修回日期: 2012-05-05
接受日期: 2012-06-29
在线出版日期: 2012-07-18
目的: 探讨胃癌中MMP-9和TIMP-1蛋白表达与ZHX2蛋白表达的关系, 并分析其与胃癌患者临床病理特征的相关性及其临床意义.
方法: 应用免疫组织化学Envision二步法检测62例胃癌组织及癌旁正常胃组织中MMP-9、TIMP-1及ZHX2蛋白的表达. 用SPSS13.0统计软件分析MMP-9和TIMP-1蛋白表达与ZHX2蛋白表达的相关性, 并分析其与患者的临床病理特征的关系.
结果: MMP-9蛋白在62例胃癌组织及癌旁正常胃组织中阳性表达率分别为66.13%(41/62)和29.03%(18/62)(P<0.01), TIMP-1蛋白在胃癌组织及癌旁正常胃组织中阳性表达率分别为41.94%(26/62)和74.19%(46/62)(P<0.01).MMP-9蛋白表达在ZHX2阳性和阴性表达组中的阳性率分别为: 84.44%(38/45)和17.65%(3/17)(P<0.05), 与患者的年龄、性别、肿瘤大小、发生部位、大体类型、分化程度、淋巴结转移、远处转移、TNM分期均无关(P>0.05), 而与肿瘤的浸润深度有关(P<0.05); TIMP-1蛋白表达在ZHX2阳性和阴性组中阳性率分别为: 28.89%(13/45)和76.47%(13/17)(P<0.05), 与患者的年龄、性别、肿瘤大小、发生部位、大体类型、分化程度、浸润深度、淋巴结转移、远处转移、TNM分期均无关(P>0.05).
结论: MMP-9高表达可以作为评估胃癌恶性生物学行为及预后的指标, MMP-9/TIMP-1表达失衡与ZHX2表达相关, MMP-9和TIMP-1可为临床选择化疗药物提供参考依据.
引文著录: 陈其军, 吕自力, 党裔武, 魏晶晶. MMP-9和TIMP-1在胃癌组织中的表达失衡及其与ZHX2的相关性. 世界华人消化杂志 2012; 20(20): 1832-1837
Revised: May 5, 2012
Accepted: June 29, 2012
Published online: July 18, 2012
AIM: To detect the expression of matrix metalloproteinase 9 (MMP-9), tissue inhibitor of metalloproteinase-l (TIMP-1) and ZHX2 in gastric carcinoma and to analyze their association with pathological features.
METHODS: Immunohistochemistry was used to examine the expression of MMP-9, TIMP-1, and ZHX2 proteins in 62 cases of gastric carcinoma and matched tumor-adjacent tissue specimens. The correlation of MMP-9, TIMP-1 and ZHX2 protein expression with clinicopathological characteristics of gastric carcinoma was then analyzed.
RESULTS: The positive rate of MMP-9 expression was significantly higher (66.13% vs 29.03%, P < 0.01), and that of TIMP-1 was significantly lower (41.94% vs 74.19%, P < 0.01) in gastric carcinoma than in tumor-adjacent tissue. The positive rate of MMP-9 expression was significantly higher in the ZHX2-positive group than in the ZHX2-negative group (84.44% vs 17.65%, P < 0.05). MMP-9 expression was not associated with age, gender, tumor size, tumor location, general type, tumor differentiation, lymph node metastasis, distant metastasis, or TNM stage, but was significantly associated with depth of invasion (P < 0.05). The positive rate of TIMP-1 expression was significantly lower in the ZHX2-positive group than in the ZHX2-negative group (28.89% vs 76.49%, P < 0.05). TIMP-1 expression was not associated with age, gender, tumor size, tumor location, general type, tumor differentiation, depth of invasion, lymph node metastasis, distant metastasis, or TNM stage.
CONCLUSION: Detection of MMP-9 protein expression may be used to assess the malignant biological behavior and prognosis of gastric carcinoma. MMP-9/TIMP-1 imbalance may be related to the expression of ZHX2 in gastric carcinoma.
- Citation: Chen QJ, Lv ZL, Dang YW, Wei JJ. Correlation between MMP-9/TIMP-1 imbalance and ZHX2 expression in gastric carcinoma. Shijie Huaren Xiaohua Zazhi 2012; 20(20): 1832-1837
- URL: https://www.wjgnet.com/1009-3079/full/v20/i20/1832.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v20.i20.1832
胃癌(gastric carcinoma)是最常见的消化系统肿瘤, 其浸润和转移是影响生存质量和预后的主要因素, 也是影响治疗效果的重要因素之一[1-4]. 近年来的研究表明基质金属蛋白酶(matrix metalloproteinases, MMPs)家族与基质金属蛋白酶组织抑制剂(tissue inhibitor of metalloproteinases, TIMPs)家族表达失衡与多种肿瘤的浸润和转移密切相关[5-11], 我们既往的研究发现ZHX2的过表达与胃癌的浸润深度有关[12]. 那么胃癌组织中是否存在MMP-9/TIMP-1蛋白表达失衡? 这种失衡与ZHX2蛋白过表达是否有关? 与胃癌的临床病理特征是否有关? 因此, 本研究采用免疫组织化学方法检测62例胃癌组织及癌旁正常胃组织中MMP-9、TIMP-1及ZHX2蛋白的表达, 现报道如下.
收集2010-04/2011-06在广西医科大学第一附属医院接受胃大部切除术的胃癌标本62例作为试验组, 其中男42例, 女20例, 年龄26-81岁, 平均58.71岁; 收集癌旁正常胃组织(均距肿瘤边缘5 cm以上)62例作为对照组. 所有病例术前均未行放化疗. 胃癌TNM分期采用美国癌症联合会(AJCC)标准(2002), 其中Ⅰ期7例, Ⅱ期33例, Ⅲ期14例, Ⅳ期8例. 组织学分级: 其中高分化腺癌5例, 中分化腺癌14例, 低分化腺癌43例, 高-中分化组包括乳头状腺癌和管状腺癌, 低分化组中包括低分化腺癌、印戒细胞癌和黏液腺癌. 兔抗人MMP-9及TIMP-1即用型抗体购自北京中杉金桥生物技术有限公司; 免疫组织化学二步法Envision system、DAB显色液、即用型二抗购自上海长岛生物技术有限公司; 所有抗体试剂均按说明书要求保存和使用.
1.2.1 免疫组织化学Envision二步法: 标本离体后立即40 g/L甲醛固定, 常规石蜡包埋制作切片, 切片烤片后二甲苯脱蜡, 乙醇水化, 柠檬酸抗原修复液高压修复, 3%过氧化氢灭活内源性过氧化物酶, MMP-9及TIMP-1即用型抗体室温2 h, 二抗室温20 min, DAB显色, 流水冲洗, 苏木素复染, 常规脱水/透明后中性树胶封片. 以PBS代替一抗作阴性对照.
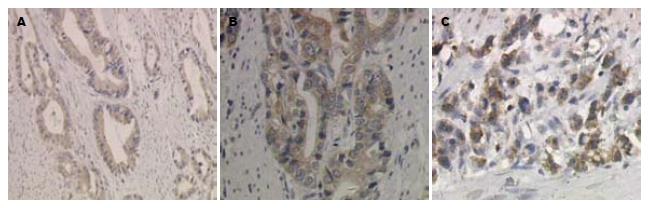
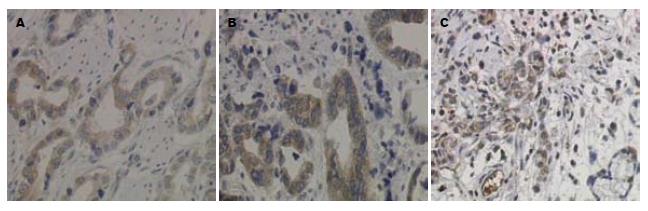
1.2.2 结果判定: MMP-9及TIMP-1阳性染色均呈棕黄色颗粒, 定位于胃癌细胞及胃黏膜上皮细胞的胞浆, 对免疫组织化学结果的评判: 以阳性细胞<10%为阴性(-), ≥10%的为阳性, 并根据阳性细胞所占比例分为: 10%-25%(+), 26%-50%(++), 51%-75%(+++), >75%(++++). ZHX2的阳性判断方法同前[12].
统计学处理 应用SPSS13.0软件进行统计处理, 组间比较采用χ2检验或Fisher确切概率法, 等级相关性采用Spearman等级相关分析.
MMP-9阳性表达呈棕黄色颗粒, 定位于胃癌细胞浆(图1)及胃黏膜上皮细胞浆. 在62例胃癌组织中MMP-9蛋白阳性表达率为66.13%(41/62), 明显高于在癌旁正常胃组织中的表达(29.03%, 18/62, P<0.01). MMP-9蛋白表达与患者的年龄、性别、肿瘤大小、发生部位、大体类型、分化程度、淋巴结转移、远处转移、TNM分期均无关(P>0.05), 而与肿瘤的浸润深度有关(P<0.05, 表1).
| 临床病理特征 | n | +(n) | -(n) | 阳性率(%) | P值 |
| 性别 | 0.897 | ||||
| 男 | 42 | 28 | 14 | 66.67 | |
| 女 | 20 | 13 | 7 | 65.00 | |
| 年龄(岁) | 0.794 | ||||
| <60 | 28 | 19 | 9 | 67.86 | |
| ≥60 | 34 | 22 | 12 | 64.71 | |
| 肿瘤大小(cm) | 0.798 | ||||
| <5 | 37 | 24 | 13 | 64.86 | |
| ≥5 | 25 | 17 | 8 | 68.00 | |
| 发生部位 | 0.326 | ||||
| 胃底/贲门 | 12 | 8 | 4 | 66.67 | |
| 胃体 | 13 | 6 | 7 | 46.15 | |
| 胃窦/幽门 | 37 | 26 | 11 | 70.27 | |
| 大体类型 | 0.669 | ||||
| 隆起型 | 8 | 6 | 2 | 75.00 | |
| 溃疡型 | 46 | 31 | 15 | 67.39 | |
| 浸润型 | 8 | 4 | 4 | 50.00 | |
| 分化程度 | 0.554 | ||||
| 低分化 | 43 | 27 | 16 | 62.79 | |
| 中分化 | 14 | 11 | 3 | 78.57 | |
| 高分化 | 5 | 3 | 2 | 60.00 | |
| 浸润深度 | 0.035 | ||||
| T1 | 3 | 0 | 3 | 0.00 | |
| T2 | 10 | 9 | 1 | 90.00 | |
| T3 | 41 | 30 | 11 | 73.17 | |
| T4 | 8 | 6 | 2 | 75.00 | |
| 淋巴结转移 | 0.823 | ||||
| N0 | 21 | 15 | 6 | 71.43 | |
| N1 | 23 | 15 | 8 | 65.22 | |
| N2 | 7 | 5 | 2 | 71.43 | |
| N3 | 11 | 6 | 5 | 54.55 | |
| 远处转移 | 1.000 | ||||
| M0 | 54 | 36 | 18 | 66.67 | |
| M1 | 8 | 5 | 3 | 62.50 | |
| TNM分期 | 0.450 | ||||
| Ⅰ期 | 7 | 3 | 4 | 42.86 | |
| Ⅱ期 | 33 | 24 | 9 | 72.73 | |
| Ⅲ期 | 14 | 9 | 5 | 64.29 | |
| Ⅳ期 | 8 | 5 | 3 | 62.50 |
TIMP-1阳性表达呈棕黄色颗粒, 定位于胃癌细胞浆(图2)及胃黏膜上皮细胞浆. 在62例胃癌组织中TIMP-1蛋白阳性表达率为41.94%(26/62), 明显低于在癌旁正常胃组织中的表达(74.19%, 46/62, P<0.01). TIMP-1蛋白表达与患者的年龄、性别、肿瘤大小、发生部位、大体类型、分化程度、浸润深度、淋巴结转移、远处转移、TNM分期均无关(P>0.05, 表2).
| 临床病理特征 | n | +(n) | -(n) | 阳性率(%) | P值 |
| 性别 | 0.831 | ||||
| 男 | 42 | 18 | 24 | 42.86 | |
| 女 | 20 | 8 | 12 | 40.00 | |
| 年龄(岁) | 0.243 | ||||
| <60 | 28 | 14 | 14 | 50.00 | |
| ≥60 | 34 | 12 | 22 | 35.29 | |
| 肿瘤大小(cm) | |||||
| <5 | 37 | 18 | 19 | 48.65 | 0.193 |
| ≥5 | 25 | 8 | 17 | 32.00 | |
| 发生部位 | 0.813 | ||||
| 胃底/贲门 | 12 | 6 | 6 | 50.00 | |
| 胃体 | 13 | 5 | 8 | 38.46 | |
| 胃窦/幽门 | 37 | 15 | 22 | 40.54 | |
| 大体类型 | 0.160 | ||||
| 隆起型 | 8 | 3 | 5 | 37.50 | |
| 溃疡型 | 46 | 17 | 29 | 36.96 | |
| 浸润型 | 8 | 6 | 2 | 75.00 | |
| 分化程度 | 0.542 | ||||
| 低分化 | 43 | 20 | 23 | 46.51 | |
| 中分化 | 14 | 4 | 10 | 28.57 | |
| 高分化 | 5 | 2 | 3 | 40.00 | |
| 浸润深度 | 0.904 | ||||
| T1 | 3 | 1 | 2 | 33.33 | |
| T2 | 10 | 5 | 5 | 50.00 | |
| T3 | 41 | 16 | 25 | 39.02 | |
| T4 | 8 | 4 | 4 | 50.00 | |
| 淋巴结转移 | 0.312 | ||||
| N0 | 21 | 10 | 11 | 47.62 | |
| N1 | 23 | 10 | 13 | 43.48 | |
| N2 | 7 | 4 | 3 | 57.14 | |
| N3 | 11 | 2 | 9 | 18.18 | |
| 远处转移 | 0.710 | ||||
| M0 | 54 | 22 | 32 | 40.74 | |
| M1 | 8 | 4 | 4 | 50.00 | |
| TNM分期 | 0.362 | ||||
| Ⅰ期 | 7 | 3 | 4 | 42.86 | |
| Ⅱ期 | 33 | 16 | 17 | 48.48 | |
| Ⅲ期 | 14 | 3 | 11 | 21.43 | |
| Ⅳ期 | 8 | 4 | 4 | 50.00 |
肿瘤的发生和发展是一个多步骤、多基因、多因素参与的复杂过程. 其中MMPs和TIMPs在降解细胞外基质(extracellular matrix, ECM)过程中起着重要作用, 参与恶性肿瘤的浸润和转移, 是恶性肿瘤浸润和转移调节的主要蛋白[13-15]. MMPs是一类结构中含有Zn2+的肽链内切酶家族, 几乎能降解ECM的所有成分, 体内存在许多抑制物可以特异性或者非特异性抑制MMPs的活性, 主要是TIMPs[16]. TIMPs由分泌MMPs的细胞同时分泌, 是MMPs生理性抑制物, 活性的MMPs受特定的TIMPs调节. 在活化的MMPs阶段, TIMPs可直接与活化的MMPs形成紧密的1:1复合物, 抑制其活性[17,18].
MMPs家族中, MMP-9是重要的一员, 而其抑制剂TIMPs中, TIMP-1作用最强, 与MMP-9共同作用使ECM降解及修复维持动态平衡, 对抑制肿瘤的浸润转移有重要作用[19-22]. 本研究显示MMP-9蛋白在胃癌组织中存在高表达, 而TIMP-1蛋白在胃癌组织中表达却下调, 表明在胃癌组织中存在MMP-9/TIMP-1蛋白表达失衡, 这与许多学者报道相符[23-30]. 本研究还发现MMP-9蛋白阳性表达与患者的年龄、性别、肿瘤大小、发生部位、大体类型、分化程度、淋巴结转移、远处转移、TNM分期均无关(P>0.05), 而与肿瘤的浸润深度有关(P<0.05). 而TIMP-1蛋白阳性表达与患者的年龄、性别、肿瘤大小、发生部位、大体类型、分化程度、浸润深度、淋巴结转移、远处转移、TNM分期均无关(P>0.05). 表明在胃癌浸润过程中, TIMP-1并不是MMP-9唯一的抑制剂.
我们既往的研究发现ZHX2的过表达与胃癌的浸润深度有关, 为了进一步的研究ZHX2在胃癌中的作用机制, 我们将本研究结果MMP-9和TIMP-1蛋白在胃癌中的表达与前期同组胃癌病例ZHX2蛋白表达, 进行相关性检验分析发现: 在62例胃癌组织中MMP-9蛋白的表达与ZHX2蛋白表达呈正相关(r = 0.630, P<0.01), 在ZHX2阳性和阴性表达组中的MMP-9阳性表达率分别为: 84.44%(38/45)和17.65%(3/17)(P<0.05); TIMP-1蛋白的表达与ZHX2蛋白表达呈负相关(r = -0.430, P<0.01), 在ZHX2阳性和阴性组中TIMP-1阳性表达率分别为: 28.89%(13/45)和76.47%(13/17)(P<0.05), 表明MMP-9/TIMP-1蛋白表达失衡与ZHX2蛋白表达相关. 关于MMP-9和TIMP-1蛋白表达与ZHX2蛋白表达的相关性研究, 目前国内外均未见相关文献报道, 具体机制也有待进一步研究. 分析其原因可能为: ZHX2通过与NF-Y结合, 影响其下游基因MMPs/TIMPs的表达, 从而调节胃癌的浸润和转移. 其详细机制有待于进一步研究.
总之, 联合检测胃癌组织中MMP-9、TIMP-1、ZHX2蛋白表达具有重要的临床意义, 一方面有助于揭示胃癌发生、发展、浸润和转移的机制, 并能作为评估胃癌恶性生物学行为及预后的指标之一; 另一方面可以为临床选择化疗药物提供参考依据. 对于三者在胃癌发生、发展、浸润和转移过程中的相互作用机制还有待进一步的研究.
近年来的研究表明基质金属蛋白酶(MMPs)家族与基质金属蛋白酶组织抑制剂(TIMPs)家族表达失衡与多种肿瘤的浸润和转移密切相关, 此外, ZHX2的过表达与胃癌的浸润深度有关. 本研究旨在探讨胃癌组织中是否存在MMPs/TIMPs表达失衡? 这种失衡与ZHX2蛋白过表达有无相关性.
邵先玉, 教授, 泰山医学院附属医院消化内科
有关胃癌的浸润和转移是当前研究的热点. 关于MMP-9和TIMP-1蛋白表达与ZHX2蛋白表达的相关性研究, 目前国内外均未见相关文献报道.
众多学者研究发现MMP-9蛋白在胃癌组织中高表达, 而TIMP-1蛋白在胃癌组织中表达却下调, 表明在胃癌组织中存在MMP-9/TIMP-1蛋白表达失衡.
本实验通过免疫组织化学法首次探讨了MMP-9/TIMP-1蛋白与ZHX2蛋白在人胃癌组织中表达的相关性.
本研究初步探讨了MMP-9和TIMP-1蛋白表达与ZHX2蛋白表达的相关性, 有助于揭示胃癌发生、发展、浸润和转移的机制, 并能作为评估胃癌恶性生物学行为及预后的指标之一; 另一方面可以为临床选择化疗药物提供参考依据.
本文对GISTs的分子学特征及其类型, 靶向治疗药物的原理机制及耐药情况进行了综述, 从基础到临床应用较全面, 有一定指导意义.
编辑: 曹丽鸥 电编:闫晋利
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. [DOI] |
| 2. | Lukaszewicz-Zajac M, Mroczko B, Szmitkowski M. [The significance of metalloproteinases and their inhibitors in gastric cancer]. Postepy Hig Med Dosw (Online). 2009;63:258-265. [PubMed] |
| 3. | Singh RD, Haridas N, Patel JB, Shah FD, Shukla SN, Shah PM, Patel PS. Matrix metalloproteinases and their inhibitors: correlation with invasion and metastasis in oral cancer. Indian J Clin Biochem. 2010;25:250-259. [PubMed] [DOI] |
| 4. | Fernandez-Gomez J, Escaf S, Gonzalez LO, Suarez A, Gonzalez-Reyes S, González J, Miranda O, Vizoso F. Relationship between metalloprotease expression in tumour and stromal cells and aggressive behaviour in prostate carcinoma: Simultaneous high-throughput study of multiple metalloproteases and their inhibitors using tissue array analysis of radical prostatectomy samples. Scand J Urol Nephrol. 2011;45:171-176. [PubMed] [DOI] |
| 5. | O'Grady A, Dunne C, O'Kelly P, Murphy GM, Leader M, Kay E. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2 in non-melanoma skin cancer: implications for tumour progression. Histopathology. 2007;51:793-804. [PubMed] [DOI] |
| 6. | Altadill A, Rodríguez M, González LO, Junquera S, Corte MD, González-Dieguez ML, Linares A, Barbón E, Fresno-Forcelledo M, Rodrigo L. Liver expression of matrix metalloproteases and their inhibitors in hepatocellular carcinoma. Dig Liver Dis. 2009;41:740-748. [PubMed] [DOI] |
| 7. | Vizoso FJ, González LO, Corte MD, Rodríguez JC, Vázquez J, Lamelas ML, Junquera S, Merino AM, García-Muñiz JL. Study of matrix metalloproteinases and their inhibitors in breast cancer. Br J Cancer. 2007;96:903-911. [PubMed] [DOI] |
| 8. | Adithi M, Nalini V, Kandalam M, Krishnakumar S. Expression of matrix metalloproteinases and their inhibitors in retinoblastoma. J Pediatr Hematol Oncol. 2007;29:399-405. [PubMed] [DOI] |
| 9. | Christopoulos TA, Papageorgakopoulou N, Ravazoula P, Mastronikolis NS, Papadas TA, Theocharis DA, Vynios DH. Expression of metalloproteinases and their tissue inhibitors in squamous cell laryngeal carcinoma. Oncol Rep. 2007;18:855-860. [PubMed] |
| 10. | Gentner B, Wein A, Croner RS, Zeittraeger I, Wirtz RM, Roedel F, Dimmler A, Dorlaque L, Hohenberger W, Hahn EG. Differences in the gene expression profile of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in primary colorectal tumors and their synchronous liver metastases. Anticancer Res. 2009;29:67-74. [PubMed] |
| 11. | Groblewska M, Siewko M, Mroczko B, Szmitkowski M. The role of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in the development of esophageal cancer. Folia Histochem Cytobiol. 2012;50:12-19. [PubMed] [DOI] |
| 13. | Görögh T, Beier UH, Bäumken J, Meyer JE, Hoffmann M, Gottschlich S, Maune S. Metalloproteinases and their inhibitors: influence on tumor invasiveness and metastasis formation in head and neck squamous cell carcinomas. Head Neck. 2006;28:31-39. [PubMed] [DOI] |
| 14. | Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010;20:161-168. [PubMed] [DOI] |
| 15. | Watanabe H. [Extracellular matrix--regulation of cancer invasion and metastasis]. Gan To Kagaku Ryoho. 2010;37:2058-2061. [PubMed] |
| 16. | Kim HS, Kim HJ, Park KG, Kim YN, Kwon TK, Park JY, Lee KU, Kim JG, Lee IK. Alpha-lipoic acid inhibits matrix metalloproteinase-9 expression by inhibiting NF-kappaB transcriptional activity. Exp Mol Med. 2007;39:106-113. [PubMed] |
| 17. | Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562-573. [PubMed] [DOI] |
| 18. | Rydlova M, Holubec L, Ludvikova M, Kalfert D, Franekova J, Povysil C, Ludvikova M. Biological activity and clinical implications of the matrix metalloproteinases. Anticancer Res. 2008;28:1389-1397. [PubMed] |
| 19. | Vempati P, Karagiannis ED, Popel AS. A biochemical model of matrix metalloproteinase 9 activation and inhibition. J Biol Chem. 2007;282:37585-37596. [PubMed] [DOI] |
| 20. | Boyd S, Tolvanen K, Virolainen S, Kuivanen T, Kyllönen L, Saarialho-Kere U. Differential expression of stromal MMP-1, MMP-9 and TIMP-1 in basal cell carcinomas of immunosuppressed patients and controls. Virchows Arch. 2008;452:83-90. [PubMed] [DOI] |
| 21. | Hu XX, Li L, Li DR, Zhang W, Cheng XQ, Zhang JQ, Tang BJ. [Expression of matrix metalloproteinases-9,2,7,and tissue inhibitor of metalloproteinases-1,2,3 mRNA in ovarian tumors and their clinical significance]. Ai Zheng. 2004;23:1194-1198. [PubMed] |
| 22. | Dragutinović V, Izrael-Zivković L, Radovanović N. Relation of matrix metalloproteinase-9 to different stages of tumors in the serum of gastric cancer. Dig Dis Sci. 2009;54:1203-1207. [PubMed] [DOI] |
| 23. | Lukaszewicz-Zając M, Mroczko B, Szmitkowski M. Gastric cancer - The role of matrix metalloproteinases in tumor progression. Clin Chim Acta. 2011;412:1725-1730. [PubMed] [DOI] |
| 24. | Kemik O, Kemik AS, Sümer A, Dulger AC, Adas M, Begenik H, Hasirci I, Yilmaz O, Purisa S, Kisli E. Levels of matrix metalloproteinase-1 and tissue inhibitors of metalloproteinase-1 in gastric cancer. World J Gastroenterol. 2011;17:2109-2112. [PubMed] [DOI] |
| 25. | Zhang M, Zhu GY, Gao HY, Zhao SP, Xue Y. Expression of tissue levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in gastric adenocarcinoma. J Surg Oncol. 2011;103:243-247. [PubMed] [DOI] |
| 26. | Mroczko B, Lukaszewicz-Zajac M, Groblewska M, Czyzewska J, Gryko M, Guzińska-Ustymowicz K, Kemona A, Kedra B, Szmitkowski M. Expression of tissue inhibitors of metalloproteinase 1 (TIMP-1) in gastric cancer tissue. Folia Histochem Cytobiol. 2009;47:511-516. [PubMed] [DOI] |
| 27. | de Mingo M, Morán A, Sánchez-Pernaute A, Iniesta P, Díez-Valladares L, Pérez-Aguirre E, de Juan C, García-Aranda C, Díaz-López A, García-Botella A. Expression of MMP-9 and TIMP-1 as prognostic markers in gastric carcinoma. Hepatogastroenterology. 2007;54:315-319. [PubMed] |
| 29. | Shim KN, Jung SA, Joo YH, Yoo K. Clinical significance of tissue levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in gastric cancer. J Gastroenterol. 2007;42:120-128. [PubMed] [DOI] |
| 30. | Yang S, Zhao Z, Wu R, Lu H, Zhang X, Huan C, Wang C, Wu X, Guan G. Expression and biological relationship of vascular endothelial growth factor-A and matrix metalloproteinase-9 in gastric carcinoma. J Int Med Res. 2011;39:2076-2085. [PubMed] |