修回日期: 2011-01-20
接受日期: 2011-01-26
在线出版日期: 2011-03-08
目的: 探讨胰岛素(Ins)对大鼠结肠平滑肌细胞(SMCs)增殖及其表达干细胞因子(SCF)的影响.
方法: 酶解法结合机械刮除法分离培养SD大鼠结肠SMCs, α-actin免疫荧光鉴定. 将SMC随机分为Ins剂量组(0、2.5、5、20、40、80 mg/L)与时间组(0、8、16、24 h), 观察Ins作用下SMCs的增殖及其合成SCF的情况. MTT法检测SMCs的增殖, Western blot及RT-PCR法检测SCF的表达.
结果: (1)5 mg/L时Ins即有显著的促SMCs增殖效应(0.052±0.006 vs 0.018±0.006, P<0.05); (2)低、中剂量Ins(2.5、5、20 mg/L)能促进结肠SMCs表达SCF(蛋白: 0.735±0.035, 0.754±0.057, 0.741±0.051 vs 0.658±0.024; mRNA: 0.688±0.077, 0.690±0.080, 0.698±0.074 vs 0.528±0.053, 均P<0.05); 高剂量Ins(40 mg/L)使SCF的表达达到最高峰(蛋白: 0.899±0.048 vs 0.658±0.024; mRNA: 0.938±0.117 vs 0.528±0.053; 均P<0.05); (3)SMC表达SCF在Ins作用16 h后达到峰值(蛋白: 0.899±0.011 vs 0.628±0.015; mRNA: 1.038±0.053 vs 0.709±0.042; 均P<0.05).
结论: Ins能促进结肠SMCs增殖, 并在一定范围内呈剂量与时间依赖性促进结肠SMCs表达SCF.
引文著录: 余盈娟, 袁玉丰, 林琳. 胰岛素对结肠平滑肌细胞增殖及其表达干细胞因子的影响. 世界华人消化杂志 2011; 19(7): 674-679
Revised: January 20, 2011
Accepted: January 26, 2011
Published online: March 8, 2011
AIM: To investigate the effect of insulin on the expression of stem cell factor (SCF) in rat colonic smooth muscle cells.
METHODS: Rat colonic smooth muscle cells (SMCs) were separated by mechanical and enzymatic methods and identified by immunofluorescence staining of α-actin. Identified colonic SMCs were randomly divided into two groups: cells treated with different concentrations of insulin (0, 2.5, 5, 20, 40, 80 mg/L) and those treated with insulin for different durations (0, 8, 16, 24 h). The expression of SCF was detected by Western blot and RT-PCR. MTT assay was used to measure the proliferation of colonic SMCs.
RESULTS: At a concentration of 5 mg/L, insulin remarkably promoted the proliferation of colonic SMCs (0.052 ± 0.006 vs 0.018 ± 0.006, P < 0.05). Insulin at a concentration of 2.5, 5 or 20 mg/L promoted SCF expression in colonic SMCs (protein: 0.735 ± 0.035, 0.754 ± 0.057, 0.741 ± 0.051 vs 0.658 ± 0.024; mRNA: 0.688 ± 0.077, 0.690 ± 0.080, 0.698 ± 0.074 vs 0.528 ± 0.053; all P < 0.05), but there were no marked differences in the expression levels of SCF protein and mRNA among these three groups of cells. When the dosage of insulin was elevated to 40 mg/L, SCF expression reached its peak (protein: 0.899 ± 0.048 vs 0.658 ± 0.024; mRNA: 0.938 ± 0.117 vs 0.528 ± 0.053; both P < 0.05). The expression of SCF reached the peak at 16 hours after insulin treatment (protein: 0.899 ± 0.011 vs 0.628 ± 0.015; mRNA: 1.038 ± 0.053 vs 0.709 ± 0.042; both P < 0.05).
CONCLUSION: Insulin promotes cell proliferation and up-regulates SCF expression in rat colonic SMCs.
- Citation: Yu YJ, Yuan YF, Lin L. Insulin regulates the expression of stem cell factor in rat colonic smooth muscle cells. Shijie Huaren Xiaohua Zazhi 2011; 19(7): 674-679
- URL: https://www.wjgnet.com/1009-3079/full/v19/i7/674.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v19.i7.674
Cajal间质细胞(interstitial cells of Cajal, ICC)、平滑肌细胞(smooth muscle cell, SMC)和肠神经系统(enteric nervous system, ENS)组成特殊的功能元件调节胃肠运动. ICC是一种呈网络状遍布于胃肠肌层的特殊间质细胞, 作为胃肠起搏细胞[1,2], 兼有感受平滑肌压力、传导神经信号的作用[3,4], 其形态与功能需要多种细胞因子的维持[5,6], 其中干细胞因子(stem cell factor, SCF)起着重要作用[7-9]. SCF是一种髓源性的多功能细胞生长因子, 亦能由胃肠SMC合成[10], 对多种细胞的生长与发育有重要调控作用. 研究离体胃肠组织发现[11,12], 胰岛素(insulin, Ins)对ICC有保护作用, 同时伴有SCF表达的增加, 但ICC表面缺乏胰岛素受体(insulin receptor, IR), 推测Ins可能通过促进SCF的表达进而对ICC起保护作用. 在胃肠道, 营养并维持ICC的SCF主要来自平滑肌[11], 该研究旨在从细胞水平探讨Ins是否能促进大鼠结肠SMC合成SCF并探讨Ins对结肠SMC增殖的影响.
SD大鼠, 雌雄不限, 体质量150-200 g, 由南京医科大学实验动物中心提供. DMEM培养液、胎牛血清(Gibco, USA), Ⅱ型胶原酶、大豆胰蛋白酶抑制剂、牛胰岛素(Sigma, USA), SCF抗体(Santa Cruz, USA), α-actin抗体(北京博奥森公司), SCF引物(南京博尔迪生物科技公司).
1.2.1 结肠SMC的分离和培养: SD大鼠10%水合氯醛麻醉致死, 自肛门上2 cm取结肠肠段10 cm左右, 用含抗生素的Hepes-Ringer缓冲液反复冲洗, 机械刮除黏膜层和浆膜层. 将平滑肌组织剪碎匀浆, 置入消化液(0.1%的Ⅱ型胶原酶和0.01%的大豆胰蛋白酶抑制剂)中, 30 ℃孵育20 min、离心、弃消化液(此过程重复1次), 加含100 mL/L胎牛血清的DMEM培养液中止消化、离心, DMEM培养液重悬细胞, 过筛; 台盼蓝染色确认细胞活力>90%, 于37 ℃ 950 mL/L O2和50 mL/L CO2条件下培养, SMC长至致密单层时, 传代培养, 采用第3-5代SMC进行实验.
1.2.2 结肠SMC的鉴定: 通过光学显微镜观察细胞形态及免疫荧光法进行鉴定. 细胞培养至7-10 d光镜下观察SMC的形态; 取对数生长的SMC, 胰蛋白酶消化, 制成单细胞悬液; 将SMC接种到放置载玻片的培养皿中, 培养1-3 d, 待SMC长至单层时, 取出载玻片. PBS缓冲液冲洗、丙酮固定, 3% H2O2阻断内源性过氧化物酶; 滴加α-actin一抗(1:100), 4 ℃过夜, PBS冲洗, 滴加罗丹明标记的羊抗兔IgG二抗, 室温避光湿盒中孵育1 h, PBS冲洗, 滴加Hoechst染核, PBS缓冲液冲洗、封片, 观察特异性荧光.
1.2.3 MTT比色法检测SMC的增殖: 结肠SMC按4×103/孔接种至96孔培养板, 贴壁后换入Ins浓度分别为0、2.5、5、20、40、80 mg/L的培养液, 24 h后加入5 g/L MTT溶液20 μL/孔, 继续孵育4 h, 吸弃上清液, 加入二甲基亚砜(DMSO)150 μL/孔, 37 ℃振荡10 min, 置酶标仪492 nm波长处比色, 记录每孔吸光度(A值). 每组设5复孔, 去掉最大值与最小值.
1.2.4 分组与处理: 取对数生长期的SMC加入无胎牛血清的DMEM培养液饥饿24 h, 研究Ins对结肠SMC合成SCF的影响. (1)Ins不同浓度组: SMC+Ins(0、2.5、5、20、40、80 mg/L)分别培养16 h; (2)Ins不同时间组: SMC+Ins(40 mg/L)分别培养0、8、16、24 h; 以上实验所用细胞来自同一大鼠, 每次实验重复3次.
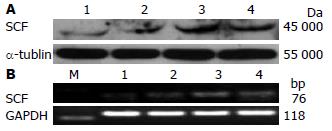
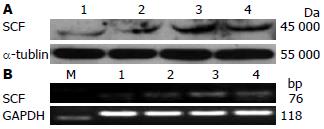
1.2.5 Western blot法检测SMC中SCF蛋白表达: 蛋白裂解液提取各组细胞蛋白, 离心、超声破碎后BCA法测定蛋白浓度. 60 μg蛋白/泳道加样, 恒流30 mA电泳, 恒压100 V转膜70 min, 封闭2 h. 加入SCF一抗(1:100), 4 ℃过夜; 二抗1:5 000, 37 ℃孵育, 曝光、显影.
1.2.6 RT-PCR法检测SMC中SCF mRNA表达: 按TRIzol试剂说明提取各组细胞总mRNA, 逆转录为cDNA, 以此cDNA为模板行PCR扩增. PCR反应条件: 94 ℃预变性5 min、94 ℃变性15 s、55 ℃退火30 s、72 ℃延伸15 s、共循环30次, 最后于72 ℃延伸8 min, PCR产物经2%琼脂糖凝胶电泳, 观测、拍照. SCF上游引物: 5'-TTC GCT TGT AAT TGG CTT TGC-3'; 下游引物: 5'-CAA CTG CCC TTG TAA GAC TTGA-3'(76 bp, NM_021843); GAPDH上游引物: 5'-CCC CCA ATG TAT CCG TTG TG-3'; 下游引物: 5'-TAG CCC AGG ATG CCC TTT AGT-3'(118 bp, BC059110).
统计学处理 所有数据录入SPSS10.0软件包分析, 以mean±SD表示, 采用方差分析和成组t检验, P<0.05为有显著性差异.
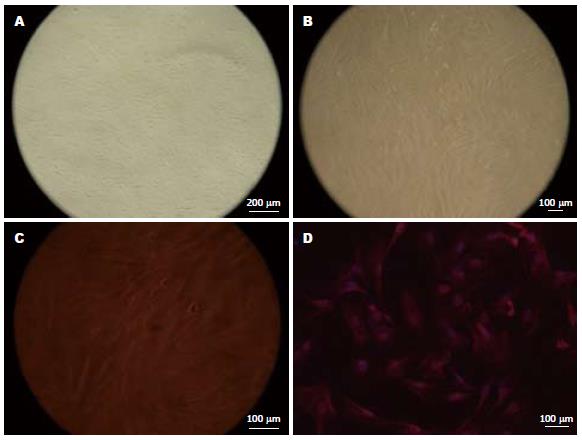
2.1.1 结肠SMC显微镜下观察: 培养24 h后, 可见细胞贴壁, 呈三角形或梭形, 有少量突起, 3-5 d开始增殖(图1A); 14 d后细胞密集, 呈峰谷样生长(图1B); 200倍显微镜下SMC的突起清晰可见(图1C).
2.1.2 α-actin免疫荧光阳性反应: 大部分多角形细胞胞质内见红色荧光, 细胞核Hoechst染色呈蓝色(图1D).
2.5 mg/L Ins对结肠SMC的增殖无影响(0.023±0.012 vs 0.018±0.006, P>0.05), 5 mg/L时能显著地促进SMC生长(0.052±0.006 vs 0.018±0.006, P<0.05), Ins在20、40、80 mg/L与5 mg/L时促SMC的增殖效应无差异(0.052±0.009, 0.046±0.013, 0.040±0.004 vs 0.052±0.006, P>0.05).
低剂量Ins(2.5 mg/L)即开始促进结肠SMC表达SCF, 至40 mg/L时SCF的表达量最大(P<0.05, 表1, 图2), 80 mg/L时SCF的表达量与40 mg/L时无差异(P>0.05, 表1, 图2).
Ins(40 mg/L)诱导SMC合成SCF的峰值在16 h后(P<0.05, 表2, 图3), 24 h与16 h时SCF的表达无统计学差异(P>0.05, 表2, 图3).
糖尿病(diabetes mellitus, DM)相关的胃肠动力障碍常见的包括消化不良、胃轻瘫、便秘, 可累及全胃肠道, 严重影响患者的生活质量[13], 其发生与ICC的异常密切相关. 2型DM人体胃肠标本研究显示ICC核膜皱缩、内质网扩张、自噬体增加并有慢波电节律的异常[14]; DM模型鼠亦发现有ICC及SCF的减少[15,16]. ICC的发育与功能的维持与c-Kit信号系统密切相关[17,18], 阻断c-Kit受体后, ICC几乎消失并伴有慢波的丧失. c-Kit的天然配体SCF是一种重要的造血生长因子, 在多种组织和细胞中均有表达, 具有多种生物学功能, 如维持造血干细胞和肥大细胞的生存[19], 调节红细胞、黑素、精子的生成并调节T淋巴细胞的分化[20,21], 调节其他一些细胞的生长周期[22], 在肿瘤与炎症疾病的发生发展中亦可能有一定的作用[23,24]. 无论是阻断c-Kit信号通路后还是在DM动物模型, 添加外源性SCF后ICC的数量、超微结构、慢波的振幅与频率均得到不同程度的改善[25,26]. 对离体胃肠组织的研究表明[27], 胃肠道平滑肌、肠神经均表达IR, 但仅在平滑肌表面检测到SCF; 另有研究显示[11], 在仅发生肠神经元缺失的小鼠胃肠道内, ICC的分布、SCF的表达、慢波的活动均无明显异常, 离体的肠道平滑肌依然能检测到SCF的表达. 综上研究推测在胃肠道维持ICC功能的SCF主要由SMC合成. Ins是胰岛β细胞分泌的蛋白质激素, 相对分子质量约为5 700 Da, 由两条氨基酸肽链组成, 由其前体Ins原在高尔基复合体内转化而成, 最主要的生理作用是调节机体细胞对葡萄糖、氨基酸、脂肪酸等的摄取、利用和储存. IR是一种酪氨酸激酶, 目前发现4种亚型IR1、IR2、IR3、IR4, 在机体不同组织及不同发育阶段表达量亦不相同[28], Ins与IR结合后IR进一步磷酸化Ins底物受体IRS[29], 进而发挥其生物学效应. Horváth等[12]对离体胃肠组织的培养中发现Ins/IGF-1信号通路的缺失同样能导致ICC数量的减少与功能异常; 外源性Ins/IGF-1能一定程度地改善DM时ICC的数量/结构异常, 并同时增加SCF的表达[30,31]. 但是ICC缺乏IR, Ins可能通过其他途径间接地对ICC起保护作用, 例如通过诱导SMC合成SCF. 本实验研究显示, 2.5 mg/L Ins即能一定程度地促进SMC表达SCF, 40 mg/L时达到最高峰, 并在16 h后作用最明显, 提示Ins在一定的范围内能呈剂量与时间依赖方式诱导SMC合成SCF. 另外, Ins对SMC尚有一定的促增殖作用, 2.5 mg/L Ins对SMC的促增殖效应与空白对照组无统计学差异, 但是5 mg/L Ins即有显著的促进SMC增殖的效应, 20、40、80 mg/L其效应与5 mg/L时无差异, 可能是IR饱和的原因. 本实验中Ins对SMC的促增殖效应与促SCF合成效应并不完全一致, 考虑MTT及Western blot是两种不同的实验方法, 其评估目的亦不相同; 另外还有可能由于实验条件所限存在一定的实验误差. 胰岛素样生长因子(insulin like growth factor 1, IGF-1)是Ins样生长因子家族中的一种, 其分子结构与Ins类似, 在某些方面有与Ins类似的生理功能. 本课题组研究显示IGF-1能促进结肠SMC分泌SCF, 与Ins一样在第16小时作用最强[32]. Ins与IGF-1有共同的信号通路PI3K/AKT与MEK/ERKs[33,34], 结肠SMC研究显示, IGF-1可能通过MAPK信号通路促进SMC表达SCF[35], Ins可能的信号通路是什么, 是否与PI3K、MAPK两条通路中的一条或两条或是其他Ins信号通路有关则有待进一步的研究与证实.
总之, 本实验从细胞水平证实Ins能促进SMC增殖并合成SCF, 为以后更进一步地探讨Ins、IGF-1、SCF、SMC、ICC之间的关系提供了一定的实验依据, 为研究糖尿病胃肠动力障碍提供理论依据.
糖尿病、胃肠病严重影响患者生活质量, 目前其机制尚不明确, 胰岛素信号通路的缺失及其引发的Cajal间质细胞功能障碍可能与之有关.
许文燮, 教授, 上海交通大学生命科学院生物医学工程系
胃肠动力障碍与胃肠起搏细胞ICC密切相关, 对ICC及其相关因素的研究成为热点.
本文首次探讨了Ins对结肠SMC增殖的影响, 并首次在细胞水平探讨Ins对结肠SMC合成SCF的影响, 为下一步探讨其可能机制奠定基础.
本研究证实, Ins能促进结肠SMC增殖, 并能诱导SMC表达SCF, 为胃肠动力障碍性疾病的治疗提供一定的理论依据.
本文选题恰当, 对研究胃肠道平滑肌起搏细胞Cajal间质细胞与胃肠动力障碍关系研究具有一定的理论意义.
编辑: 曹丽鸥 电编:何基才
| 1. | Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of cajal from the murine small intestine. J Physiol. 1998;513:203-213. [PubMed] [DOI] |
| 2. | Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347-349. [PubMed] [DOI] |
| 3. | Iino S, Horiguchi K, Nojyo Y, Ward SM, Sanders KM. Interstitial cells of Cajal contain signalling molecules for transduction of nitrergic stimulation in guinea pig caecum. Neurogastroenterol Motil. 2009;21:542-50, e12-e13. [PubMed] |
| 4. | Beckett EA, Takeda Y, Yanase H, Sanders KM, Ward SM. Synaptic specializations exist between enteric motor nerves and interstitial cells of Cajal in the murine stomach. J Comp Neurol. 2005;493:193-206. [PubMed] [DOI] |
| 5. | Choi KM, Gibbons SJ, Roeder JL, Lurken MS, Zhu J, Wouters MM, Miller SM, Szurszewski JH, Farrugia G. Regulation of interstitial cells of Cajal in the mouse gastric body by neuronal nitric oxide. Neurogastroenterol Motil. 2007;19:585-595. [PubMed] [DOI] |
| 6. | Wouters MM, Gibbons SJ, Roeder JL, Distad M, Ou Y, Strege PR, Szurszewski JH, Farrugia G. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2B receptors. Gastroenterology. 2007;133:897-906. [PubMed] [DOI] |
| 7. | Takeda M, Takayama I, Terada N, Baba T, Ward SM, Ohno S, Fujino MA. Immunoelectron-microscopic study of Kit-expressing cells in the jejunum of wildtype and Ws/Ws rats. Cell Tissue Res. 2001;304:21-30. [PubMed] [DOI] |
| 8. | Torihashi S, Nishi K, Tokutomi Y, Nishi T, Ward S, Sanders KM. Blockade of kit signaling induces transdifferentiation of interstitial cells of cajal to a smooth muscle phenotype. Gastroenterology. 1999;117:140-148. [PubMed] [DOI] |
| 9. | Nakahara M, Isozaki K, Vanderwinden JM, Shinomura Y, Kitamura Y, Hirota S, Matsuzawa Y. Dose-dependent and time-limited proliferation of cultured murine interstitial cells of Cajal in response to stem cell factor. Life Sci. 2002;70:2367-2376. [PubMed] [DOI] |
| 10. | Morimoto M. Intestinal Smooth Muscle Cells Enhance Stem Cell Factor (SCF) Production Locally against Gastrointestinal Nematode Infections. J Vet Med Sci. 2011; Jan 7. [Epub ahead of print]. [PubMed] |
| 11. | Ward SM, Ordög T, Bayguinov JR, Horowitz B, Epperson A, Shen L, Westphal H, Sanders KM. Development of interstitial cells of Cajal and pacemaking in mice lacking enteric nerves. Gastroenterology. 1999;117:584-594. [PubMed] [DOI] |
| 12. | Horváth VJ, Vittal H, Ordög T. Reduced insulin and IGF-I signaling, not hyperglycemia, underlies the diabetes-associated depletion of interstitial cells of Cajal in the murine stomach. Diabetes. 2005;54:1528-1533. [PubMed] [DOI] |
| 14. | Kim ER, Kim KM, Lee JY, Joo M, Kim S, Noh JH, Ward SM, Koh SD, Rhee PL. The clue of Interstitial Cell of Cajalopathy (ICCpathy) in human diabetic gastropathy The ultrastructural and electrical clues of ICCpathy in human diabetic gastropathy. Exp Toxicol Pathol. 2010; Dec 22. [Epub ahead of print]. [PubMed] |
| 15. | Yamamoto T, Watabe K, Nakahara M, Ogiyama H, Kiyohara T, Tsutsui S, Tamura S, Shinomura Y, Hayashi N. Disturbed gastrointestinal motility and decreased interstitial cells of Cajal in diabetic db/db mice. J Gastroenterol Hepatol. 2008;23:660-667. [PubMed] [DOI] |
| 16. | 罗 云, 林 琳, 张 红杰, 李 学良, 吴 高珏, 王 美峰. 糖尿病慢传输运动结肠Cajal间质细胞和干细胞因子的变化. 世界华人消化杂志. 2007;15:458-463. [DOI] |
| 20. | Tsiftsoglou AS, Vizirianakis IS, Strouboulis J. Erythropoiesis: model systems, molecular regulators, and developmental programs. IUBMB Life. 2009;61:800-830. [PubMed] [DOI] |
| 21. | Ray P, Krishnamoorthy N, Ray A. Emerging functions of c-kit and its ligand stem cell factor in dendritic cells: regulators of T cell differentiation. Cell Cycle. 2008;7:2826-2832. [PubMed] [DOI] |
| 22. | Skaar JR, Pagano M. Control of cell growth by the SCF and APC/C ubiquitin ligases. Curr Opin Cell Biol. 2009;21:816-824. [PubMed] [DOI] |
| 23. | Ali S, Ali S. Role of c-kit/SCF in cause and treatment of gastrointestinal stromal tumors (GIST). Gene. 2007;401:38-45. [PubMed] [DOI] |
| 24. | Reber L, Da Silva CA, Frossard N. Stem cell factor and its receptor c-Kit as targets for inflammatory diseases. Eur J Pharmacol. 2006;533:327-340. [PubMed] [DOI] |
| 25. | Tong W, Jia H, Zhang L, Li C, Ridolfi TJ, Liu B. Exogenous stem cell factor improves interstitial cells of Cajal restoration after blockade of c-kit signaling pathway. Scand J Gastroenterol. 2010;45:844-851. [PubMed] [DOI] |
| 27. | Horváth VJ, Vittal H, Lörincz A, Chen H, Almeida-Porada G, Redelman D, Ordög T. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759-770. [PubMed] [DOI] |
| 28. | Caruso MA, Blaufuss PC, Kittilson JD, Raine J, Sheridan MA. Isolation and characterization of a mRNA encoding a novel insulin receptor (IR) subtype, IR2, from rainbow trout (Oncorhynchus mykiss) and patterns of expression of the four IR subtypes, IR1-IR4, in tissues and during embryonic development. Gen Comp Endocrinol. 2010;169:258-268. [PubMed] [DOI] |
| 31. | 林 琳, 徐 丽明, 罗 云, 吴 高珏, 汤 玉蓉, 张 红杰, 李 学良. 糖尿病结肠动力障碍时Cajal间质细胞和干细胞因子的变化以及胰岛素的干预效应. 胃肠病学. 2008;13:200-204. |
| 32. | 宁 月季, 张 蔚, 成 家飞, 李 学良, 王 美峰, 林 琳. 胰岛素样生长因子1对大鼠结肠平滑肌细胞中干细胞因子表达的影响. 世界华人消化杂志. 2009;17:3502-3506. [DOI] |
| 33. | Picinato MC, Hirata AE, Cipolla-Neto J, Curi R, Carvalho CR, Anhê GF, Carpinelli AR. Activation of insulin and IGF-1 signaling pathways by melatonin through MT1 receptor in isolated rat pancreatic islets. J Pineal Res. 2008;44:88-94. [PubMed] |
| 34. | Lessmann E, Grochowy G, Weingarten L, Giesemann T, Aktories K, Leitges M, Krystal G, Huber M. Insulin and insulin-like growth factor-1 promote mast cell survival via activation of the phosphatidylinositol-3-kinase pathway. Exp Hematol. 2006;34:1532-1541. [PubMed] [DOI] |