修回日期: 2012-07-12
接受日期: 2012-08-01
在线出版日期: 2012-08-28
目的: 研究活化转录因子2(activating transcription factor-2, ATF2)在人胰腺癌细胞系Capan2中高表达能否促使转化生长因子β1(transforming growth factor-β1, TGF-β1)诱导Capan2细胞上皮-间充质转化(epithelial-mesenchymal transition, EMT).
方法: 应用PGEX-ATF2质粒瞬时转染Capan2细胞系, 并继续用TGF-β1诱导, 以仅加DMSO组为空白对照组, 倒置显微镜观察细胞形态学变化, Western blot检测E-cadherin、vimentin、ATF2的表达. Transwell小室侵袭实验验证侵袭能力的变化.
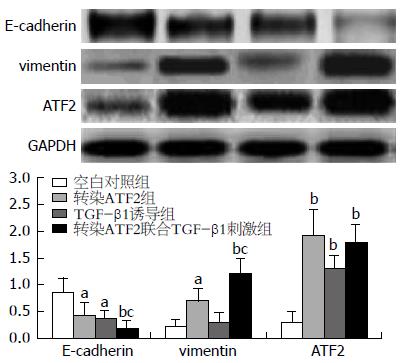
结果: 与空白对照组细胞相比, 转染ATF2并用TGF-β1诱导的Capan2细胞系能够明显改变细胞形态, 并且使得E-cadherin的蛋白水平表达明显下调, vimentin、ATF2的蛋白水平表达则显著上调, 细胞侵袭能力也显著增加(P = 0.008).
结论: ATF2能够与TGF-β1联合共同诱导Capan2细胞系产生EMT, 从而对胰腺癌的分子靶向治疗找到新的方向.
引文著录: 许元鸿, 刘哲, 郭克建, 杜瑞霞. 一个新的促进人胰腺癌细胞上皮-间充质转化因子: ATF-2. 世界华人消化杂志 2012; 20(24): 2265-2269
Revised: July 12, 2012
Accepted: August 1, 2012
Published online: August 28, 2012
AIM: To investigate whether ATF2 together with TGF-β1 can induce epithelial-mesenchymal transition (EMT) in human pancreatic cancer cell line Capan2.
METHODS: Capan2 cells were induced with TGF-β1 after transfection with PGEX-ATF2, and the negative control group was treated with DMSO. Cell morphological alternations were examined by phase contrast microscopy. The expression of mesenchymal marker vimentin and epithelial markers E-cadherin and ATF2 were detected by Western blot. Cell migration was determined by Transwell motility assay.
RESULTS: Compare to the negative control group, cells transfected with ATF2 and treated with TGF-β1 showed loss of cell-cell contacts, fibroblastic morphology, decreased expression of E-cadherin, up-regulated expression of vimentin and ATF2, and increased migration ability (P = 0.008).
CONCLUSION: ATF2 together with TGF-β1 can induce EMT in human pancreatic cancer cell line Capan2. ATF2 may be a new molecular target for therapy of pancreatic cancer.
- Citation: Xu YH, Liu Z, Guo KJ, Du RX. ATF2, a novel factor promoting epithelial-mesenchymal transition in human pancreatic cancer cells. Shijie Huaren Xiaohua Zazhi 2012; 20(24): 2265-2269
- URL: https://www.wjgnet.com/1009-3079/full/v20/i24/2265.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v20.i24.2265
在美国, 胰腺癌在各种癌症死因中列第4位. 其早期诊断困难, 对化疗及其他疗法相对不敏感, 5年生存率仅为3%-5%[1]. 进一步解析胰腺癌发生、发展的分子机制, 对胰腺癌的诊断和治疗将产生深远的影响. 活化转录因子2(activating transcription factor-2, ATF2), 作为AP-1成员之一, 其亮氨酸拉链结构(bZIP)由C端的亮氨酸二聚化结合区和N端的碱性DNA结合区组成[2,3]. 鼠NMuMG细胞由TGF-β介导的细胞上皮-间充质转化(epithelial-mesenchymal transition, EMT)可由于P38 MAPK通路特异性的抑制剂SB202190的加入而使磷酸化的ATF2表达降低[4]. EMT自20余年前被提出以来, 一直受到广泛的关注. 经典的诱导EMT的因子为TGF-β, 另外还有表皮生长因子(epidermal growth factor, EGF)、肝细胞生长因子(hepatocyte growth factor, HGF)、成纤维细胞生长因子(fibroblast growth factor, FGF)等. EMT使细胞失去极性, 丢失细胞间紧密连接和黏附连接, 获得了浸润性和游走迁移能力. 目前已经在乳腺癌、口腔鳞癌、肝癌、胰腺癌等多种癌症中得到证明[5-8]. 本实验中, 我们证明转染ATF2后的Capan2细胞对TGF-β1的刺激更加敏感, 形成EMT. 我们推测ATF2可以与TGF-β1协同作用使得EMT的产生变得更加容易.
兔抗羊E-cadherin、vimentin单克隆抗体及兔抗ATF2多克隆抗体购自Santa Cruz公司; TGF-β1购自Repro tech公司.
1.2.1 细胞培养及转染: 人胰腺癌细胞株Capan2由中国医科大学普外实验室盛伟伟博士馈赠. 培养于含100 mL/L胎牛血清的DMEM培养基中, 置于37 ℃、50 mL/L CO2培养箱中, 每2 d换液1次, 每3-4 d传代1次. 表达ATF2质粒pGEX-ATF2由美国(Center for Cancer and Stem Cell Biology, Houston, USA) Jin C.教授馈赠. 当细胞在6孔板上培养达到70%-80%融合时, 用Lipofectamine 2000将PGEX-ATF2或PGEX-质粒按照说明书瞬时转染至Capan2细胞中, 48 h后转染ATF2及空白PGEX质粒的Capan2细胞继续用含有10 μg/L TGF-β1的DMEM培养基培养, 空白对照组仅加等量的DMSO, 48 h后, 倒置相差显微镜观察细胞形态变化.
1.2.2 Western blot检测: Western blot蛋白印迹检测ATF2、E-cadherin、vimentin表达: 严格按照RIPA蛋白裂解液试剂盒说明书进行各组蛋白的提取, 将蛋白与5×蛋白上样缓冲液以4:1混合后煮沸变性5 min, 置于10% SDS-聚丙烯酰胺凝胶电泳分离蛋白, 分离的蛋白转移至PVDF膜上之后, 用5%脱脂奶粉配置的TBST室温封闭2 h, 加入ATF2(1:800)、E-cadherin(1:800)、vimentin(1:1 000)、GAPDH(1:1 500)一抗后4 ℃封闭过夜, 辣根过氧化物酶标记的二抗室温2 h, TBST洗膜, 5 min×4次, 化学发光法(electrochemiluminescence, ECL)显色. 实验重复3次.
1.2.3 Transwell小室侵袭实验: Transwell小室上铺100 mg/L Matrigel生物胶50 μL, 下室中加入含150 mL/L胎牛血清的DMEM培养基600 μL. 所有实验细胞均调整密度为1×106个/孔, 种于小室上室, 放于培养箱中培养24 h. 取出PBS冲洗后, 4%多聚甲醛固定, 0.1%结晶紫染色, 200倍显微镜下计数, 任意选取5个视野, 以其平均细胞数为侵袭至膜上的细胞数, 每组重复3次实验, 求取平均数为结果.
统计学处理 所有计量资料均采用mean±SD表示, 采用SPSS13.0软件对结果进行分析, 应用t检验统计分析; P<0.05有统计学意义.
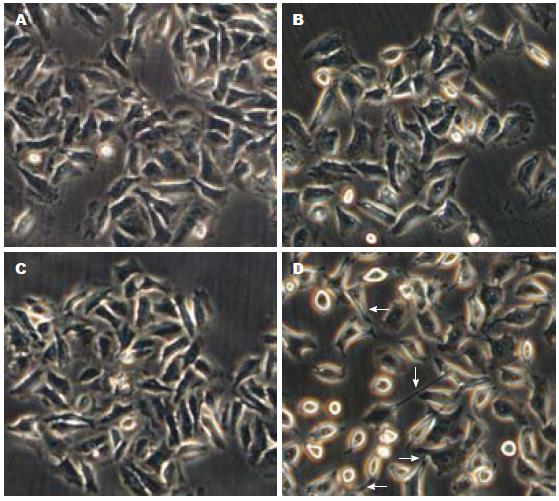
与空白对照组相比, Capan2细胞在转染ATF2后更容易受到TGF-β1的刺激, 大部分细胞变成梭形, 呈现明显的间叶细胞的形态(图1A, D). 单独ATF2转染或单独TGF-β1刺激组则没有出现明确细胞形态学的变化(图1B, C).
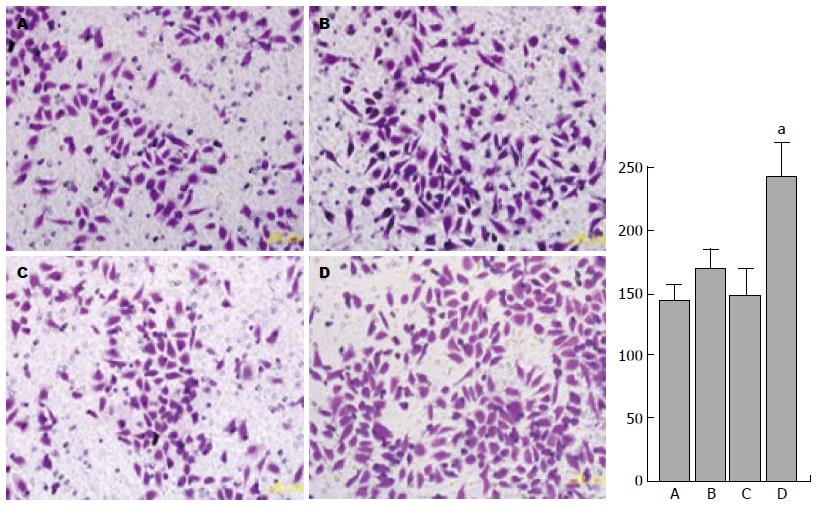
应用Western blot的方法检测上皮相关标志物E-cadherin和间质相关标志物vimentin以及ATF2的蛋白表达. 与空白对照组、转染ATF2组及TGF-β1诱导组细胞相比, 转染ATF2联合TGF-β1刺激组细胞vimentin、ATF2表达显著升高, 而E-cadherin的表达则是显著降低. 细胞的侵袭能力增高(图2), 提示明确的上皮向间质转化的趋势. 细胞的侵袭能力也是EMT的标志之一, 为此我们应用Transwell细胞侵袭实验验证各组细胞的侵袭能力. AT细胞的侵袭能力明显大于其他组细胞(图3).
在本实验中, 我们在已经转染ATF2的Capan2细胞培养基中加入10 μg/L TGF-β1, 48 h后观察到大部分细胞形态学发生显著变化, 同时Western blot也检测到了vimentin、ATF2表达的升高及E-cadherin表达的降低, 而在A或T细胞中这种变化不明显. 这提示我们, ATF2可以与TGF-β1协同作用使Capan2细胞产生EMT. EMT目前受到了广泛的关注, 他与肿瘤的侵袭、转移, 特别是早期转移有极为密切的关系, 而且可以通过多条途径诱导产生. TGF-β与其受体结合后, 激活细胞内Smad信号通路, 通过抑制E-cadherin的表达来诱导EMT发生[9,10]; 韩磊等[11]则发现, Genistein具有明显的抑制EMT的作用; 而在人胰腺癌中, Collagen I通过激活JNK1而使得胰腺癌的N-cadherin表达上调从而增加其侵袭能力[12], 单独转染带有SNAIL的质粒便可使BxPC3细胞产生EMT[13]. 癌基因k-Ras的突变是胰腺癌发生早期关键事件之一, 大约有30%的早期胰腺癌及几乎100%进展期胰腺癌伴有此基因的突变[14-16]. 被激活的k-Ras可以通过包括MEKK/SEK/JNK/AP-1(ATF-2/Jun)途径在内的多种传导途径来诱导细胞的转化[14-16]. c-Jun在胰腺癌中的表达增高, 证明Ras/AP-1传导途径有可能参与胰腺癌的发生.
而ATF2作为AP-1成员之一, 他可以同c-Jun、CREB形成二聚体[17,18], 在黑色素瘤细胞中, ATF2的表达与患者的预后密切相关[19]. 在乳腺癌中ATF2稳定表达则与肿瘤细胞对DNA的损伤耐受有关[20]. ATF2对脑的正常发育非常关键, 因为敲除ATF2的小鼠大脑发育异常, 同时造成内耳、小脑及脑室的结构缺陷[21]. 在人结直肠癌细胞系中加入托芬那酸(tolfenamic acid, TA)刺激后, 磷酸化的ATF2表达升高, 同时伴有ATF3的表达水平的升高. 我们在前期研究中还发现作为AP-1类因子的抑制因子, JDP2可以逆转由TGF-β1诱导产生的人胰腺癌Panc-1细胞系的EMT[22]. 而且ATF2的表达升高是通过P38 MAPK、JNK、ERK通路的激活来实现的[23]. 由上所述, ATF2极可能通过诸如ERK、JNK等通路与胰腺癌的EMT之间存在关联, 但是其确切机制目前研究仍是空白.
总之, 转染ATF2的胰腺癌Capan2细胞对TGF-β1的诱导变得敏感, 产生EMT. 由此可见, ATF2与胰腺癌的侵袭、转移密切相关, 将为胰腺癌的分子靶向治疗提供新的方向.
胰腺癌是常见的恶性肿瘤, 由于其早期发生微转移及其临床症状出现较晚而令其治疗效果不佳; 活化转录因子2(ATF2), 作为AP-1类的因子之一, 在多种恶性肿瘤中均有不同程度的表达, 极可能成为胰腺癌研究的新方向.
陈光, 教授, 吉林大学第一医院消化器官外科
关于恶性肿瘤侵袭、转移机制的研究目前已经成为研究肿瘤发生与发展的热点; ATF2, 作为与EMT密切相关的一种因子, 极可能为胰腺癌特别是针对胰腺癌早期转移的治疗提供新的靶点.
OPN及VEGF-C在乳腺癌、胰腺癌、肺癌等多种肿瘤组织中表达较正常组织表达增高, 且其表达增高与肿瘤的淋巴结转移密切相关.
本研究将PGEX-ATF2质粒转染至胰腺癌细胞系Capan2中, 而此高表达ATF2的细胞系则促使TGF-β1诱导形成EMT, 此研究国内外未见报道.
本研究主要探讨过表达ATF2的胰腺癌细胞系Capan2在TGF-β1的诱导下形成了典型的EMT, 为进一步研究胰腺癌的治疗提供了新的方向.
该论文设计合理, 实验数据及图表详实可靠, 方法具有一定创新性, 为临床治疗胰腺癌提供了很好的借鉴.
编辑: 张姗姗 电编:闫晋利
| 1. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106-130. [PubMed] [DOI] |
| 2. | Landschulz WH, Johnson PF, McKnight SL. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988;240:1759-1764. [PubMed] [DOI] |
| 3. | Glover JN, Harrison SC. Crystal structure of the heterodimeric bZIP transcription factor c-Fos-c-Jun bound to DNA. Nature. 1995;373:257-261. [PubMed] [DOI] |
| 4. | Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci. 2002;115:3193-3206. [PubMed] |
| 5. | Wendt MK, Smith JA, Schiemann WP. Transforming growth factor-β-induced epithelial-mesenchymal transition facilitates epidermal growth factor-dependent breast cancer progression. Oncogene. 2010;29:6485-6498. [PubMed] [DOI] |
| 6. | Maseki S, Ijichi K, Tanaka H, Fujii M, Hasegawa Y, Ogawa T, Murakami S, Kondo E, Nakanishi H. Acquisition of EMT phenotype in the gefitinib-resistant cells of a head and neck squamous cell carcinoma cell line through Akt/GSK-3β/snail signalling pathway. Br J Cancer. 2012;106:1196-1204. [PubMed] [DOI] |
| 7. | Giangreco A, Lu L, Vickers C, Teixeira VH, Groot KR, Butler CR, Ilieva EV, George PJ, Nicholson AG, Sage EK. β-Catenin determines upper airway progenitor cell fate and preinvasive squamous lung cancer progression by modulating epithelial-mesenchymal transition. J Pathol. 2012;226:575-587. [PubMed] [DOI] |
| 8. | Wang Z, Li Y, Ahmad A, Banerjee S, Azmi AS, Kong D, Sarkar FH. Pancreatic cancer: understanding and overcoming chemoresistance. Nat Rev Gastroenterol Hepatol. 2011;8:27-33. [PubMed] [DOI] |
| 9. | Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A. Transforming growth factor-beta employs HMGA2 to elicit epithelial-mesenchymal transition. J Cell Biol. 2006;174:175-183. [PubMed] [DOI] |
| 10. | Thuault S, Tan EJ, Peinado H, Cano A, Heldin CH, Moustakas A. HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition. J Biol Chem. 2008;283:33437-33446. [PubMed] [DOI] |
| 12. | Shintani Y, Hollingsworth MA, Wheelock MJ, Johnson KR. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 2006;66:11745-11753. [PubMed] [DOI] |
| 13. | Nishioka R, Itoh S, Gui T, Gai Z, Oikawa K, Kawai M, Tani M, Yamaue H, Muragaki Y. SNAIL induces epithelial-to-mesenchymal transition in a human pancreatic cancer cell line (BxPC3) and promotes distant metastasis and invasiveness in vivo. Exp Mol Pathol. 2010;89:149-157. [PubMed] [DOI] |
| 14. | Torrisani J, Bournet B, Cordelier P, Buscail L. [New molecular targets in pancreatic cancer]. Bull Cancer. 2008;95:503-512. [PubMed] |
| 15. | Mackenzie RP, McCollum AD. Novel agents for the treatment of adenocarcinoma of the pancreas. Expert Rev Anticancer Ther. 2009;9:1473-1485. [PubMed] [DOI] |
| 16. | Furukawa T. Molecular pathology of pancreatic cancer: implications for molecular targeting therapy. Clin Gastroenterol Hepatol. 2009;7:S35-S39. [PubMed] [DOI] |
| 17. | Benbrook DM, Jones NC. Heterodimer formation between CREB and JUN proteins. Oncogene. 1990;5:295-302. [PubMed] |
| 18. | van Dam H, Castellazzi M. Distinct roles of Jun Fos and Jun ATF dimers in oncogenesis. Oncogene. 2001;20:2453-2464. [PubMed] [DOI] |
| 19. | Berger AJ, Kluger HM, Li N, Kielhorn E, Halaban R, Ronai Z, Rimm DL. Subcellular localization of activating transcription factor 2 in melanoma specimens predicts patient survival. Cancer Res. 2003;63:8103-8107. [PubMed] |
| 20. | Papassava P, Gorgoulis VG, Papaevangeliou D, Vlahopoulos S, van Dam H, Zoumpourlis V. Overexpression of activating transcription factor-2 is required for tumor growth and progression in mouse skin tumors. Cancer Res. 2004;64:8573-8584. [PubMed] [DOI] |
| 21. | Mei Y, Yuan Z, Song B, Li D, Ma C, Hu C, Ching YP, Li M. Activating transcription factor 3 up-regulated by c-Jun NH(2)-terminal kinase/c-Jun contributes to apoptosis induced by potassium deprivation in cerebellar granule neurons. Neuroscience. 2008;151:771-779. [PubMed] [DOI] |