修回日期: 2011-01-04
接受日期: 2011-01-11
在线出版日期: 2011-02-08
目的: 研究高氧对肠上皮细胞分泌片(SC)表达的影响.
方法: 采用细胞计数和Giemsa染色方法检测不同氧浓度对细胞生长和细胞分裂能力的影响, 采用免疫组织化学方法检测不同氧浓度对Caco-2表达SC的影响.
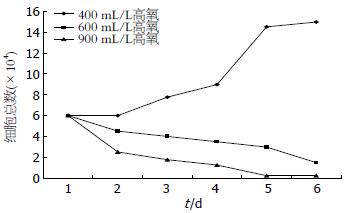
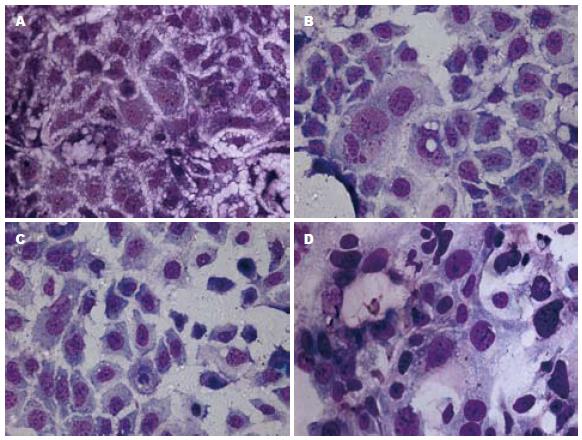
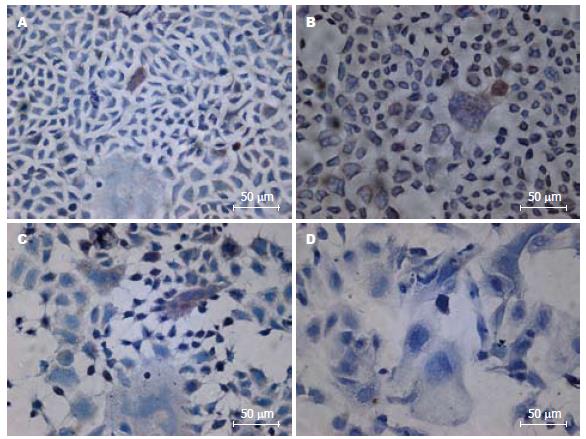
结果: 细胞计数显示400 mL/L氧浓度利于细胞生长; 600、900 mL/L氧浓度导致细胞迅速死亡. 不同氧浓度干预细胞3 d细胞分裂指数有明显不同, 分裂细胞百分数分别为正常氧浓度2.5%; 400 mL/L氧浓度为3.3%; 600 mL/L氧浓度为1.3%; 900 mL/L氧浓度大部分细胞死亡. 与正常氧浓度相比, 氧浓度为400、600 mL/L时SC表达增强, 900 mL/L氧浓度时SC表达明显减弱, 甚为阴性, 但600 mL/L氧浓度SC表达较400 mL/L氧浓度减弱.
结论: 适度的高氧促进细胞生长和促进肠上皮细胞表达SC, 严重高氧则抑制肠上皮细胞SC表达及肠上皮细胞生长, 肠上皮细胞SC表达增多有助于保护肠黏膜及平衡肠黏膜作用, 阻滞细菌入侵肠道.
引文著录: 刘冬妍, 苗佳宁. 高氧对肠上皮细胞表达分泌片的影响. 世界华人消化杂志 2011; 19(4): 362-366
Revised: January 4, 2011
Accepted: January 11, 2011
Published online: February 8, 2011
AIM: To explore the effect of hyperoxia on the expression of secretory component (SC) in human intestinal epithelial Caco-2 cells.
METHODS: The number of Caco-2 cells was counted with a hemacytometer, and cell division was determined by Giemsa staining. The changes in the expression levels of SC in Caco-2 cells were detected by immunocytochemistry.
RESULTS: Caco-2 cells exhibited exponential growth in air containing 400 mL/L O2. Cell growth was partially inhibited when the proportion of oxygen in air was elevated to 600 mL/L, and completely inhibited when elevated to 900 mL/L. The division index of treated cells was 2.5% in air, 3.3% in 400 mL/L O2, and 1.3% in 600 mL/L O2. Many cells died in 900 mL/L O2. Compared with cells incubated in air, the expression of SC was up-regulated in 400 mL/L and 600 mL/L O2. The ability of intestinal epithelial cells to express SC was limited in 900 mL/L O2. Compared with cells incubated in 400 mL/L O2, the expression of SC was down-regulated in 600 mL/L O2.
CONCLUSION: Moderately high concentrations of oxygen promote cell growth and SC expression in Caco-2 cells, whereas extremely high concentrations of oxygen inhibit cell growth and SC expression. High levels of SC are beneficial to maintaining and balancing the intestinal mucosa and inhibiting bacterial invasion.
- Citation: Liu DY, Miao JN. Effect of hyperoxia on the expression of secretory component in human intestinal epithelial Caco-2 cells. Shijie Huaren Xiaohua Zazhi 2011; 19(4): 362-366
- URL: https://www.wjgnet.com/1009-3079/full/v19/i4/362.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v19.i4.362
单层Caco-2细胞来源于人体结肠癌细胞株, 此细胞类似正常人肠上皮细胞, 在形态学上与人体肠上皮细胞相同, 并可分泌与人体相同的酶类、转化因子等[1], 目前有文献报道高氧可影响新生儿肠道形态和屏障功能[2,3], 本研究中我们利用高氧对体外培养的Caco-2细胞进行干预, 研究高氧对肠上皮细胞生长和表达分泌片(secretory component, SC)的影响.
Caco-2细胞株(上海生物研究所), 抗人SC抗体(Sigma公司).
1.2.1 细胞培养: 应用含有200 mL/L胎牛血清、青霉素-链霉素双抗液、pH值为7.2的DMEM培养液在37 ℃、50 mL/L CO2条件下培养Caco-2细胞, 每天换液1次.
1.2.2 采用细胞计数方法计数不同氧浓度对细胞生长影响: 当细胞生长达融合状态时, 消化传代, 计数, 均匀传代6瓶, 放入37 ℃ 50 mL/L CO2孵箱培养24 h, 细胞贴壁后, 放入400 mL/L氧浓度孵箱后, 分别在第1、2、3、4、5、6天消化计数细胞, 重复上述方法分别将6瓶细胞放入600、900 mL/L氧浓度孵箱计数细胞.
1.2.3 采用Giemsa染色方法检测不同氧浓度对细胞分裂能力的影响: 取同代细胞接种于盖玻片上, 放入37 ℃、50 mL/L CO2孵箱培养24 h贴壁后, 放入400、600、900 mL/L氧浓度孵箱内, 分别于第3天、第6天用PBS漂洗数次, 用甲醇:冰乙酸(3:1)固定30 min, Giemsa染液染色10 min, 自来水冲洗, 晾干后镜检. 计算细胞分裂指数(细胞分裂指数 = 分裂细胞数/总细胞数×100%).
1.2.4 免疫组织化学检测不同氧浓度对Caco-2表达SC影响: 取同代细胞接种于盖玻片上, 待细胞生长达融合状态时用上述不同氧浓度处理24 h, 取出盖玻片用甲醛固定, 然后依次加入H2O2、兔血清、抗SC抗体、生物素标记的IgG抗体、酶标记的卵白素, 最后用DAB显色.
400 mL/L氧浓度时细胞于贴壁后第2天开始增多, 第4、5天呈指数增多, 第6天趋于平稳; 600 mL/L氧浓度时细胞随时间延长逐渐减少; 900 mL/L氧浓度时细胞随时间延长迅速死亡(图1).
不同氧浓度干预细胞3 d, 细胞分裂指数有明显不同, 正常氧浓度时细胞密集分布, 部分细胞正在分裂, 分裂细胞百分数为2.5%; 400 mL/L氧浓度时与正常氧浓度相比, 处于正分裂的细胞数增多, 分裂细胞百分数为3.3%; 600 mL/L氧浓度时与正常氧浓度相比, 处于正分裂的细胞数减少, 分裂细胞百分数为1.3%; 900 mL/L氧浓度时大部分细胞死亡, 存活细胞与正常氧浓度细胞相比, 处于正分裂的细胞数明显减少, 难见, 但可见大核细胞. 不同氧浓度干预6 d后细胞终止分裂, 400 mL/L氧浓度细胞分裂指数与正常氧比较无明显不同, 600 mL/L氧浓度细胞分裂指数与正常氧比较无明显不同, 但部分细胞核变大; 900 mL/L氧浓度时大部分细胞死亡, 存活细胞与正常氧浓度细胞相比, 处于正分裂的细胞数与正常氧比较无明显不同, 但部分细胞核变大(图2).
SC主要位于Caco-2细胞的细胞质和细胞膜上, 在正常氧浓度中SC-阳性细胞数为0.1%-0.5%; 在400 mL/L氧浓度中SC-阳性细胞为0.4%-0.8%. 在600 mL/L氧浓度中 SC-阳性细胞数为0.2%-0.7%. 氧浓度>600 mL/L后SC的表达明显减少, 在900 mL/L氧浓度中不见SC阳性细胞(图3).
SIgA是肠道第一线的免疫防御, 是肠道免疫屏障的重要组成, 他覆盖保护肠黏膜免受有害细菌入侵, 阻止慢性炎症发生[4,5]. 当细菌黏附和入侵肠道时, 可激活黏膜免疫诱导SIgA大量分泌. SIgA在肠道主要是发挥免疫排除作用[6,7]和免疫平衡作用[8], 减少细菌易位, 限制细菌穿透肠上皮[8]. 他由浆细胞产生的pIgA和J链与肠上皮细胞分泌的pIgR组成. pIgR细胞外部分称为SC, 是黏膜免疫系统的一种重要转膜糖蛋白, 对肠黏膜有保护作用[9]. SC可抵抗蛋白酶对SIgA的降解, 加强由SIgA提供的黏膜免疫. SC上有大量不同的糖基, 这是细菌的重要配体, 因此SC作为微生物的重要清除因子可保护上皮免受侵袭[10]. 游离SC是防御细菌(大肠杆菌、梭状芽孢杆菌、肺炎球菌)、抵御原虫和中和霍乱毒素的重要因子[11-15], 可作为微生物的非特异清除物和限制炎症过程起到保护肠黏膜及平衡肠黏膜作用[16].
高氧有"双刃剑"的作用, 高氧可阻止细菌对上皮表面的入侵, 加强黏膜IgA的产生, 但是长期高氧供应可引起一定的毒性作用, 使机体抵抗力下降, 如高氧可加重流感病毒对新生鼠的感染性[17]. 长期高氧治疗在出现支气管肺发育异常的同时有生长迟缓现象, 该学者认为这是高氧影响肠空泡形成使吸收能力下降引起的, 并且高氧可影响其他器官, 如引起肾小管坏死、肿胀和再生, 以及肠浆膜和亚黏膜血管舒张[18]. 高氧环境中能引起机体信号通路的改变[2], 如激活NF-κB信号通路[19]等, 高氧还可使肠黏膜厚度变薄, 一氧化氮合酶Ⅱ(nitric oxide synthase Ⅱ, NOSⅡ)蛋白含量减少, 肠绒毛结构的改变和NOS调整可能影响新生鼠肠道屏障功能, 使肠道易遭细菌侵袭[2]. 国内富建华等[20]报道高氧使新生大鼠肠道自由基损伤, 本课题组亦报道高氧导致新生大鼠肠道SIgA发生变化[3], 本研究中体外用不同浓度氧处理肠上皮细胞, 发现肠上皮细胞在40%氧浓度中细胞迅速生长, 分裂指数达3.3%; 在60%氧浓度中生长减慢, 分裂指数也降低; 在90%氧浓度中细胞生长受抑并死亡. 肠上皮细胞分泌SC受许多因素的影响, 如细胞因子[21-26]、维生素A[27,28]、NO[29]、激素和微生物[30]均影响SC的表达. 本研究发现适度的高氧(在40%氧浓度SC阳性细胞数为0.4%-0.8%, 在60%氧浓度SC阳性细胞数为0.2%-0.7%)可诱导肠上皮细胞分泌SC, 但是严重高氧(90%)限制SC的分泌. 这证明严重高氧抑制肠上皮细胞生长并杀伤肠上皮细胞, 这样导致肠黏膜受损, 肠道屏障削弱. 同时对肠道有保护作用的SC在高氧作用下表达减少, 进一步削弱肠道屏障, 使得细菌易于入侵肠道.
总之, 适度的高氧促进细胞生长和促进肠上皮细胞表达SC, 严重高氧则抑制肠上皮细胞SC表达及肠上皮细胞生长, 肠上皮细胞SC表达增多有助于保护肠黏膜及平衡肠黏膜作用, 阻滞细菌入侵肠道.
当前临床抢救新生儿呼吸衰竭最有效的方法是氧疗法, 即机械通入高浓度的氧, 此疗法虽挽救患儿生命, 但长时间高氧治疗会引起肺、脑、眼等近隔器官损伤, 近年陆续报道长期高氧治疗还引起新生儿肾、肝、肠等远隔器官损伤.
白雪巍, 副教授, 哈尔滨医科大学第一临床医学院肝胆胰外科
国内外研究新生儿呼吸衰竭高氧治疗对消化系统的影响重点集中在形态学变化、NOS和NO变化, 目前对肠道屏障的损伤成为研究的热点.
刘冬妍等报道高氧引起新生儿肠道SIgA变化; 国外Torbati等和Giannone等分别报道高氧对新生儿肠道造成损伤.
本研究证实高氧影响肠上皮细胞生长和分泌功能, 为探讨高氧对肠道屏障的变化及其机制作了铺垫, 为进一步研究高氧对远隔器官影响奠定基础, 为深入探索防治氧疗后遗症新途径奠定实验基础.
本文学术价值较高, 具有较好的科研及临床意义.
编辑: 李薇 电编:何基才
| 1. | Yokomizo A, Moriwaki M. Transepithelial permeability of myricitrin and its degradation by simulated digestion in human intestinal Caco-2 cell monolayer. Biosci Biotechnol Biochem. 2005;69:1774-1776. [PubMed] [DOI] |
| 2. | Giannone PJ, Bauer JA, Schanbacher BL, Reber KM. Effects of hyperoxia on postnatal intestinal development. Biotech Histochem. 2007;82:17-22. [PubMed] [DOI] |
| 4. | Forchielli ML, Walker WA. The role of gut-associated lymphoid tissues and mucosal defence. Br J Nutr. 2005;93 Suppl 1:S41-S48. [PubMed] [DOI] |
| 5. | Shi HN, Walker A. Bacterial colonization and the development of intestinal defences. Can J Gastroenterol. 2004;18:493-500. [PubMed] |
| 6. | Boullier S, Tanguy M, Kadaoui KA, Caubet C, Sansonetti P, Corthésy B, Phalipon A. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J Immunol. 2009;183:5879-5885. [PubMed] [DOI] |
| 7. | Corthësy B. Secretory immunoglobulin A: well beyond immune exclusion at mucosal surfaces. Immunopharmacol Immunotoxicol. 2009;31:174-179. [PubMed] [DOI] |
| 8. | Macpherson AJ, Slack E. The functional interactions of commensal bacteria with intestinal secretory IgA. Curr Opin Gastroenterol. 2007;23:673-678. [PubMed] [DOI] |
| 9. | Murthy AK, Dubose CN, Banas JA, Coalson JJ, Arulanandam BP. Contribution of polymeric immunoglobulin receptor to regulation of intestinal inflammation in dextran sulfate sodium-induced colitis. J Gastroenterol Hepatol. 2006;21:1372-1380. [PubMed] |
| 10. | Perrier C, Sprenger N, Corthésy B. Glycans on secretory component participate in innate protection against mucosal pathogens. J Biol Chem. 2006;281:14280-14287. [PubMed] [DOI] |
| 11. | de Araújo AN, Giugliano LG. Lactoferrin and free secretory component of human milk inhibit the adhesion of enteropathogenic Escherichia coli to HeLa cells. BMC Microbiol. 2001;1:25. [PubMed] [DOI] |
| 12. | Dallas SD, Rolfe RD. Binding of Clostridium difficile toxin A to human milk secretory component. J Med Microbiol. 1998;47:879-888. [PubMed] [DOI] |
| 13. | Hammerschmidt S, Talay SR, Brandtzaeg P, Chhatwal GS. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol Microbiol. 1997;25:1113-1124. [PubMed] [DOI] |
| 14. | Davids BJ, Palm JE, Housley MP, Smith JR, Andersen YS, Martin MG, Hendrickson BA, Johansen FE, Svärd SG, Gillin FD. Polymeric immunoglobulin receptor in intestinal immune defense against the lumen-dwelling protozoan parasite Giardia. J Immunol. 2006;177:6281-6290. [PubMed] |
| 15. | Uren TK, Wijburg OL, Simmons C, Johansen FE, Brandtzaeg P, Strugnell RA. Vaccine-induced protection against gastrointestinal bacterial infections in the absence of secretory antibodies. Eur J Immunol. 2005;35:180-188. [PubMed] [DOI] |
| 16. | Phalipon A, Corthésy B. Novel functions of the polymeric Ig receptor: well beyond transport of immunoglobulins. Trends Immunol. 2003;24:55-58. [PubMed] [DOI] |
| 17. | O'Reilly MA, Marr SH, Yee M, McGrath-Morrow SA, Lawrence BP. Neonatal hyperoxia enhances the inflammatory response in adult mice infected with influenza A virus. Am J Respir Crit Care Med. 2008;177:1103-1110. [PubMed] [DOI] |
| 18. | Torbati D, Tan GH, Smith S, Frazier KS, Gelvez J, Fakioglu H, Totapally BR. Multiple-organ effect of normobaric hyperoxia in neonatal rats. J Crit Care. 2006;21:85-93; discussion 93-94. [PubMed] [DOI] |
| 19. | Wright CJ, Zhuang T, La P, Yang G, Dennery PA. Hyperoxia-induced NF-kappaB activation occurs via a maturationally sensitive atypical pathway. Am J Physiol Lung Cell Mol Physiol. 2009;296:L296-L306. [PubMed] [DOI] |
| 21. | Liu DY, Wang XL, Liu P. Tumor necrosis factor-alpha upregulates the expression of immunoglobulin secretory component. J Investig Allergol Clin Immunol. 2007;17:101-106. [PubMed] |
| 22. | Sollid LM, Kvale D, Brandtzaeg P, Markussen G, Thorsby E. Interferon-gamma enhances expression of secretory component, the epithelial receptor for polymeric immunoglobulins. J Immunol. 1987;138:4303-4306. [PubMed] |
| 23. | Kvale D, Løvhaug D, Sollid LM, Brandtzaeg P. Tumor necrosis factor-alpha up-regulates expression of secretory component, the epithelial receptor for polymeric Ig. J Immunol. 1988;140:3086-3089. [PubMed] |
| 24. | Phillips JO, Everson MP, Moldoveanu Z, Lue C, Mestecky J. Synergistic effect of IL-4 and IFN-gamma on the expression of polymeric Ig receptor (secretory component) and IgA binding by human epithelial cells. J Immunol. 1990;145:1740-1744. [PubMed] |
| 25. | Denning GM. IL-4 and IFN-gamma synergistically increase total polymeric IgA receptor levels in human intestinal epithelial cells. Role of protein tyrosine kinases. J Immunol. 1996;156:4807-4814. [PubMed] |
| 26. | Hayashi M, Takenouchi N, Asano M, Kato M, Tsurumachi T, Saito T, Moro I. The polymeric immunoglobulin receptor (secretory component) in a human intestinal epithelial cell line is up-regulated by interleukin-1. Immunology. 1997;92:220-225. [PubMed] [DOI] |
| 27. | Liu DY. TNF-α and VA Upregulate the Expression of Secretory Component in Caco-2 Cells. Am Biotech Lab. 2008;26:34-38. |
| 28. | Takenouchi-Ohkubo N, Asano M, Chihaya H, Chung-Hsuing WU, Ishikasa K, Moro I. Retinoic acid enhances the gene expression of human polymeric immunoglobulin receptor (pIgR) by TNF-alpha. Clin Exp Immunol. 2004;135:448-454. [PubMed] [DOI] |
| 30. | Kaetzel CS. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev. 2005;206:83-99. [PubMed] [DOI] |