修回日期: 2012-04-23
接受日期: 2012-05-18
在线出版日期: 2012-06-18
目的: 评价瞬时弹性成像(Fibroscan, FS)在非酒精性脂肪性肝病(nonalcoholic fatty liver disease, NAFLD)肝纤维化诊断中的作用.
方法: 选取2008-04/2011-02在北京佑安医院住院, 且经病理检查诊断为NAFLD的患者83例, 临床检测肝功能、空腹血糖、血脂、尿酸, 同时应用FS进行肝脏硬度检测. 以病理检查结果为金标准, 分析肝脏硬度、生化学指标及病理肝纤维化程度的相关性, 采用受试者工作特征(ROC)曲线分析FS诊断NAFLD肝纤维化的准确性.
结果: 不同程度肝纤维化分期的肝脏硬度值, S0期: 4.28 kPa±1.32 kPa, S1期: 7.40 kPa±2.13 kPa, S2期: 11.52 kPa±3.86 kPa, S3期: 19.99 kPa±5.42 kPa. 肝脏硬度与肝纤维化程度呈正相关, Spearman相关系数为0.768, P<0.001. Pearson相关分析显示, FS肝脏硬度检测值与ALT、AST呈正相关, 与HDL、ApoA呈负相关(P<0.05). FS诊断S0-S1、S2、S3期的ROC曲线下面积分别为0.889(0.813, 0.965)、0.838(0.729, 0.948)、0.938(0.000, 1.000), 诊断界值分别为8.95 kPa、10.60 kPa、15.66 kPa.
结论: FS对NAFLD肝纤维化有较高的诊断价值, 可作为NAFLD患者诊断和动态随访的依据.
引文著录: 范丽娟, 廖慧钰, 姜太一, 黄云丽, 刘燕敏. 瞬时弹性成像与非酒精性脂肪性肝病病理纤维化分期的相关性. 世界华人消化杂志 2012; 20(17): 1515-1519
Revised: April 23, 2012
Accepted: May 18, 2012
Published online: June 18, 2012
AIM: To evaluate the role of Fibroscan (FS) in the diagnosis of liver fibrosis in patients with nonalcoholic fatty liver disease (NAFLD).
METHODS: A total of 83 patients who were pathologically diagnosed with NAFLD and underwent liver stiffness measurement using FibroScan at Beijing Youan Hospital from April 2008 to February 2011 were included in this study. Staging of liver fibrosis based on a liver biopsy was performed in all patients. Other clinical tests included liver function, FBG, blood lipids and UA The correlation between liver stiffness and liver fibrosis degree was analyzed. The receive operating characteristic (ROC) curve was used to analyze the accuracy of Fibroscan in diagnosing liver fibrosis with NAFLD.
RESULTS: The liver stiffness differed among patients with different stages of liver fibrosis. The Fibroscan values were 4.28 kPa ± 1.32 kPa, 7.40 kPa ± 2.13 kPa, 11.52 kPa ± 3.86 kPa, and 19.99 kPa ± 5.42 kPa for patients with S0 to S3 liver fibrosis, respectively, and liver stiffness was closely related to stage of liver fibrosis (r = 0.768, P < 0.001). Fibroscan score was positively correlated with ALT and AST, but negatively with HDL and ApoA (all P < 0.05). The area under the ROC curve for FibroScan score in assessing liver fibrosis was 0.889 (0.813, 0.965) in patients with S1 liver fibrosis, 0.838 (0.729, 0.948) in those with S2, and 0.938 (0.000, 1.000) in those with S3. The cut off values were 8.95 kPa, 10.60 kPa and 15.66 kPa, respectively.
CONCLUSION: Fibroscan is valuable for the diagnosis of liver fibrosis in patients with NAFLD.
- Citation: Fan LJ, Liao HY, Jiang TY, Huang YL, Liu YM. Correlation between liver stiffness measurement by Fibroscan and liver fibrosis staging based on a liver biopsy in patients with NAFLD. Shijie Huaren Xiaohua Zazhi 2012; 20(17): 1515-1519
- URL: https://www.wjgnet.com/1009-3079/full/v20/i17/1515.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v20.i17.1515
非酒精性脂肪性肝病(nonalcoholic fatty liver disease, NAFLD)是21世纪全球重要的公共健康问题之一, 亦是我国愈来愈重要的慢性肝病问题. 目前肝活检被认为是诊断金标准. 但有病理学学者认为NAFLD肝损伤后肝脂肪变及纤维化在肝脏分布是不均匀的, 肝穿所获样本的病理变化差异是造成误差的根本原因[1], 且因其为侵入性操作, 在临床应用中受到一定限制. 瞬时弹性成像(Fibroscan, FS)是一项无创测量肝组织纤维化程度的新方法, 其工作原理为以超声检查为基础, 脉冲回声测出低频弹性波在肝脏组织中的传导速度, 通过计算得到组织的弹性数值, 以千帕(kPa)表示[2]. 文献报道FS检测与病理活检具有良好的相关性[3]. 本文以NAFLD的病理学检查结果为金标准, 探讨FS在NAFLD肝纤维化诊断中的价值.
选取2008-04/2011-02在首都医科大学附属北京佑安医院住院, 且经病理检查诊断为NAFLD的患者83例, 男62例, 女21例, 年龄33.71岁±10.64岁, 平均年龄16-63岁. 其中单纯性脂肪肝患者42例, 非酒精性脂肪性肝炎患者41例, 诊断符合2010年修订版《非酒精性脂肪性肝病诊疗指南》[4]. 排除条件: 急性炎症、肿瘤、病毒性肝炎、药物性肝病、全胃肠外营养、肝豆状核变性、自身免疫性肝病等可导致脂肪肝的特定疾病; 摄入乙醇量>140 g/wk(女性>70 g/wk); 年龄>70岁或<16岁.
全部患者入院后详细询问病史, 进行体格检查, 测量身高、体质量、血压, 根据公式计算BMI(kg/m2) = 体质量(kg)/身高(m2). 肝组织活检前后3 d内常规检测肝功能、血脂、空腹血糖、尿酸. 血生化由OLYMPUS AU5400全自动生化分析仪进行检测. 肝组织活检后10 d内应用瞬时弹性扫描仪(Fibroscan; Echosens, Paris, France)进行肝脏硬度检测(liver stiffness measurement, LSM)值测量. 检测方法: 检测区域选择右侧腋前线至腋中线第7、8、9肋间, 连续检测, 要求成功检测10次, 取中位数作为最终测定结果, 并以弹性值kPa表示, 最终检测要求成功率≥60%, 四分位间距(IQR)低于测量值中位数的1/3. FS检查由接受过专业培训的操作熟练医师完成. 肝组织病理标本由我院病理科进行纤维化评分. 肝组织炎症分级(G)分为G0、G1、G2、G3、G4共5级, 肝纤维化分期(S)分为S0、S1、S2、S3、S4共5期.
统计学处理 应用SPSS17.0进行统计学分析, 计量资料用mean±SD表示. 肝脏硬度与肝纤维化程度的相关性用Spearman秩相关分析, 检验水准α = 0.05. 肝脏硬度与临床生化特征的相关性分析采用Pearson相关分析. 用受试者工作特征(ROC)曲线分析FS诊断肝纤维化的准确性.
本研究入组病例83例, 其中S0期9例, S1期42例, S2期24例, S3期8例, 无S4期病例. FS检测失败率为9.6%(8/83). 不同程度肝纤维化分期的肝脏硬度值, S0期4.28 kPa±1.32 kPa, S1期7.40 kPa±2.13 kPa, S2期11.52 kPa±3.86 kPa, S3期19.99 kPa±5.42 kPa. 肝脏硬度与肝纤维化程度呈正相关, Spearman相关系数为0.768(P<0.001, 表1).
| S0期 | S1期 | S2期 | S3期 | |
| n | 9 | 42 | 24 | 8 |
| FS检测失败(n) | 0 | 2 | 4 | 2 |
| FS检测值(kPa) | 4.28± 1.32 | 7.40± 2.13 | 11.52± 3.86 | 19.99± 5.42 |
| r值 | 0.768 | |||
| P值 | <0.001 | |||
Pearson相关分析显示, FS肝脏硬度检测值与血清丙氨酸氨基转移酶(alanine aminotransferase, ALT)、门冬氨酸氨基转移酶(aspartate aminotransferase, AST)呈正相关, 与HDL、ApoA呈负相关(表2).
| mean±SD | r值 | P值 | |
| 年龄(岁) | 33.71±10.64 | -0.015 | 0.911 |
| 收缩压(mmHg) | 125.77±18.64 | 0.128 | 0.248 |
| 舒张压(mmHg) | 69.66±14.14 | -0.080 | 0.471 |
| BMI(kg/m2) | 26.35±2.54 | 0.178 | 0.170 |
| ALT(U/L) | 79.46±50.34 | 0.253 | 0.049 |
| AST(U/L) | 48.77±25.12 | 0.439 | 0.000 |
| TBIL(μmol/L) | 16.95±6.81 | -0.990 | 0.446 |
| GGT(U/L) | 81.83±56.04 | 0.032 | 0.805 |
| ALP(U/L) | 79.95±28.47 | 0.052 | 0.690 |
| TG(mmol/L) | 2.15±1.07 | -0.118 | 0.336 |
| CHO(mmol/L) | 4.23±0.98 | 0.090 | 0.142 |
| LDL(mmol/L) | 3.14±0.91 | 0.018 | 0.892 |
| HDL(mmol/L) | 1.27±10.40 | -2.56 | 0.046 |
| ApoA(mmol/L) | 104.54±25.03 | -0.364 | 0.004 |
| ApoB(mmol/L) | 87.95±21.33 | 0.060 | 0.643 |
| Glu(mmol/L) | 4.98±1.64 | -0.037 | 0.743 |
| UA(μmol/L) | 345.00±79.02 | 0.147 | 0.184 |
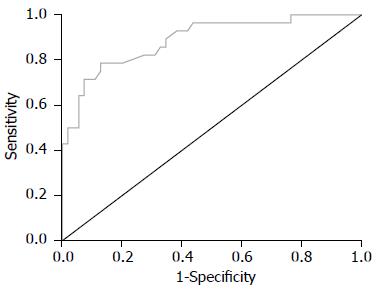
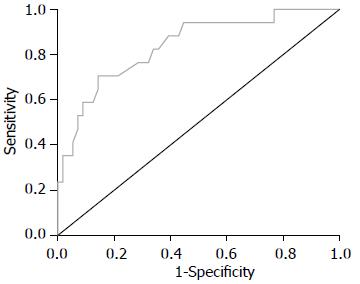
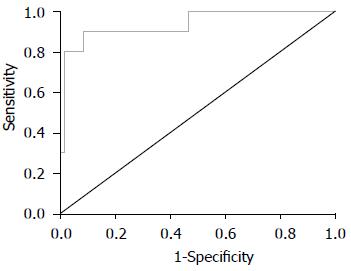
FS诊断S0-S1、S2、S3期的ROC曲线如图1-3所示, 诊断评价结果见表3. 各阶段肝纤维化的ROC曲线下面积均>0.8, 敏感度及特异度较高, 表明FS检查对NAFLD有较高的诊断价值.
| 肝纤维化分期 | ROC曲线下面积 | 95%CI | 诊断界值(kPa) | 敏感度 | 特异度 |
| S0-1 | 0.889 | 0.813, 0.965 | 8.95 | 0.786 | 0.836 |
| S2 | 0.838 | 0.729, 0.948 | 10.60 | 0.706 | 0.857 |
| S3 | 0.938 | 0.000, 1.000 | 15.66 | 0.900 | 0.918 |
目前NAFLD的诊断主要根据询问病史、体检、生物化学检测、影像诊断及肝组织活检. 生物化学检测诊断本病特异性不高, B型超声检查诊断脂肪肝的特异性仅为62%, 且当肝脂肪变程度低于30%时, 超声检出能力大大降低[5]. 肝组织活检是诊断NAFLD的金标准, 但该检查为侵入性, 且存在抽样误差和标本穿刺偏倚现象等缺点[6,7], 亦不适宜作为动态监测及评估病情的检查方法. 肝脏FS测定方法已经在慢性乙型肝炎及肝硬化、慢性丙型肝炎肝纤维化的无创性诊断方面做了大量探索, 研究表明FS与肝组织纤维化程度强相关[8]. 但目前FS检测在NAFLD肝纤维化诊断的应用研究尚少, 国内外尚未建立针对NAFLD的肝纤维化分期诊断标准. 本研究对NAFLD应用FS检测的诊断肝纤维化价值进行总结分析.
FS是一种非侵入性的检测方法, 其测量信息的采集来源为肝脏内约1 cm×2 cm×5 cm的区域, 约是肝穿刺活检组织的100倍, 受样本误差的几率降低, 观察者组内及组间重复性好[9-11], 且较肝组织活检更适用于对慢性肝病的动态随访, 反映肝组织纤维化这一动态的发展过程[12-14].
文献报道FS诊断(慢性丙型肝炎)S≥2、S≥3、S = 4的阈值分别为7.1-8.8、9.5-9.6、12.5-14.6, 且肝纤维化程度愈严重, FS诊断准确性愈高[15-18]. 本研究结果显示, NAFLD肝脏硬度与肝脏纤维化程度呈正相关, 不同程度肝纤维化分期的肝脏硬度值, S0期4.28 kPa±1.32 kPa, S1期7.40 kPa±2.13 kPa, S2期11.52 kPa±3.86 kPa, S3期19.99 kPa±5.42 kPa. S0-1期、S2期、S3期的诊断界值分别为8.95 kPa、10.60 kPa、15.66 kPa, 较文献报道的慢性病毒性肝炎的诊断界值稍高, 因脂肪组织对低频剪切波和超声波具有强烈的衰减作用[19], 故肝组织脂肪变可能导致FS检测值较同一纤维化分期的其他病因引起的慢性肝病更高. 亦有研究者报道, 未发现肝脏脂肪变性对FS测定值有显著影响[20,21], 与本文结果不同, 有待于进一步进行大样本量的研究分析.
本研究Pearson相关分析显示, FS肝脏硬度检测值与ALT、AST呈正相关, 与HDL、ApoA呈负相关. 目前已有研究认为FS弹性值与肝组织炎症(ALT)相关, 同一纤维化分期患者中, 生化指标改善者弹性值相应降低[12,22-24], 与本研究结论相符合. 故可考虑进行大样本量的研究以观察FS弹性值是否能够较敏感地区分单纯性脂肪肝和非酒精性脂肪性肝炎. HDL及ApoA反映机体的脂代谢失衡程度, NAFLD存在较高比例的脂代谢异常, 故可认为FS弹性值与NAFLD的临床生化学指标有较好的相关性. FS诊断NAFLD各期肝纤维化的ROC曲线下面积均>0.8, 敏感度及特异度较高, 表明FS检查对NAFLD肝纤维化有较高的诊断价值.
总之, FS诊断NAFLD各期肝纤维化与肝组织病理有较好的相关性, 与人体学指标(如BMI、腰围)、生化学指标、B型超声结合分析, 可部分替代肝组织活检, 且具有可重复性, 可作为动态观察随访手段监测肝组织纤维化和炎症程度. 但FS检测亦具有一定的局限性, 文献报道有2.4%-9.4%的失败率[11]. 本研究检测失败率为9.6%(8/83). 影响检测成功率可能的因素有BMI>28、糖尿病、年龄>50岁、肋间隙相对狭窄的女性, 其中BMI>30是影响FS弹性测定的最主要因素[25-27].
瞬时弹性成像(FS)是近年来研发的无创、简便的测定肝脏纤维化的新方法, 已经在慢性乙型肝炎及肝硬化、慢性丙型肝炎肝纤维化的无创性诊断方面做了大量的探索.
李健丁, 教授, 山西医科大学第一医院放射科CT室
研究表明FS与肝组织纤维化程度相关. 但目前国内外尚未建立FS弹性测定针对非酒精性脂肪性肝病(NAFLD)的肝纤维化分期诊断标准.
本研究结果显示, NAFLD肝脏硬度与肝脏纤维化程度呈正相关, 对NAFLD肝纤维化有较高的诊断价值.
瞬时弹性成像(FS): 一项无创测量肝组织纤维化程度的新方法, 其工作原理是以超声检查为基础, 脉冲回声测出低频弹性波在肝脏组织中的传导速度, 通过计算得到组织的弹性数值, 以千帕(kPa)表示.
本文就FS与NAFLD病理纤维化分期的相关性作了深入研究, 方法科学, 逻辑性强, 具有一定的临床应用前景.
编辑: 张姗姗 电编:鲁亚静
| 1. | Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898-1906. [PubMed] [DOI] |
| 3. | Sporea I, Sirli R, Deleanu A, Tudora A, Curescu M, Cornianu M, Lazar D. Comparison of the liver stiffness measurement by transient elastography with the liver biopsy. World J Gastroenterol. 2008;14:6513-6517. [PubMed] [DOI] |
| 5. | Roldan-Valadez E, Favila R, Martínez-López M, Uribe M, Méndez-Sánchez N. Imaging techniques for assessing hepatic fat content in nonalcoholic fatty liver disease. Ann Hepatol. 2008;7:212-220. [PubMed] |
| 7. | Friedrich-Rust M, Hadji-Hosseini H, Kriener S, Herrmann E, Sircar I, Kau A, Zeuzem S, Bojunga J. Transient elastography with a new probe for obese patients for non-invasive staging of non-alcoholic steatohepatitis. Eur Radiol. 2010;20:2390-2396. [PubMed] [DOI] |
| 8. | Yoneda M, Yoneda M, Mawatari H, Fujita K, Endo H, Iida H, Nozaki Y, Yonemitsu K, Higurashi T, Takahashi H. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD). Dig Liver Dis. 2008;40:371-378. [PubMed] [DOI] |
| 9. | Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193-2213. [PubMed] [DOI] |
| 10. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [PubMed] [DOI] |
| 11. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. [PubMed] [DOI] |
| 12. | Ganne-Carrié N, Ziol M, de Ledinghen V, Douvin C, Marcellin P, Castera L, Dhumeaux D, Trinchet JC, Beaugrand M. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology. 2006;44:1511-1517. [PubMed] [DOI] |
| 15. | Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. [PubMed] [DOI] |
| 16. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. [PubMed] [DOI] |
| 17. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [PubMed] [DOI] |
| 18. | Yeshua H, Oren R. Non invasive assessment of liver fibrosis. Ann Transplant. 2008;13:5-11. [PubMed] |
| 19. | Kettaneh A, Marcellin P, Douvin C, Poupon R, Ziol M, Beaugrand M, de Lédinghen V. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol. 2007;46:628-634. [PubMed] [DOI] |
| 20. | Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360-369. [PubMed] [DOI] |
| 21. | Boursier J, Konate A, Guilluy M, Gorea G, Sawadogo A, Quemener E, Oberti F, Reaud S, Hubert-Fouchard I, Dib N. Learning curve and interobserver reproducibility evaluation of liver stiffness measurement by transient elastography. Eur J Gastroenterol Hepatol. 2008;20:693-701. [PubMed] [DOI] |
| 22. | Chan HL, Wong GL, Choi PC, Chan AW, Chim AM, Yiu KK, Chan FK, Sung JJ, Wong VW. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat. 2009;16:36-44. [PubMed] [DOI] |
| 23. | Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380-384. [PubMed] [DOI] |
| 24. | Jung KS, Kim SU, Ahn SH, Park YN, Kim do Y, Park JY, Chon CY, Choi EH, Han KH. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology. 2011;53:885-894. [PubMed] [DOI] |
| 25. | Foucher J, Castéra L, Bernard PH, Adhoute X, Laharie D, Bertet J, Couzigou P, de Lédinghen V. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol. 2006;18:411-412. [PubMed] [DOI] |
| 26. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [PubMed] [DOI] |
| 27. | Chang PE, Lui HF, Chau YP, Lim KH, Yap WM, Tan CK, Chow WC. Prospective evaluation of transient elastography for the diagnosis of hepatic fibrosis in Asians: comparison with liver biopsy and aspartate transaminase platelet ratio index. Aliment Pharmacol Ther. 2008;28:51-61. [PubMed] [DOI] |