修回日期: 2010-12-14
接受日期: 2010-12-21
在线出版日期: 2011-02-08
目的: 研究体外香菇多糖(Lentinan)对多药耐药基因表达的影响和促进顺铂抑制胃癌细胞增殖的作用.
方法: 分别用顺铂(Cisplatin)、香菇多糖和顺铂联合香菇多糖处理SGC-7901胃癌细胞, 将其分为4组: 对照组(Con组), 香菇多糖组(Len组), 顺铂组(Cis组)和顺铂联合香菇多糖组(L+C)组. 应用RT-PCR检测多药耐药基因MDR1、MRP1和LRP基因mRNA表达; 应用CCK-8试剂盒检测Con组和药物处理组前后的胃癌细胞增殖状态.
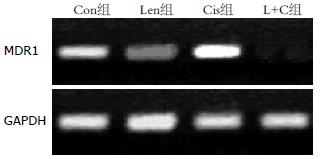
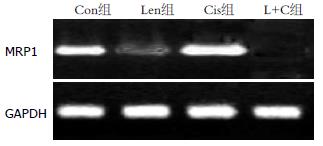
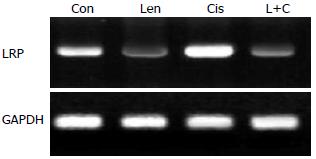
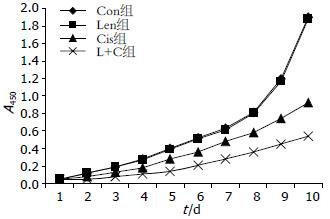
结果: 正常SGC-7901胃癌细胞中多药耐药基因MDR1、MRP1和LRP均表达mRNA, 香菇多糖能显著降低多药耐药基因表达而对细胞增殖无明显影响; 顺铂明显增加多药耐药基因表达, 同时抑制细胞增殖(P<0.05); 香菇多糖联合顺铂作用后, MDR1和MRP1基因表达完全受到抑制, LRP表达显著降低, 细胞增殖速度明显低于Con组、Len组和Cis组, 差异具有统计学意义(10 d: 0.54 vs 1.90, 1.88, 0.92, 均P<0.05).
结论: 香菇多糖联合顺铂后因抑制多药耐药基因表达而显著增强顺铂的抑制细胞增殖作用.
引文著录: 伍海鹰, 陈一明, 林龙, 林友刚, 邱庆安, 刘宁. 体外香菇多糖对顺铂抑制胃癌细胞增殖的促进作用. 世界华人消化杂志 2011; 19(4): 344-348
Revised: December 14, 2010
Accepted: December 21, 2010
Published online: February 8, 2011
AIM: To investigate whether lentinan enhances cisplatin-mediated inhibition of cell proliferation in human gastric cancer cell line SGC-7901 and to explore its effect on the expression of multidrug resistance genes.
METHODS: SGC-7901 cells were divided into four groups: untreated cells (control group), those treated with lentinan alone (lentinan group), those treated with cisplatin alone (cisplatin group), and those treated with both lentinan and cisplatin (lentinan + cisplatin group). RT-PCR was applied to detect the mRNA expression of MDR1, MRP1, and LRP in SGC-7901 cells. The proliferation of SGC-7901 cells was detected using the Cell Counting Kit-8.
RESULTS: High expression of multidrug resistance genes MDR1, MRP1 and LRP was detected in untreated SGC-7901 cells. Treatment with lentinan significantly decreased the mRNA expression of multidrug resistance genes but had no effect on cell proliferation (P > 0.05). Cisplatin treatment lessened cell proliferation and promoted the expression of multidrug resistance genes. Treatment with lentinan + cisplatin completely suppressed the mRNA expression of MDR1 and MRP1 and significantly decreased LRP expression and cell proliferation compared with the control group, lentinan group, and cisplatin group (10 d: 0.54 vs 1.90, 1.88, 0.92, all P < 0.05).
CONCLUSION: Lentinan combined with cisplatin can significantly inhibit the expression of multidrug resistance genes and strongly enhance cisplatin-mediated inhibition of the proliferation of SGC-7901 cells.
- Citation: Wu HY, Chen YM, Lin L, Lin YG, Qiu QA, Liu N. Lentinan enhances cisplatin-mediated inhibition of cell proliferation in human gastric cancer cell line SGC-7901. Shijie Huaren Xiaohua Zazhi 2011; 19(4): 344-348
- URL: https://www.wjgnet.com/1009-3079/full/v19/i4/344.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v19.i4.344
香菇多糖(Lentinan)为担子菌纲伞菌目伞菌科香菇菌属香菇菌提取的有效成分, 主要成分为甘露糖甘肽, 其余为多种糖分和各种氨基酸等[1,2]. 香菇多糖在治疗胃癌[3-5]、结肠癌[6]、肺癌[7]等肿瘤具有良好的疗效, 特别在胃癌和结直肠癌治疗中, 能显著延长患者存活时间. 目前对香菇多糖的抗肿瘤机制尚未清楚, 认为与其典型的T淋巴细胞激活作用相关, 体内外均能促进细胞毒T淋巴细胞(cytotoxic T lymphocyte, CTL)的产生, 提高CTL细胞杀伤力, 增强免疫功能并促进抗体依赖性细胞毒作用[8,9]. 国内研究表明香菇多糖能抑制肺癌细胞血管内皮生长因子(vascular endothelial growth factor, VEGF)表达, 减少肿瘤组织周围血管增生, 在体外能抑制肿瘤细胞增殖[10]. 研究表明肿瘤细胞通过表达多药耐药基因, 编码具有生物泵功能的蛋白质, 将进入肿瘤细胞的化疗药物不断地泵出细胞而产生耐药作用[11-14]. 本文研究香菇多糖和顺铂(Cisplatin)对SGC-7901胃癌细胞多药耐药基因表达的影响, 并探讨对胃癌细胞增殖的作用, 探索香菇多糖增强抗癌药物抗肿瘤作用的可能机制.
SGC-7901胃癌细胞购于美国标准生物品收藏中心(ATCC); RPMI 1640和胎牛血清购于Hyclone公司; CCK-8试剂盒购于碧云天公司; RNA提取试剂盒和RT-PCR试剂盒购于天根公司; 香菇多糖采用南京康海药业有限公司的香菇多糖冻干粉剂(天地欣); 顺铂购于辉瑞公司; 引物由上海生工公司合成.
1.2.1 细胞培养和药物处理: SGC-7901胃癌细胞完全培养基为100 mL/L胎牛血清、89% RPMI 1640和1%双抗, 培养在37 ℃、50 mL/L CO2恒温培养箱中, 实验时取对数生长期细胞. 香菇多糖冻干粉剂采用生理盐水配制成1 mmol/L浓度工作液, 顺铂用生理盐水配制成200 mg/L浓缩存储液, 使用时加入完全培养基配制如下浓度处理液: Len组(单独香菇多糖, 终浓度0.05 µmol/L), Cis组(单独顺铂, 终浓度2 mg/L), L+C组(香菇多糖和顺铂, 终浓度分别为0.05 µmol/L和2 mg/L). 取约60%汇合度的对数生长期胃癌细胞分别加入3组处理液(Con组仅加入完全培养基作为对照), 药物处理24 h, PBS清洗后更换新鲜培养基, 部分细胞继续培养做细胞增殖实验, 部分细胞提取RNA进行RT-PCR.
1.2.2 RT-PCR检测多药耐药基因表达: 按照RNA提取试剂盒说明提取SGC-7901胃癌细胞总RNA, 以GAPDH作为内参照进行RT-PCR扩增, 引物序列见表1. PCR反应参数: 94 ℃预变性5 min, 94 ℃变性1 min, 表1基因对应退火温度退火45 s, 72 ℃延伸45 s, 共35个循环, 最后一轮72 ℃延伸10 min, PCR产物经20 g/L琼脂糖凝胶电泳后检测.
| 基因 | 引物序列 | 退火温度(℃) | PCR产物长度(bp) |
| MDR1 | Sense: GTCATTGTGGAGAAAGGAAATCATG | 64 | 479 |
| Antisense: ATTCCAAGGGCTAGAAACAATAGTG | |||
| MRP1 | Sense: CTGTTTTGTTTTCGGGTTCC | 62 | 498 |
| Antisense: CCAAGGCCTTCCAAATCTC | |||
| LRP | Sense: GAGGATAAAGATGGAGACAA | 58 | 467 |
| Antisense: GAGAATCACGCAGTAGTTGTGG |
1.2.3 CCK-8检测SGC-7901胃癌细胞增殖: Len、Cis和L+C药物处理组及Con组的SGC-7901胃癌细胞以每孔1 000个细胞的密度接种于96孔板, 每孔加入10 µL CCK-8溶液, 以仅加入细胞培养液和CCK-8溶液但没有加入细胞的孔为空白对照, 每组均设置4个复孔. 在细胞培养箱内孵育1 h后, 450 nm条件下测定吸光度, 每天同一时间检测细胞吸光度(A值), 连续检测10 d. 每孔细胞绝对A值 = 每孔A值-空白对照孔A值, 每组细胞每天平均A值 = (主要孔绝对A值+4复孔绝对A值)/5, 所得数据以时间为横坐标、A值为纵坐标采用Excel 2010绘制细胞增殖曲线.
统计学处理 采用SPSS12.0进行数据处理与统计分析, 对SGC-7901胃癌细胞A值进行Friedman检验, 检验水准α = 0.05, P<0.05认为差异具有统计学意义.
Con组SGC-7901胃癌细胞可检测到MDR1、MRP1和LRP基因mRNA表达. 单独香菇多糖处理后, MDR1、MRP1和LRP基因mRNA表达显著降低; 单独顺铂作用能显著提高多药耐药基因MDR1、MRP1和LRP表达; 顺铂联合香菇多糖作用后, SGC-7901胃癌细胞MDR1、MRP1和LRP基因表达显著降低(前2者完全受到抑制, 后者显著降低), 具体结果见图1-3.
对照未处理组SGC-7901胃癌细胞表现为对数增殖状态, 单独香菇多糖对胃癌细胞增殖无明显影响(P>0.05); 顺铂能明显抑制胃癌细胞增殖, 细胞增殖速度较对照组降低, 差异具有统计学意义(P<0.05); 顺铂联合香菇多糖作用后, 胃癌细胞增殖速度显著降低, 细胞增殖曲线变平缓, 细胞增殖速度显著低于其他3组, 差异具有统计学意义(P<0.05, 表2, 图4).
| 分组 | 时间(d) | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Con组 | 0.050 | 0.120 | 0.190 | 0.280 | 0.400 | 0.520 | 0.630 | 0.810 | 1.200 | 1.900 |
| Len组 | 0.050 | 0.118 | 0.187 | 0.270 | 0.390 | 0.510 | 0.610 | 0.800 | 1.170 | 1.880 |
| Cis组 | 0.050 | 0.085 | 0.130 | 0.179 | 0.280 | 0.360 | 0.480 | 0.580 | 0.740 | 0.920ac |
| L+C组 | 0.050 | 0.054 | 0.080 | 0.110 | 0.140 | 0.210 | 0.280 | 0.360 | 0.450 | 0.540ac |
化疗是目前肿瘤治疗的主要方法之一, 临床上接受化疗的患者在首次或再次接受化疗时可出现耐药而导致疗效变差或完全无疗效现象[15], 目前认为与肿瘤细胞获得性耐药机制主要和多药耐药基因高表达有关[16-18]. 多药耐药相关基因主要包括MDR1、MRP1和LRP, 在肿瘤耐药机制中扮演重要角色. MDR1基因编码的产物P-糖蛋白是一种跨膜蛋白, 由1 281个氨基酸组成的2个完全相同的单体构成, 每个单体均有6个跨膜区和1个ATP结合位点, 跨膜区作为膜通道有利于药物转运, 而ATP结合位点与能量供应有关, 能将肿瘤药物逆浓度从细胞内泵出细胞外[12,19-20]. MRP1基因编码的多药耐药相关蛋白1属于跨膜糖蛋白, 具有ATP依赖泵作用, 能将带负电荷的药物分子逆浓度泵到细胞外, 减少细胞内药物浓度, 导致肿瘤耐药的发生, 还可通过改变细胞质及细胞器的pH值, 使药物到达作用部位的靶位点时浓度减少, 从而产生肿瘤耐药, 并直接参与肿瘤的转移和复发[21-26]. LRP广泛分布于正常组织, 阻止以胞核为效应点的药物转运到胞质中, 并将进入胞质的药物转运到运输囊泡中, 起隔绝药物作用, 并以胞吐的方式排出体外, 从而产生耐药现象[27,28].
顺铂作用于增殖细胞的DNA, 有类似烷化剂双功能基团的作用, 可以和细胞内的碱基结合, 使DNA分子链内和链间交叉键联, 失去功能而不能复制, 高浓度时也能抑制RNA及蛋白质的合成[29,30]. 本研究表明SGC-7901胃癌细胞表达多药耐药基因MDR1、MRP1和LRP, 单独顺铂作用后, 3种多药耐药基因表达均增加, 可能是SGC-7901胃癌细胞接触顺铂后, 受刺激而诱导性表达增加. 有研究表明, 体外顺铂梯度浓度刺激胃癌细胞可产生顺铂耐受的胃癌细胞系[31,32], 该现象可能与多药耐药基因诱导性高表达机制有关[33]. 香菇多糖对胃癌细胞增殖无明显影响, 但能显著降低多药耐药基因表达, 甚至达到完全抑制状态; 联合顺铂共同作用后, SGC-7901胃癌细胞多药耐药基因表达受到抑制, 呈低表达或无表达状态, 细胞增殖缓慢, 增殖曲线变平缓, 其机制可能是香菇多糖抑制多药耐药基因表达而导致顺铂抗肿瘤作用增强. 对于香菇多糖降低多药耐药基因表达的具体机制目前仍然不清楚, 有待于进一步实验研究. 本研究的体外实验表明香菇多糖能显著改善肿瘤细胞对化疗药物的耐药作用, 提高抗肿瘤疗效, 为临床上香菇多糖联合化疗药物治疗肿瘤提供了理论依据.
香菇多糖主要成分为甘露糖甘肽, 目前在肿瘤特别是胃癌和结直肠癌治疗中, 能显著提高疗效, 但其抗肿瘤机制尚未清楚. 多药耐药基因是肿瘤发生耐药的主要机制, 可能与香菇多糖抗肿瘤作用有关.
张俊, 副教授, 上海交通大学医学院附属瑞金医院外科; 王志刚, 副主任医师, 上海市第六人民医院普外科
采用香菇多糖和顺铂体外作用胃癌细胞, 探索其对胃癌细胞多药耐药基因表达和细胞增殖的影响, 揭示了香菇多糖通过抑制多药耐药基因表达而抗肿瘤的作用机制.
本实验揭示SGC-7901胃癌细胞表达多药耐药基因, 顺铂可诱导其表达增加. 香菇多糖能显著抑制多药耐药基因表达, 可能通过抑制多药耐药基因表达而增强顺铂的抗肿瘤作用.
本文设计合理, 新颖性较好, 对中药的肿瘤治疗作用提供了理论依据.
编辑: 李薇 电编:何基才
| 3. | Yoshino S, Watanabe S, Imano M, Suga T, Nakazawa S, Hazama S, Oka M. Improvement of QOL and prognosis by treatment of superfine dispersed lentinan in patients with advanced gastric cancer. Hepatogastroenterology. 2010;57:172-177. [PubMed] |
| 4. | Oba K, Kobayashi M, Matsui T, Kodera Y, Sakamoto J. Individual patient based meta-analysis of lentinan for unresectable/recurrent gastric cancer. Anticancer Res. 2009;29:2739-2745. [PubMed] |
| 6. | Ng ML, Yap AT. Inhibition of human colon carcinoma development by lentinan from shiitake mushrooms (Lentinus edodes). J Altern Complement Med. 2002;8:581-589. [PubMed] [DOI] |
| 7. | Matsusaki K, Hirose N, Yamada T, Morita M, Okamoto F, Kawano T, Miura O, Okazaki Y, Toda T, Minamisono Y. [Case of gastric cancer with recurrence of carcinomatous lymphangiosis of the lung 7.6 years after surgery and successfully treated with S-1/low-dose CDDP/Lentinan combination therapy]. Gan To Kagaku Ryoho. 2008;35:995-997. [PubMed] |
| 8. | Hamuro J. [Anticancer immunotherapy with perorally effective lentinan]. Gan To Kagaku Ryoho. 2005;32:1209-1215. [PubMed] |
| 11. | Liu FS. Mechanisms of chemotherapeutic drug resistance in cancer therapy--a quick review. Taiwan J Obstet Gynecol. 2009;48:239-244. [PubMed] [DOI] |
| 12. | Habibollahi P, Ghahremani MH, Azizi E, Ostad SN. Multi Drug Resistance-1 (MDR1) Expression in Response to Chronic Diazinon Exposure: An In vitro Study on Caco-2 Cells. Bull Environ Contam Toxicol. 2011;86:105-109. [PubMed] [DOI] |
| 13. | Li Y, Revalde JL, Reid G, Paxton JW. Modulatory effects of curcumin on multi-drug resistance-associated protein 5 in pancreatic cancer cells. Cancer Chemother Pharmacol. 2010; Nov 30. [Epub ahead of print]. [PubMed] |
| 14. | Hoffmann K, Franz C, Xiao Z, Mohr E, Serba S, Büchler MW, Schemmer P. Sorafenib modulates the gene expression of multi-drug resistance mediating ATP-binding cassette proteins in experimental hepatocellular carcinoma. Anticancer Res. 2010;30:4503-4508. [PubMed] |
| 15. | 邱 红, 丁 方勇, 熊 慧华, 张 明生, 李 瑞超, 陈 元. 胃癌耐药细胞株OCUM-2M/VP16的建立及其耐药机制. 世界华人消化杂志. 2009;17:1809-1814. [DOI] |
| 16. | Zhang WX, Chen B, Chen H, Cai Q, Cai WM. Co-regulation of mRNA level of UDP glucuronosyltransferase 1A9 and multi-drug resistance protein 2 in Chinese human liver. Clin Chim Acta. 2010;411:119-121. [PubMed] [DOI] |
| 17. | Tanaka M, Okazaki T, Suzuki H, Abbruzzese JL, Li D. Association of multi-drug resistance gene polymorphisms with pancreatic cancer outcome. Cancer. 2010; Oct 4. [Epub ahead of print]. [PubMed] |
| 18. | Smith MG, Jordan D, Chapman TA, Chin JJ, Barton MD, Do TN, Fahy VA, Fairbrother JM, Trott DJ. Antimicrobial resistance and virulence gene profiles in multi-drug resistant enterotoxigenic Escherichia coli isolated from pigs with post-weaning diarrhoea. Vet Microbiol. 2010;145:299-307. [PubMed] [DOI] |
| 19. | Kimura Y, Morita SY, Matsuo M, Ueda K. Mechanism of multidrug recognition by MDR1/ABCB1. Cancer Sci. 2007;98:1303-1310. [PubMed] [DOI] |
| 20. | Kugawa F, Suzuki T, Miyata M, Tomono K, Tamanoi F. Construction of a model cell line for the assay of MDR1 (multi drug resistance gene-1) substrates/inhibitors using HeLa cells. Pharmazie. 2009;64:296-300. [PubMed] |
| 21. | Cole SP, Deeley RG. Transport of glutathione and glutathione conjugates by MRP1. Trends Pharmacol Sci. 2006;27:438-446. [PubMed] [DOI] |
| 22. | Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204:216-237. [PubMed] [DOI] |
| 23. | Fischer S, Pietsch M, Schirmer K, Luckenbach T. Identification of multi-drug resistance associated proteins MRP1 (ABCC1) and MRP3 (ABCC3) from rainbow trout (Oncorhynchus mykiss). Mar Environ Res. 2010;69 Suppl:S7-S10. [PubMed] [DOI] |
| 24. | Colabufo NA, Berardi F, Perrone MG, Cantore M, Contino M, Inglese C, Niso M, Perrone R. Multi-drug-resistance-reverting agents: 2-aryloxazole and 2-arylthiazole derivatives as potent BCRP or MRP1 inhibitors. ChemMedChem. 2009;4:188-195. [PubMed] [DOI] |
| 25. | Matsunaga S, Asano T, Tsutsuda-Asano A, Fukunaga Y. Indomethacin overcomes doxorubicin resistance with inhibiting multi-drug resistance protein 1 (MRP1). Cancer Chemother Pharmacol. 2006;58:348-353. [PubMed] [DOI] |
| 26. | Friedrich RE, Punke C, Reymann A. Expression of multi-drug resistance genes (mdr1, mrp1, bcrp) in primary oral squamous cell carcinoma. In Vivo. 2004;18:133-147. [PubMed] |
| 27. | Langlois B, Emonard H, Martiny L, Dedieu S. [Multiple involvements of LRP-1 receptor in tumor progression]. Pathol Biol (Paris). 2009;57:548-554. [PubMed] [DOI] |
| 28. | Valera ET, Scrideli CA, Queiroz RG, Mori BM, Tone LG. Multiple drug resistance protein (MDR-1), multidrug resistance-related protein (MRP) and lung resistance protein (LRP) gene expression in childhood acute lymphoblastic leukemia. Sao Paulo Med J. 2004;122:166-171. [PubMed] [DOI] |
| 29. | Boulikas T. Clinical overview on Lipoplatin: a successful liposomal formulation of cisplatin. Expert Opin Investig Drugs. 2009;18:1197-1218. [PubMed] [DOI] |
| 30. | Tsang RY, Al-Fayea T, Au HJ. Cisplatin overdose: toxicities and management. Drug Saf. 2009;32:1109-1122. [PubMed] [DOI] |
| 32. | 张 敏, 李 勇莉, 高 建凯, 王 国栋, 高 福莲. 靶向mdr1不同位点的siRNA对两种耐药细胞MDR的逆转效果. 世界华人消化杂志. 2009;17:3387-3393. [DOI] |
| 33. | Leonhardt K, Gebhardt R, Mössner J, Lutsenko S, Huster D. Functional interactions of Cu-ATPase ATP7B with cisplatin and the role of ATP7B in the resistance of cells to the drug. J Biol Chem. 2009;284:7793-7802. [PubMed] [DOI] |