protects cancer cells from DNA-damage-induced cytotoxicity during chemotherapy. Nucleic Acids Res. 2009;37:4371-4384. [PubMed] [DOI] |
| 16. | Akaboshi S, Watanabe S, Hino Y, Sekita Y, Xi Y, Araki K, Yamamura K, Oshima M, Ito T, Baba H. HMGA1 is induced by Wnt/beta-catenin pathway and maintains cell proliferation in gastric cancer. Am J Pathol. 2009;175:1675-1685. [PubMed] [DOI] |
| 17. | Chiappetta G, Ferraro A, Vuttariello E, Monaco M, Galdiero F, De Simone V, Califano D, Pallante P, Botti G, Pezzullo L. HMGA2 mRNA expression correlates with the malignant phenotype in human thyroid neoplasias. Eur J Cancer. 2008;44:1015-1021. [PubMed] [DOI] |
| 18. | Venkatesan N, Kandalam M, Pasricha G, Sumantran V, Manfioletti G, Ono SJ, Reddy MA, Krishnakumar S. Expression of high mobility group A2 protein in retinoblastoma and its association with clinicopathologic features. J Pediatr Hematol Oncol. 2009;31:209-214. [PubMed] [DOI] |
| 19. | Meyer B, Loeschke S, Schultze A, Weigel T, Sandkamp M, Goldmann T, Vollmer E, Bullerdiek J. HMGA2 overexpression in non-small cell lung cancer. Mol Carcinog. 2007;46:503-511. [PubMed] [DOI] |
| 20. | Li AY, Lin HH, Kuo CY, Shih HM, Wang CC, Yen Y, Ann DK. High-mobility group A2 protein modulates hTERT transcription to promote tumorigenesis. Mol Cell Biol. 2011;31:2605-2617. [PubMed] [DOI] |
| 21. | Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y, Muramatsu K, Fukuda Y, Ogura S, Yamaguchi K. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One. 2010;5:e13247. [PubMed] [DOI] |
| 24. | Gao Y, Shan ZY, Wang H, Zhang HM, Teng WP. Inhibitory effect of shRNA targeting GPR48 on invasion and metastasis of human cervical carcinoma cell line HeLa. Ai Zheng. 2009;28:104-107. [PubMed] |
| 25. | Bluff JE, Amarzguioui M, Slattery J, Reed MW, Brown NJ, Staton CA. Anti-tissue factor short hairpin RNA inhibits breast cancer growth in vivo. Breast Cancer Res Treat. 2011;128:691-701. [PubMed] [DOI] |
| 26. | Zhang D, Liu J, Wang Y, Chen J, Chen T. shRNA-mediated silencing of Gli2 gene inhibits proliferation and sensitizes human hepatocellular carcinoma cells towards TRAIL-induced apoptosis. J Cell Biochem. 2011;112:3140-3150. [PubMed] [DOI] |
| 27. | De Martino I, Visone R, Wierinckx A, Palmieri D, Ferraro A, Cappabianca P, Chiappetta G, Forzati F, Lombardi G, Colao A. HMGA proteins up-regulate CCNB2 gene in mouse and human pituitary adenomas. Cancer Res. 2009;69:1844-1850. [PubMed] [DOI] |
| 28. | Tessari MA, Gostissa M, Altamura S, Sgarra R, Rustighi A, Salvagno C, Caretti G, Imbriano C, Mantovani R, Del Sal G. Transcriptional activation of the cyclin A gene by the architectural transcription factor HMGA2. Mol Cell Biol. 2003;23:9104-9116. [PubMed] [DOI] |
| 29. | Motoyama K, Inoue H, Nakamura Y, Uetake H, Sugihara K, Mori M. Clinical significance of high mobility group A2 in human gastric cancer and its relationship to let-7 microRNA family. Clin Cancer Res. 2008;14:2334-2340. [PubMed] [DOI] |