修回日期: 2012-07-26
接受日期: 2012-08-06
在线出版日期: 2012-08-28
目的: 评价骨桥蛋白(osteopontin, OPN)和血管内皮生长因子C(vascular endothelial growth factor C, VEGF-C)在胃癌中表达及与临床病理参数之间相关性, 进一步探讨二者的共表达在胃癌淋巴结转移中的机制.
方法: 收集复旦大学附属中山医院手术切除胃癌组织标本及患者相关临床资料, 对纳入研究的93例胃癌原发灶标本采用免疫组织化学染色法(EnVision二步法), 检测OPN与VEGF-C蛋白的表达.
结果: 93例胃癌组织中OPN与VEGF-C的阳性表达率分别为64.5%(60/93)和69.9%(65/93), 在非肿瘤性胃黏膜中均未见其阳性表达; OPN和VEGF-C的表达与胃癌浆膜侵犯、TNM分期以及淋巴结转移呈明显相关(P<0.05). 蛋白之间的相关性分析显示, OPN与VEGF-C之间存在正相关(r = 0.493, P<0.01).
结论: 联合检测OPN与VEGF-C有助于阐述胃癌发生发展、浸润转移的机制, OPN可能通过上调VEGF-C的表达促进胃癌的淋巴结转移.
引文著录: 刘秀平, 陈世耀, 高虹, 潘勤聪. OPN和VEGF-C在胃癌中的表达及其临床意义. 世界华人消化杂志 2012; 20(24): 2243-2247
Revised: July 26, 2012
Accepted: August 6, 2012
Published online: August 28, 2012
AIM: To evaluate the expression of osteopontin (OPN) and vascular endothelial growth factor C (VEGF-C) in gastric cancer and to analyze their correlation with clinical pathological characteristics of gastric cancer.
METHODS: Surgical specimens of gastric cancer and related clinical data were collected from the Affiliated Zhongshan Hospital of Fudan University. The expression of OPN and VEGF-C proteins in 93 gastric cancer specimens was examined by immunohistochemistry.
RESULTS: The positive rates of OPN and VEGF-C expression in 93 gastric cancer specimens were 64.5% (60/93) and 69.9% (65/93), respectively. The expression of OPN and VEGF-C was significantly correlated with serosal invasion, tumor TNM stage, and lymph node metastasis (all P < 0.05). Moreover, the expression of OPN was positively correlated with that of VEGF-C in gastric cancer (r = 0.493, P < 0.01).
CONCLUSION: OPN and VEGF-C protein expression might be associated with the development, invasion, and metastasis of gastric cancer. OPN may play a role in the lymph node metastasis of gastric cancer by up-regulating the expression of VEGF-C.
- Citation: Liu XP, Chen SY, Gao H, Pan QC. Clinical significance of OPN and VEGF-C expression in gastric cancer. Shijie Huaren Xiaohua Zazhi 2012; 20(24): 2243-2247
- URL: https://www.wjgnet.com/1009-3079/full/v20/i24/2243.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v20.i24.2243
胃癌是一种严重危害人类健康, 高发病率、高死亡率的恶性肿瘤. 淋巴转移是胃癌的主要扩散途径, 相当多的患者因出现淋巴广泛转移丧失了根治的机会. 因此除了实现胃癌的早期诊断外, 采取有效的治疗措施, 防止胃癌的淋巴转移意义重大, 但目前胃癌淋巴转移的机制尚不明确. 骨桥蛋白(osteopontin, OPN)是一种多功能分泌型钙结合磷酸化糖蛋白, 在多种实体瘤如肝癌、乳腺癌、头颈部肿瘤等大部分肿瘤及其转移复发组织中表达明显升高, 且OPN的过表达与肿瘤的高侵袭力、转移潜力以及预后差密切相关[1-3]. 血管内皮生长因子(vascular endothelial growth factor, VEGF)是具旁分泌功能的生长因子, 强烈促进内皮细胞分化增生, VEGF-C是VEGF家族中的新成员, 在淋巴管生成及淋巴转移中起到重要作用. 我们采用免疫组织化学方法研究OPN、VEGF-C在胃癌组织中的表达与胃癌临床病理因素的关系, 以及二者之间的相关性来探索OPN在胃癌淋巴转移中的作用.
病理标本为住院接受胃癌手术患者. 入选标准包括: (1)术前胃镜及病理证实胃癌患者; (2)接受胃癌根治术; (3)年龄≥18岁. 排除标准包括: (1)术前已接受放疗、化疗患者; (2)姑息性切除患者; (3)术前合并其他恶性肿瘤或有恶性肿瘤病史者; (4)已行胃大部切除的残胃癌患者. 共93例, 发生淋巴转移47例, 术中也发现肿大淋巴结但术后病理未发现癌细胞46例; 胃癌癌灶累及浆膜64例, 28例未累及浆膜; TNM分期Ⅰ-Ⅱ期47例, Ⅲ-Ⅳ期46例.
1.2.1 免疫组织化学染色: 采用免疫组织化学染色(EnVision二步法), 同时以PBS替代一抗作为阴性对照, 已知阳性切片作为阳性对照. 一抗分别为鼠抗人OPN单克隆抗体(购自Santa Cruz公司)、兔抗人多克隆抗体VEGF-C(购自美国Zymed公司), DAB显色.
1.2.2 结果判定: OPN和VEGF-C阳性细胞均以胞浆内出现明显棕黄色颗粒为主. OPN结果判定: 参照说明书进行, 选择5个高倍视野, 按阳性细胞数占同类细胞的百分比, 将结果分为: 阴性(-), <5%; 弱阳性(+), 5%-25%; 阳性(++), 26%-50%; 强阳性(+++), >50%, 其中(-, +)为OPN不表达或弱表达, (++, +++)为OPN高表达. VEGF-C结果判定: 以至少阳性细胞数≥30%为VEGF-C表达阳性[4].
统计学处理 应用SPSS11.5统计软件进行数据处理. 比较胃癌不同临床分期、不同浸润深度及有无淋巴结转移组间OPN、VEGF-C蛋白表达率差异采用χ2检验; OPN与VEGF-C之间的关系采用Spearman等级相关分析.
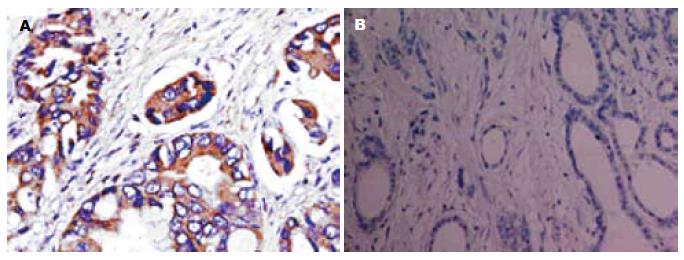
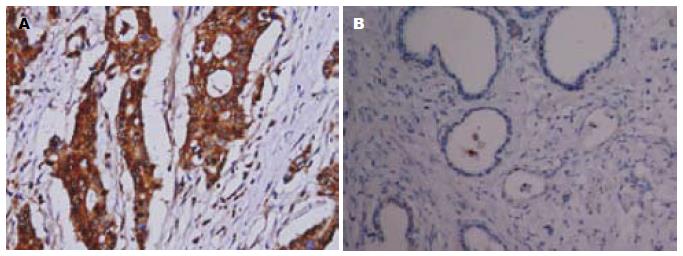
OPN和VEGF-C阳性染色均定位于细胞浆, 呈棕黄色颗粒, 其表达呈明显异质性. 但在癌旁非肿瘤组织中未见二者过表达(图1, 2).
93例胃癌组织中OPN阳性表达率为64.5%(60/93). OPN在伴发淋巴结转移者中的表达率高于无淋巴结转移者, 并与胃癌的临床分期、有无浆膜侵犯相关(P<0.05, 表1).
| 临床病理因素 | n | OPN | P值 | |
| 阳性n(%) | 阴性n(%) | |||
| 浆膜侵犯 | ||||
| 无 | 28 | 16(57.1) | 12(42.9) | 0.000 |
| 有 | 64 | 43(67.2) | 21(32.8) | |
| 淋巴结转移 | ||||
| 无 | 46 | 23(50.0) | 23(50.0) | 0.000 |
| 有 | 47 | 37(78.7) | 10(21.3) | |
| TNM分期 | ||||
| I/II | 47 | 29(61.7) | 18(38.3) | 0.000 |
| III/IV | 46 | 31(67.4) | 15(32.6) | |
93例胃癌组织中VEGF-C阳性表达率69.9%(65/93). VEGF-C同样在伴发淋巴结转移者中的阳性表达率高于无淋巴结转移者, 其表达与胃癌的临床分期、有无浆膜侵犯相关(P<0.05, 表2).
| 临床病理因素 | n | VEGF-C | P值 | |
| 阳性n(%) | 阴性n(%) | |||
| 浆膜侵犯 | ||||
| 无 | 28 | 18(64.3) | 10(35.7) | 0.000 |
| 有 | 64 | 47(73.4) | 17(26.6) | |
| 淋巴结转移 | ||||
| 无 | 46 | 26(56.5) | 20(43.5) | 0.000 |
| 有 | 47 | 39(83.0) | 8(17.0) | |
| TNM分期 | ||||
| I/II | 47 | 30(63.8) | 17(37.2) | 0.000 |
| III/IV | 46 | 35 (76.1) | 11(23.9) | |
OPN是一种与恶性转化有关的钙结合磷酸化蛋白, 其分子中心有一个特殊的RGD序列(Arg-Gly-Asp), 是某些种类的整合素(如整合素αvβ3, αvβ5)和CD44的配体, 介导细胞-基质的相互作用及相关的细胞信号途径, 从而在体内发挥其促进细胞黏附趋化、抑制凋亡、促进肿瘤转移等作用. VEGF-C是VEGF家族成员, 主要产生于肿瘤细胞, 其受体是VEGFR-3, 主要表达于淋巴管内皮, VEGF-C可与淋巴管内皮细胞表面的VEGFR-3受体特异性结合, 继而诱导毛细淋巴管的增殖和生长, 促进肿瘤内淋巴管生成, 并降低内皮细胞间的黏附, 增加淋巴管的通透性, 最终导致淋巴结转移的发生[5,6]. OPN及VEGF-C在乳腺癌[5,7]、胰腺癌[6,8]、肺癌[9]等多种肿瘤组织中表达较正常组织表达增高, 且其表达增高与肿瘤的淋巴结转移密切相关. 我们的研究显示OPN和VEGF-C在胃癌组织中高表达, 且二者的表达水平均与胃癌的TNM分期、浆膜浸润以及淋巴结转移密切相关, 与其他国内外文献报道结果一致.
OPN在胃癌淋巴转移中的作用一直引起人们的兴趣, 研究表明胃癌患者血浆及癌组织中OPN较正常对照明显升高, 在伴有淋巴管侵犯及淋巴结转移的患者中升高更为明显[10]; Ue等[11]也报道胃癌组织中OPN的mRNA表达与癌旁及正常胃黏膜组织相比表达明显增高, 免疫组织化学显示OPN与CD44的共表达与胃癌淋巴转移和远处转移有关, 且有趣的是OPN和CD44强烈的免疫反应与淋巴管集簇的形成显著相关; Allan等[12]也通过临床和实验室方法证实OPN参与了乳腺癌的淋巴管道转移过程.
一些研究表明OPN可能作为上游基因通过影响HGF、EGF、VEGF、COX-2、MMP等一些细胞因子及受体相互作用参与肿瘤的发生和转移[9,13-19]. Chakraborty等[20]研究认为OPN可通过内外分泌机制促进VEGF依赖乳腺癌血管生长及血管生成. Yang等[21]研究发现采用siRNA沉默OPN基因表达可导致转染乳腺癌细胞VEGF蛋白表达明显下调. 也有报道持相反意见认为VEGF能够诱导内皮细胞表达OPN和整合素αvβ3[22,23]. 我们的研究也提示OPN与VEGF-C在胃癌中存在共表达及正相关现象, 这与Tang等[24]的研究结果相似. 此外也有研究发现OPN与VEGF在乳腺癌、卵巢癌等其他恶性肿瘤中共表达[25,26], 提示二者可能通过相互作用促进胃癌以及其他恶性肿瘤的淋巴转移.
由于OPN在人类多种肿瘤中表达上调, 并且在一些癌前病变组织中也检测到OPN的存在, 提示OPN的过表达可能是肿瘤发生及转移过程中的早期事件. 由于OPN可激活NF-κB等信号转导通路, 该通路可进一步激活许多转移相关基因如MMP[27,28]、COX-2[29,30]等, 结合我们的实验结果猜测, 作为一个早期肿瘤转移相关蛋白, OPN可能通过次第调节VEGF-C等下游基因最终导致肿瘤淋巴转移的发生. 以上结论为我们进一步研究OPN在胃癌淋巴转移中的作用及调节的分子机制提供了思路.
骨桥蛋白(OPN)在多种肿瘤组织中表达较正常组织增高, 其表达增高与肿瘤的淋巴结转移密切相关, 其在胃癌淋巴转移中的作用一直被人们重视; 血管内皮生长因子C(VEGF-C)是VEGF家族中的新成员, 在淋巴管生成及淋巴转移中起到重要作用.
李增山, 副教授, 中国人民解放军第四军医大学病理教研室
淋巴转移是胃癌的主要扩散途径, 相当多的患者因出现淋巴广泛转移丧失了根治的机会. 因此除了实现胃癌的早期诊断外, 采取有效的治疗措施, 防止胃癌的淋巴转移意义重大, 但目前胃癌淋巴转移的机制尚不明确.
OPN及VEGF-C在乳腺癌、胰腺癌、肺癌等多种肿瘤组织中表达较正常组织表达增高, 且其表达增高与肿瘤的淋巴结转移密切相关.
本文研究了OPN和VEGF-C在胃癌中的表达, 并分析了其与胃癌临床病理特征之间的关系, 对于进一步研究两者与胃癌生物学行为之间的关系有一定的提示意义, 同时对进一步研究OPN在胃癌淋巴转移中的作用及分子机制提供了思路.
本文整体设计较为合理, 结论明确, 论述有据, 对于进一步研究OPN和VEGF-C与胃癌生物学行为之间的关系有一定提示意义.
编辑: 张姗姗 电编:闫晋利
| 1. | Lin F, Li Y, Cao J, Fan S, Wen J, Zhu G, Du H, Liang Y. Overexpression of osteopontin in hepatocellular carcinoma and its relationships with metastasis, invasion of tumor cells. Mol Biol Rep. 2011;38:5205-5210. [PubMed] [DOI] |
| 2. | Mi Z, Bhattacharya SD, Kim VM, Guo H, Talbot LJ, Kuo PC. Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis. 2011;32:477-487. [PubMed] [DOI] |
| 3. | Yang G, Zhang Y, Wu J, Xiong J, Deng H, Wang J, Yang C, Zhu Z. Osteopontin regulates growth and migration of human nasopharyngeal cancer cells. Mol Med Report. 2011;4:1169-1173. [PubMed] |
| 4. | Amioka T, Kitadai Y, Tanaka S, Haruma K, Yoshihara M, Yasui W, Chayama K. Vascular endothelial growth factor-C expression predicts lymph node metastasis of human gastric carcinomas invading the submucosa. Eur J Cancer. 2002;38:1413-1419. [PubMed] [DOI] |
| 5. | Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192-198. [PubMed] [DOI] |
| 6. | Tang RF, Itakura J, Aikawa T, Matsuda K, Fujii H, Korc M, Matsumoto Y. Overexpression of lymphangiogenic growth factor VEGF-C in human pancreatic cancer. Pancreas. 2001;22:285-292. [PubMed] [DOI] |
| 7. | Rudland PS, Platt-Higgins A, El-Tanani M, De Silva Rudland S, Barraclough R, Winstanley JH, Howitt R, West CR. Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res. 2002;62:3417-3427. [PubMed] |
| 8. | Koopmann J, Fedarko NS, Jain A, Maitra A, Iacobuzio-Donahue C, Rahman A, Hruban RH, Yeo CJ, Goggins M. Evaluation of osteopontin as biomarker for pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13:487-491. [PubMed] |
| 9. | Shijubo N, Uede T, Kon S, Maeda M, Segawa T, Imada A, Hirasawa M, Abe S. Vascular endothelial growth factor and osteopontin in stage I lung adenocarcinoma. Am J Respir Crit Care Med. 1999;160:1269-1273. [PubMed] |
| 10. | Wu CY, Wu MS, Chiang EP, Wu CC, Chen YJ, Chen CJ, Chi NH, Chen GH, Lin JT. Elevated plasma osteopontin associated with gastric cancer development, invasion and survival. Gut. 2007;56:782-789. [PubMed] [DOI] |
| 11. | Ue T, Yokozaki H, Kitadai Y, Yamamoto S, Yasui W, Ishikawa T, Tahara E. Co-expression of osteopontin and CD44v9 in gastric cancer. Int J Cancer. 1998;79:127-132. [PubMed] [DOI] |
| 12. | Allan AL, George R, Vantyghem SA, Lee MW, Hodgson NC, Engel CJ, Holliday RL, Girvan DP, Scott LA, Postenka CO. Role of the integrin-binding protein osteopontin in lymphatic metastasis of breast cancer. Am J Pathol. 2006;169:233-246. [PubMed] [DOI] |
| 13. | Zhang QH, Qian K, Li XJ, Pu J, Wu XT. [Experimental study of the hepatocyte growth factor contributing to lymphangiogenesis and lymphatic metastasis in gastric cancer]. Zhonghua Weichang Waike Zazhi. 2007;10:212-216. [PubMed] |
| 14. | Zhang G, He B, Weber GF. Growth factor signaling induces metastasis genes in transformed cells: molecular connection between Akt kinase and osteopontin in breast cancer. Mol Cell Biol. 2003;23:6507-6519. [PubMed] [DOI] |
| 15. | Das R, Mahabeleshwar GH, Kundu GC. Osteopontin induces AP-1-mediated secretion of urokinase-type plasminogen activator through c-Src-dependent epidermal growth factor receptor transactivation in breast cancer cells. J Biol Chem. 2004;279:11051-11064. [PubMed] [DOI] |
| 16. | Jain S, Chakraborty G, Kundu GC. The crucial role of cyclooxygenase-2 in osteopontin-induced protein kinase C alpha/c-Src/IkappaB kinase alpha/beta-dependent prostate tumor progression and angiogenesis. Cancer Res. 2006;66:6638-6648. [PubMed] [DOI] |
| 17. | Song G, Ouyang G, Mao Y, Ming Y, Bao S, Hu T. Osteopontin promotes gastric cancer metastasis by augmenting cell survival and invasion through Akt-mediated HIF-1alpha up-regulation and MMP9 activation. J Cell Mol Med. 2009;13:1706-1718. [PubMed] [DOI] |
| 18. | hen RX, Xia YH, Xue TC, Zhang H, Ye SL. Down-regulation of osteopontin inhibits metastasis of hepatocellular carcinoma cells via a mechanism involving MMP-2 and uPA. Oncol Rep. 2011;25:803-808. [PubMed] |
| 19. | Chen RX, Xia YH, Xue TC, Ye SL. Osteopontin promotes hepatocellular carcinoma invasion by up-regulating MMP-2 and uPA expression. Mol Biol Rep. 2011;38:3671-3677. [PubMed] [DOI] |
| 20. | Chakraborty G, Jain S, Kundu GC. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 2008;68:152-161. [PubMed] [DOI] |
| 21. | Yang L, Zhao W, Zuo WS, Wei L, Song XR, Wang XW, Zheng G, Zheng MZ. Silencing of osteopontin promotes the radiosensitivity of breast cancer cells by reducing the expression of hypoxia inducible factor 1 and vascular endothelial growth factor. Chin Med J (Engl). 2012;125:293-299. [PubMed] |
| 22. | Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am J Pathol. 1996;149:293-305. [PubMed] |
| 23. | Shijubo N, Uede T, Kon S, Nagata M, Abe S. Vascular endothelial growth factor and osteopontin in tumor biology. Crit Rev Oncog. 2000;11:135-146. [PubMed] |
| 24. | Tang H, Wang J, Bai F, Zhai H, Gao J, Hong L, Xie H, Zhang F, Lan M, Yao W. Positive correlation of osteopontin, cyclooxygenase-2 and vascular endothelial growth factor in gastric cancer. Cancer Invest. 2008;26:60-67. [PubMed] [DOI] |
| 27. | Furger KA, Menon RK, Tuck AB, Bramwell VH, Chambers AF. The functional and clinical roles of osteopontin in cancer and metastasis. Curr Mol Med. 2001;1:621-632. [PubMed] [DOI] |
| 28. | Sasaki N, Morisaki T, Hashizume K, Yao T, Tsuneyoshi M, Noshiro H, Nakamura K, Yamanaka T, Uchiyama A, Tanaka M. Nuclear factor-kappaB p65 (RelA) transcription factor is constitutively activated in human gastric carcinoma tissue. Clin Cancer Res. 2001;7:4136-4142. [PubMed] |
| 29. | Cianciulli A, Calvello R, Cavallo P, Dragone T, Carofiglio V, Panaro MA. Modulation of NF-κB activation by resveratrol in LPS treated human intestinal cells results in downregulation of PGE(2) production and COX-2 expression. Toxicol In Vitro. 2012; Jul 6. [Epub ahead of print]. [PubMed] [DOI] |
| 30. | Park SY, Kim YH, Kim YH, Lee SJ. Aromatic-turmerone attenuates invasion and expression of MMP-9 and COX-2 through inhibition of NF-κB activation in TPA-induced breast cancer cells. J Cell Biochem. 2012; Jun 27. [Epub ahead of print]. [PubMed] |