修回日期: 2012-03-05
接受日期: 2012-03-25
在线出版日期: 2012-05-08
目的: 研究11,12-表氧化二十碳三烯酸(11, 12-epoxyeicosatrienoic acids, 11,12-EETs)抑制游离脂肪酸(free fatty acids, FFAs)对原代小鼠胰岛β细胞凋亡的影响, 探讨11,12-EETs保护胰岛β细胞是否通过抑制内质网应激相关转录因子ATF4与ATF6核转位来实现.
方法: 棕榈酸(palmitic acids, PA)诱导原代小鼠胰岛β细胞凋亡, 与11,12-EETs共同孵育24 h后, 采用流式细胞术检测该细胞的线粒体膜电位的变化与细胞凋亡水平的改变, 以观察11,12-EETs对原代小鼠胰岛β细胞的保护作用; 用免疫印迹法检测内质网应激(endoplasmic reticulum stress, ERS)相关转录因子ATF4与ATF6在胞浆与胞核中的蛋白表达, 以此观察二者的核转位改变.
结果: 100 nmol/L 11,12-EETs与400 μmol/L PA共同孵育胰岛β细胞24 h后, 与FFAs对照组相比, FFAs+EET组可显著提升胰岛β细胞的存活率(62.1%±7.3% vs 53.0%±6.1%), 并明显降低胰岛β细胞线粒体的去极化水平(23.6%±3.4% vs 35.2%±4.7%); 11,12-EETs可有效抑制PA诱导的细胞凋亡(24.5%±4.2% vs 40.1%±5.6%); 同时, PA刺激的ATF4与ATF6的核转位受到11,12-EETs的影响, 胞核ATF4与ATF6蛋白水平显著性降低, 胞浆ATF4与ATF6蛋白水平明显上调.
结论: 11,12-EETs可以抑制FFAs诱导的凋亡, 其分子机制可能是通过抑制FFAs引发的ATF4与ATF6转位导致内质网应激相关基因表达.
引文著录: 吴斌, 张玫, 吕瑞雪, 罗通行, 李一松, 王兰兰. 表氧化二十碳三烯酸对游离脂肪酸诱导胰岛β细胞凋亡的抑制作用. 世界华人消化杂志 2012; 20(13): 1088-1093
Revised: March 5, 2012
Accepted: March 25, 2012
Published online: May 8, 2012
AIM: To investigate the effect of 11,12-epoxyeicosatrienoic acid (EET) on free fatty acid-induced cell apoptosis and translocation of activating transcription factor 4 (ATF4) and activating transcription factor 6 (ATF6) in primarily cultured murine pancreatic β-cells.
METHODS: Primary pancreatic β-cells were isolated from murine pancreas islets and cultured. After treatment with palmitic acid (400 μmol/L), pancreatic β-cells were incubated with 11,12-EET (100 nmol/L) for 24 h. Viability of primary pancreatic β-cells was examined by WST-1 colorimetric assay. Changes in mitochondrial membrane potential were evaluated to observe depolarization of cellular mitochondria by flow cytometry. Western blot was used to determine the protein expression of cytoplasmic and nuclear ATF4 and ATF6 to observe their translocation.
RESULTS: After treatment with palmitic acid and 11,12-EET for 24 h, viability of primary pancreatic β-cells was significantly increased (62.1% ± 7.3% vs 53.0% ± 6.1%, P < 0.05), and mitochondrial depolarization (23.6% ± 3.4% vs 35.2% ± 4.7%, P < 0.05) and apoptosis rate (24.5% ± 4.2% vs 40.1% ± 5.6%, P < 0.05) were markedly decreased compared to cells treated with palmitic acid alone. Palmitic acid significantly increased cytoplasmic but decreased nuclear protein levels of ATF4 and ATF6 in pancreatic β-cells.
CONCLUSION: 11,12-EET significantly inhibits FFA-induced apoptosis of pancreatic β-cells by inhibiting the translocation of ATF4 and ATF6.
- Citation: Wu B, Zhang M, Lv RX, Luo TX, Li YS, Wang LL. 11,12-Epoxyeicosatrienoic acid inhibits free fatty acid-induced apoptosis of pancreatic β-cells through targeting nuclear ATF4 and ATF6. Shijie Huaren Xiaohua Zazhi 2012; 20(13): 1088-1093
- URL: https://www.wjgnet.com/1009-3079/full/v20/i13/1088.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v20.i13.1088
各研究显示血浆游离脂肪酸(free fatty acids, FFAs)水平上升严重削弱胰腺β细胞功能[1-4]; 同时, 循环FFAs水平急性上升可以有效地刺激β细胞分泌胰岛素[5,6]; 然而, 持续性上升则导致胰腺β细胞凋亡[6]. 11,12-表氧化二十碳三烯酸(11,12-epoxyeicosatrienoic acids, 11,12-EETs)由细胞色素P450表氧化酶代谢花生四烯酸产生, 被可溶性环氧化水解酶(soluble epoxide hydrolase, SEH)代谢为DHET(dihydroxyeicosa trienoic acids)[7]. 传统观点认为其主要作用是激活钙敏感的钾通道使平滑肌细胞处于超极化状态从而舒张血管[8]; 最近报道显示SEH基因敲除小鼠糖尿病模型胰岛β细胞功能明显改善[9], EETs水平上升显著性抑制糖尿病发病过程的胰岛β细胞凋亡[10]. 内质网应激(endoplasmic reticulum stress, ERS)削弱2型糖尿病发病过程中的胰岛β细胞胰岛素分泌功能并导致其凋亡[11,12]. 那么转录激活因子4(activating transcription factor 4, ATF4)与ATF6是否参与EETs抑制FFAs诱导的胰岛β细胞凋亡呢? 本实验以FFAs诱导胰岛β细胞凋亡, 通过11,12-EETs干预这一过程, 观察其是否有保护作用, 同时明确EETs对FFAs刺激的ATF4与ATF6转位的影响, 探讨EETs在保护糖尿病胰腺功能以及胰岛β细胞凋亡方面的分子机制.
RPMI 1640培养基、FBS和胰酶为Gibco公司产品; 11,12-EETs购自美国Cayman公司; 棕榈酸(palmitic acid, PA)、Ⅳ胶原酶、Histopaque-1077分离液和二甲基亚砜(Dimethyl sulfoxide, DMSO)均购自Sigma-Aldrich公司; 无脂肪酸牛血清白蛋白(bovine serum albumin, BSA)、WST-1细胞增殖及细胞毒性检测试剂盒和PVDF膜为德国ROCHE公司产品; 线粒体膜电位(JC-1)检测试剂盒、Annexin-V/PI细胞凋亡检测试剂盒及BCA增强型蛋白浓度检测试剂盒为中国碧云天生物技术公司产品; ATF4与ATF6抗体购自英国Abcam公司; β-actin为美国Santa-cruz公司产品; 辣根过氧化物酶标记山羊抗兔和抗鼠IgG为美国Invitrogen公司产品; 增强化学发光法(ECL)试剂盒购自美国Thermo-fisher公司; 细胞胞浆和核蛋白抽提试剂盒与蛋白Marker为Fermentas公司产品; 流式细胞仪为Beckman coulter产品(FC500型). 实验中所用其他试剂均为进口或国产分析纯.
1.2.1 胰岛分离和原代β细胞培养: 从8-10 wk的C57BL/6小鼠胰腺分离胰岛. 斩首法处死小鼠, 将含有1 g/L Ⅳ型胶原酶的(不含Ca2+)Hanks缓冲液通过导管注入已处死小鼠胰腺使其膨胀, 胰腺随即分离取出, 然后在37 ℃下孵育20 min; 加入一定比例Hitopaque-1077细胞分离液混合后采取密度梯度离心, 在解剖显微镜下手工吸出单个胰岛细胞[6,17]. 分离完全的β细胞植入6孔板、12孔板和96孔板, 用含100-200 mL/L FBS 的RPMI1640培养基(血清浓度视分离β细胞状态)置于37 ℃、50 mL/L CO2的培养箱中经相应干预处理后用于后续实验.
1.2.2 细胞实验设计和分组处理: 本实验前期预实验显示20、50、100 nmol/L 11,12-EETs在胰岛β细胞保护方面存在剂量依赖性, 100 nmol /L 11,12-EETs保护效应最为显著; 同时我们观察到100 nmol/L 11,12-EETs不会引起胰岛β细胞线粒体除极化和凋亡(结果在本文未显示). 细胞实验分组为: 空白对照组、溶媒对照组(0.4%无脂肪酸BSA和5 μL DMSO)、FFAs对照组(400 μmol/L PA)、FFAs+EET组(400 μmol/L PA+100 nmol/L 11,12-EET), 共同孵育24 h.
1.2.3 WST-1法检测细胞凋亡: 通常细胞毒性实验每孔加入100 μL(5 000个细胞), 并添加10 μL WST-1溶液. 若起始培养体积为200 μL, 则需加入20 μL WST-1溶液, 其他情况以此类推. 可以用添加相应量细胞培养液和WST-1溶液但未加细胞的孔作为空白对照. 在450 nm测定吸光度(A)值, 该数值水平反映存活细胞数量和活力高低.
1.2.4 细胞线粒体膜电位(JC-1)检测: 取适量JC-1(200×), 按照每50 μL JC-1(200×)加入8 mL超纯水的比例稀释JC-1, 加入2 mL JC-1染色缓冲液(5×), 混匀后即为JC-1染色工作液. Hanks缓冲液洗涤细胞1次, 加入0.5 mL RPMI1640细胞培养基, 再加入1 mL JC-1染色工作液, 充分混匀. 细胞培养箱中37 ℃孵育20 min; 孵育期间, 按照每1 mL JC-1染色缓冲液(5×)加入4 mL蒸馏水的比例, 配制适量的JC-1染色缓冲液(1×), 并放置于冰浴. 孵育结束后, 吸除上清, 用JC-1染色缓冲液(1×)洗涤2次, 加入1 mL细胞培养液, 流式细胞仪上机检测.
1.2.5 流式细胞术检测早期凋亡: 把细胞培养液吸出至新的1.5 mL EP管, PBS洗涤1次, 加入适量胰酶工作液(含有EDTA)消化. 若镜下观察细胞变圆即可停止消化, 1 000 r/min离心5 min, 弃掉上清; 加入前述中收集的细胞培养液, 稍混匀, 转移到离心管内, 1 000 r/min离心5 min, 弃上清, 收集细胞, 用1×PBS缓冲液轻轻吹打混匀细胞; 取5-10万重悬的细胞, 1 000 r/min离心5 min, 弃上清, 加入195 μL Annexin V-FITC结合液轻轻吹打, 室温下放置5 min; 再加入5 μL Annexin V-FITC溶液, 轻轻吹打混匀; 室温避光孵育10 min; 1 000 r/min离心5 min, 弃上清, 加入190 μL Annexin V-FITC结合液轻轻重悬细胞; 加入10 μL碘化丙啶染色液, 轻轻吹打混匀, 避光; 30 min内信号流式细胞仪检测为宜, Annexin V-FITC为绿色荧光, PI为红色荧光.
1.2.6 Western blot: 收集各组胰岛β细胞, 提取细胞胞浆蛋白与核蛋白, BCA增强蛋白浓度测定法进行蛋白定量. 以每泳道20-25 μg蛋白量上样, 经SDS-PAGE电泳分离蛋白, 4 ℃转膜过夜使蛋白转移至PVDF膜上. 室温下脱脂奶粉封闭2.5 h, 用一抗ATF4(1:2 000)、ATF6(1:2 000)孵育过夜, TBST缓冲液漂洗3-6次, 辣根过氧化物酶标记的山羊兔和鼠二抗(1:5 000)室温分别孵育2-3 h, 曝光显影成像通过凝胶分析系统软件分析. 以上实验均重复3次.
统计学处理 所有数据均以mean±SD表示, 应用SPSS13.0软件按进行统计学分析, 每个分组间的差异采用单因素方差分析(ANOVA), 以P<0.05为差异具有显著性统计学意义.
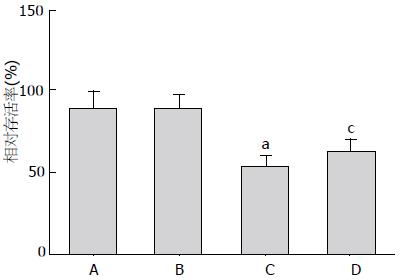
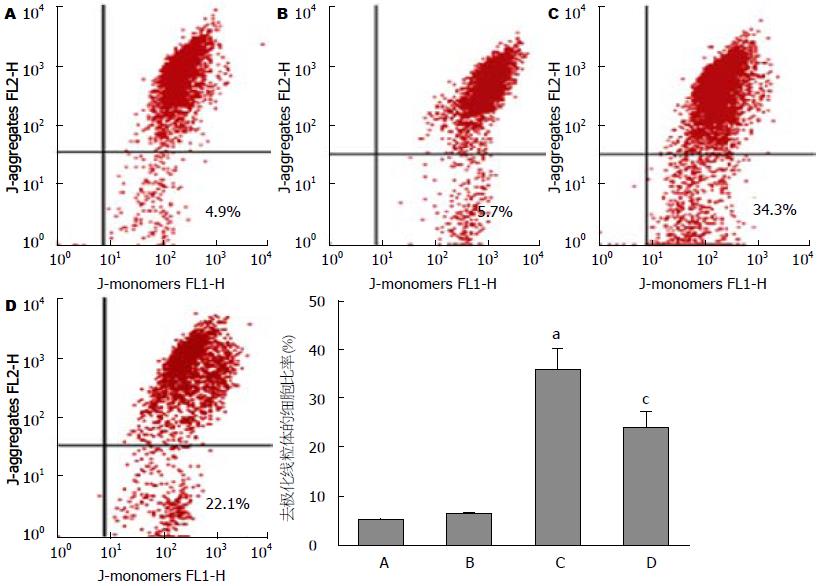
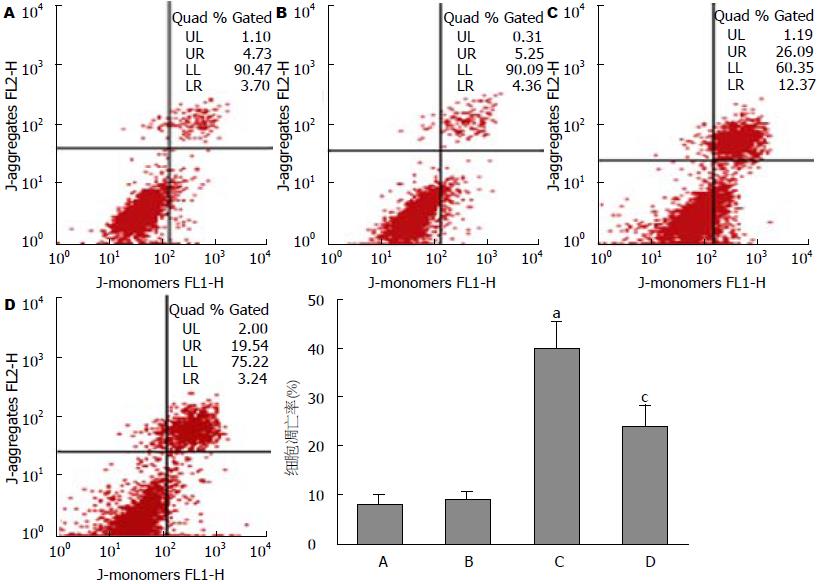
空白对照组细胞存活率为87.7%±10.3%, 溶媒对照组细胞存活率为86.2%±9.9%, FFAs对照组细胞存活率为53.0%±6.1%, FFAs+EET组细胞存活率为62.1%±7.3%. 与空白对照组相比, FFAs组细胞存活率显著性降低(P<0.05), FFAs+EET组细胞存活率较FFAs对照组增高, 两者差异具有统计学差异(P<0.05, 图1); 流式细胞术检测各分组细胞线粒体膜电位, 与空白对照组比较, FFAs对照组含有去极化线粒体的细胞比率有显著性增高(35.2%±4.70% vs 4.7%±0.9%, P<0.05), 而较FFAs对照组相比, FFAs+EET组含有去极化线粒体的细胞比率显著性降低(23.6%±3.4% vs 35.2%±4.70%, 图2); 流式细胞术检测各分组细胞凋亡, 结果显示FFAs对照组与空白对照组相比较其凋亡水平明显增高(40.1%±5.6% vs 8.2%±1.6%, P<0.05), FFAs+EET组与FFAs对照组比较其凋亡水平则明显降低(24.5%±4.2% vs 40.1%±5.6%, P<0.05, 图3). 综上结果, 11,12-EET可有效提高胰岛β细胞存活率和抑制FFAs诱导的细胞凋亡.
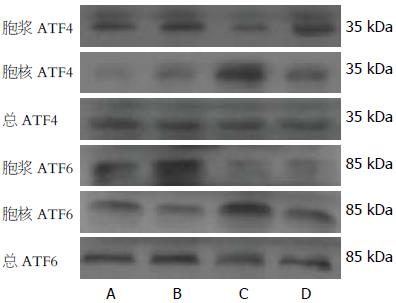
Western blot显示, FFAs对照组胞浆ATF4/ATF6水平与其他各实验分组比较显著性降低(P<0.05), 而核ATF4/ATF6水平较其他各实验分组明显升高(P<0.05); 与FFAs对照组相比, FFAs+EET组胞浆ATF4/ATF6水平升高(P<0.05), 核ATF4/ATF6水平降低(P<0.05, 图4). 综上, 11,12-EET抑制FFAs刺激的ATF4与ATF6的核转位.
11,12-EET由细胞色素P450表氧化酶代谢花生四烯酸产生, 其在胰岛素分泌和作用以及糖脂代谢中扮演重要角色[12,13]. 现已证实, EETs具有血管舒张、抗炎症、抗高血压、促血管生成、抗动脉粥样硬化和抗缺血再灌注引起的损伤等众多生理作用[14-17]. 最近其治疗胰岛素抵抗、1型和2型糖尿病的作用正愈来愈受到关注[18-20]. EETs主要通过激活PI3K-AKT信号通路从而抑制胰岛素抵抗, 抑制糖尿病引起的靶器官损伤, 但其在其他调节通路上的机制仍不明确[21,22]. 血浆FFAs升高削弱胰腺β细胞分泌胰岛素, 促进肥胖所引起的糖耐量受损[23]; 非糖尿病的健康个体持续性、生理性血浆FFAs升高预示其发展为2型糖尿病几率显著性增加[24]. 糖尿病发生、发展与胰岛β细胞功能失调、胰岛素分泌功能减退紧密相关, 内质网应激则参与了上述病程[25,26]. Kharroubi等[27]发现PA通过NF-κB诱导INS-1细胞凋亡, 同时诱发INS-1细胞内质网应激, ATF4发生选择性剪切即ATF4激活转入细胞核, ATF6转录激活; FFAs亦能诱导细胞线粒体功能失调.
FFAs引起的胰腺细胞内质网应激所致的胰岛β细胞功能失调、胰岛素分泌功能减退, 或最终引起胰岛β细胞凋亡与体内小分子活性物质相关[28-30]. 然而目前尚未有文献报道EETs在FFAs诱导的胰岛β细胞凋亡方面的作用机制. 为了探讨EETs在保护胰岛β细胞的新机制, 本文采用PA体外诱导原代小鼠胰岛β细胞凋亡, 同时采用11,12-EETs共同孵育, 利用细胞毒实验、线粒体膜电位检测和流式细胞术检测细胞凋亡以及Western blot检测内质网应激分子ATF4与ATF6核转位的变化. 细胞毒实验结果显示11,12-EETs能够明显地保护胰岛β细胞, 提高了与PA共同作用下胰岛β细胞的存活数. 线粒体膜电位检测能够直接反映该线粒体除极化细胞的水平, 而除极化水平高低反映细胞是否处于细胞凋亡早期. 那么通过其水平高低可以得出结论, 11,12- EETs可以显著性抑制FFAs所引起的胰岛β细胞早期凋亡. 进一步就上述结果, 我们通过annexin-V/PI法检测细胞凋亡, 与线粒体膜电位检测结果类似, 最后得出, 11,12-EETs可以保护处于高浓度FFAs培养下的胰岛β细胞. Western blot明确显示, 11,12-EETs抑制PA所刺激的ATF4与ATF6转位, 提示由于核转位水平下降, UPR(unfolded protein response, UPR)基因表达水平降低, 从而起到保护胰岛β细胞的作用.
总之, 本研究结果提示EETs在抑制FFAs诱导的胰岛β细胞凋亡方面起到重要的作用. 这为我们提供胰岛素抵抗、代谢综合征和糖尿病等疾病下胰腺功能保护提供新的理论依据与思路, 下一步我们将更深入地研究其分子机制和作用靶点.
研究显示血浆游离脂肪酸(FFAs)水平上升严重削弱胰腺β细胞功能, 循环FFAs水平急性上升可以有效地刺激β细胞分泌胰岛素; 然而, 持续性上升则导致胰腺β细胞凋亡.
黄昆, 教授, 华中科技大学同济药学院
11,12-表氧化二十碳三烯酸(EETs)具有血管舒张、抗炎症、抗高血压、促血管生成、抗动脉粥样硬化和抗缺血再灌注引起的损伤等众多生理作用; 最近其治疗胰岛素抵抗、1型和2型糖尿病的作用正越来越受到关注.
可溶性环氧化水解酶(SEH)基因敲除小鼠糖尿病模型胰岛β细胞功能明显改善, EETs水平上升显著性抑制糖尿病发病过程的胰岛β细胞凋亡.
本研究结果提示EETs在抑制FFAs诱导的胰岛β细胞凋亡方面起到重要的作用, 这为我们提供胰岛素抵抗、代谢综合征和糖尿病等疾病时胰腺功能的保护提供新的理论依据与思路.
本文利用FFAs诱导胰岛β细胞凋亡, 通过11,12-EETs干预这一过程确定EETs的保护性作用, 并探讨了EETs在保护糖尿病胰腺功能以及胰岛β细胞凋亡的分子机制, 文章思路较新颖, 并有较好的研究意义和科研价值.
编辑: 张姗姗 电编:闫晋利
| 1. | Carpentier A, Mittelman SD, Bergman RN, Giacca A, Lewis GF. Prolonged elevation of plasma free fatty acids impairs pancreatic beta-cell function in obese nondiabetic humans but not in individuals with type 2 diabetes. Diabetes. 2000;49:399-408. [PubMed] [DOI] |
| 2. | Eitel K, Staiger H, Brendel MD, Brandhorst H, Bretzel RG, Häring HU, Kellerer M. [Apoptosis induced by free fatty acids]. Med Klin (Munich). 2003;98:248-252. [PubMed] [DOI] |
| 3. | Kishore P, Tonelli J, Koppaka S, Fratila C, Bose A, Lee DE, Reddy K, Hawkins M. Time-dependent effects of free fatty acids on glucose effectiveness in type 2 diabetes. Diabetes. 2006;55:1761-1768. [PubMed] [DOI] |
| 4. | Wilding JP. The importance of free fatty acids in the development of Type 2 diabetes. Diabet Med. 2007;24:934-945. [PubMed] [DOI] |
| 5. | Lai E, Bikopoulos G, Wheeler MB, Rozakis-Adcock M, Volchuk A. Differential activation of ER stress and apoptosis in response to chronically elevated free fatty acids in pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2008;294:E540-E550. [PubMed] [DOI] |
| 6. | Wyne KL. Free fatty acids and type 2 diabetes mellitus. Am J Med. 2003;115 Suppl 8A:29S-36S. [PubMed] [DOI] |
| 7. | Pfister SL, Gauthier KM, Campbell WB. Vascular pharmacology of epoxyeicosatrienoic acids. Adv Pharmacol. 2010;60:27-59. [PubMed] [DOI] |
| 8. | Fleming I. DiscrEET regulators of homeostasis: epoxyeicosatrienoic acids, cytochrome P450 epoxygenases and vascular inflammation. Trends Pharmacol Sci. 2007;28:448-452. [PubMed] [DOI] |
| 9. | Luria A, Bettaieb A, Xi Y, Shieh GJ, Liu HC, Inoue H, Tsai HJ, Imig JD, Haj FG, Hammock BD. Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc Natl Acad Sci USA. 2011;108:9038-9043. [PubMed] [DOI] |
| 10. | Luo P, Wang MH. Eicosanoids, β-cell function, and diabetes. Prostaglandins Other Lipid Mediat. 2011;95:1-10. [PubMed] [DOI] |
| 11. | Back SH, Kang SW, Han J, Chung HT. Endoplasmic reticulum stress in the β-cell pathogenesis of type 2 diabetes. Exp Diabetes Res. 2012;2012:618396. [PubMed] |
| 12. | Quan W, Lim YM, Lee MS. Role of autophagy in diabetes and endoplasmic reticulum stress of pancreatic β-cells. Exp Mol Med. 2012;44:81-88. [PubMed] [DOI] |
| 13. | Mustafa S, Sharma V, McNeill JH. Insulin resistance and endothelial dysfunction: Are epoxyeicosatrienoic acids the link? Exp Clin Cardiol. 2009;14:e41-e50. [PubMed] |
| 14. | Xu X, Zhao CX, Wang L, Tu L, Fang X, Zheng C, Edin ML, Zeldin DC, Wang DW. Increased CYP2J3 expression reduces insulin resistance in fructose-treated rats and db/db mice. Diabetes. 2010;59:997-1005. [PubMed] [DOI] |
| 15. | Gauthier KM, Yang W, Gross GJ, Campbell WB. Roles of epoxyeicosatrienoic acids in vascular regulation and cardiac preconditioning. J Cardiovasc Pharmacol. 2007;50:601-608. [PubMed] [DOI] |
| 16. | Keserü B, Barbosa-Sicard E, Popp R, Fisslthaler B, Dietrich A, Gudermann T, Hammock BD, Falck JR, Weissmann N, Busse R. Epoxyeicosatrienoic acids and the soluble epoxide hydrolase are determinants of pulmonary artery pressure and the acute hypoxic pulmonary vasoconstrictor response. FASEB J. 2008;22:4306-4315. [PubMed] [DOI] |
| 17. | Loot AE, Fleming I. Cytochrome P450-derived epoxyeicosatrienoic acids and pulmonary hypertension: central role of transient receptor potential C6 channels. J Cardiovasc Pharmacol. 2011;57:140-147. [PubMed] [DOI] |
| 18. | Burgess A, Vanella L, Bellner L, Schwartzman ML, Abraham NG. Epoxyeicosatrienoic acids and heme oxygenase-1 interaction attenuates diabetes and metabolic syndrome complications. Prostaglandins Other Lipid Mediat. 2012;97:1-16. [PubMed] [DOI] |
| 19. | Chen G, Wang P, Zhao G, Xu G, Gruzdev A, Zeldin DC, Wang DW. Cytochrome P450 epoxygenase CYP2J2 attenuates nephropathy in streptozotocin-induced diabetic mice. Prostaglandins Other Lipid Mediat. 2011;96:63-71. [PubMed] [DOI] |
| 20. | Skepner JE, Shelly LD, Ji C, Reidich B, Luo Y. Chronic treatment with epoxyeicosatrienoic acids modulates insulin signaling and prevents insulin resistance in hepatocytes. Prostaglandins Other Lipid Mediat. 2011;94:3-8. [PubMed] [DOI] |
| 21. | Dhanasekaran A, Gruenloh SK, Buonaccorsi JN, Zhang R, Gross GJ, Falck JR, Patel PK, Jacobs ER, Medhora M. Multiple antiapoptotic targets of the PI3K/Akt survival pathway are activated by epoxyeicosatrienoic acids to protect cardiomyocytes from hypoxia/anoxia. Am J Physiol Heart Circ Physiol. 2008;294:H724-H735. [PubMed] [DOI] |
| 22. | Xu X, Tu L, Wang L, Fang X, Wang DW. CYP2J3 Gene Delivery Reduces Insulin Resistance via Upregulation of eNOS in Fructose-treated Rats. Cardiovasc Diabetol. 2011;10:114. [PubMed] [DOI] |
| 23. | Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11:351-356. [PubMed] [DOI] |
| 24. | Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L, DeFronzo R, Cusi K. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 2003;52:2461-2474. [PubMed] [DOI] |
| 25. | Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752-763. [PubMed] [DOI] |
| 26. | Sari FR, Watanabe K, Thandavarayan RA, Harima M, Zhang S, Muslin AJ, Kodama M, Aizawa Y. 14-3-3 protein protects against cardiac endoplasmic reticulum stress (ERS) and ERS-initiated apoptosis in experimental diabetes. J Pharmacol Sci. 2010;113:325-334. [PubMed] [DOI] |
| 27. | Kharroubi I, Ladrière L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004;145:5087-5096. [PubMed] [DOI] |
| 28. | Maia RC, Culver CA, Laster SM. Evidence against calcium as a mediator of mitochondrial dysfunction during apoptosis induced by arachidonic acid and other free fatty acids. J Immunol. 2006;177:6398-6404. [PubMed] |
| 29. | van der Kallen CJ, van Greevenbroek MM, Stehouwer CD, Schalkwijk CG. Endoplasmic reticulum stress-induced apoptosis in the development of diabetes: is there a role for adipose tissue and liver? Apoptosis. 2009;14:1424-1434. [PubMed] [DOI] |
| 30. | Szabat M, Kalynyak TB, Lim GE, Chu KY, Yang YH, Asadi A, Gage BK, Ao Z, Warnock GL, Piret JM. Musashi expression in β-cells coordinates insulin expression, apoptosis and proliferation in response to endoplasmic reticulum stress in diabetes. Cell Death Dis. 2011;2:e232. [PubMed] [DOI] |