修回日期: 2011-05-20
接受日期: 2011-05-24
在线出版日期: 2011-06-18
目的: 探讨白介素-10(IL-10)对梗阻性黄疸大鼠肝细胞凋亡的作用.
方法: Wistar♂大鼠随机分为假手术(SO)组、梗阻性黄疸(OJ)组和IL-10组. OJ组和IL-10组结扎切断胆总管建立梗阻性黄疸模型, SO组仅游离胆总管. IL-10组术后第1天开始每天腹腔内注射IL-10(4 μg/kg). 实时荧光定量PCR法检测肝组织转化生长因子β1(TGF-β1) mRNA的水平, 免疫组织化学法检测肝组织TGF-β1蛋白表达情况. 末端脱氧核甘酸介导生物素标记(TUNEL)法检测大鼠肝细胞凋亡情况, 并检测血清TBIL、DBIL、ALT和AST水平.
结果: 胆总管结扎术后3 d, 与SO组相比, OJ组大鼠血清转氨酶、肝组织TGF-β1表达水平及肝细胞凋亡率均明显升高(ALT: 91.83 U/L±21.47 U/L vs 47.67 U/L±12.79 U/L; AST: 208.67 U/L±32.36 U/L vs 75.17 U/L±11.96 U/L; TGF-β1 mRNA: 7.48±1.51 vs 1.21±0.79; TGF-β1蛋白: 6.11%±1.11% vs 1.26%±0.64%; 凋亡: 15.06%±1.17% vs 3.94%±0.46%; 均P<0.05). 胆总管结扎术后7 d, 与SO组相比, OJ组大鼠上述各项实验指标均进一步升高(ALT: 178.83 U/L±46.25 U/L vs 44.50 U/L±9.97 U/L; AST: 461.17 U/L±88.48 U/L vs 76.50 U/L±12.39 U/L; TGF-β1 mRNA: 11.98±3.05 vs 1.01±0.52; TGF-β1蛋白: 9.97%±2.84% vs 1.68%±0.71%; 凋亡: 23.49%±3.35% vs 4.31%±0.67%; 均P<0.05). 应用IL-10治疗后, 与OJ组相比, IL-10组大鼠肝功能、肝组织TGF-β1表达水平及肝细胞凋亡率均明显降低(ALT: 94.17 U/L±20.02 U/L vs 178.83 U/L±46.25 U/L; AST: 257.83 U/L±56.53 U/L vs 461.17 U/L±88.48 U/L; TGF-β1 mRNA: 7.05±1.15 vs 11.98±3.05; TGF-β1 蛋白: 7.06%±1.32% vs 9.97%±2.84%; 凋亡: 15.08%±1.69% vs 23.49%±3.35%; 均P<0.05).
结论: IL-10可通过抑制肝组织TGF-β1的表达, 而减少梗阻性黄疸大鼠肝细胞凋亡.
引文著录: 曹阳, 赵闯, 徐锋, 戴朝六. 白介素-10对梗阻性黄疸大鼠肝组织转化生长因子β1表达和肝细胞凋亡的影响. 世界华人消化杂志 2011; 19(17): 1773-1779
Revised: May 20, 2011
Accepted: May 24, 2011
Published online: June 18, 2011
AIM: To explore the effect of interleukin-10 (IL-10) on hepatocyte apoptosis in biliary-obstructed rats.
METHODS: Male Wistar rats were divided randomly into sham operation (SO) group, obstructive jaundice (OJ) group and IL-10 group. Rats of the OJ and IL-10 groups underwent ligation and severing of the common bile duct, while mobilization of the common bile duct was performed in the SO group. The IL-10 group was intraperitoneally injected with IL-10 (4 μg/kg) daily after operation. The mRNA and protein expression of transforming growth factor-β1 (TGF-β1) in liver tissue was detected by fluorescence real-time quantitative PCR and immunohistochemical staining, respectively. Blood samples were taken to measure serum total bilirubin (TBIL), direct bilirubin (DBIL), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels, while hepatic cell apoptosis was evaluated by TUNEL method.
RESULTS: Compared to the SO group, the levels of serum ALT and AST, hepatic TGF-β1 mRNA and protein expression, and hepatic cell apoptosis index significantly increased in the OJ group 3 days after operation (ALT: 91.83 U/L ± 21.47 U/L vs 47.67 U/L ± 12.79 U/L; AST: 208.67 U/L ± 32.36 U/L vs 75.17 U/L ± 11.96 U/L; TGF-β1 mRNA: 7.48 ± 1.51 vs 1.21 ± 0.79; TGF-β1 protein: 6.11% ± 1.11% vs 1.26% ± 0.64%; apoptosis: 15.06% ± 1.17% vs 3.94% ± 0.46%; all P < 0.05), and further increased 7 d after operation (ALT: 178.83 U/L ± 46.25 U/L vs 44.50 U/L ± 9.97 U/L; AST: 461.17 U/L ± 88.48 U/L vs 76.50 U/L ± 12.39 U/L; TGF-β1 mRNA: 11.98 ± 3.05 vs 1.01 ± 0.52; TGF-β1 protein: 9.97% ± 2.84% vs 1.68% ± 0.71%; apoptosis: 23.49% ± 3.35% vs 4.31% ± 0.67%; all P < 0.05). Treatment with IL-10 significantly decreased hepatic function, hepatic TGF-β1 expression, and hepatic cell apoptosis compared to the OJ group 7 d after operation (ALT: 94.17 U/L ± 20.02 U/L vs 178.83 U/L ± 46.25 U/L; AST: 257.83 U/L ± 56.53 U/L vs 461.17 U/L ± 88.48 U/L; TGF-β1 mRNA: 7.05 ± 1.15 vs 11.98 ± 3.05; TGF-β1 protein: 7.06% ± 1.32% vs 9.97% ± 2.84%; apoptosis: 15.08% ± 1.69% vs 23.49% ± 3.35%; all P < 0.05).
CONCLUSION: IL-10 could attenuate hepatocyte apoptosis by suppressing hepatic TGF-β1 expression in biliary-obstructed rats.
- Citation: Cao Y, Zhao C, Xu F, Dai CL. Interleukin-10 suppresses hepatic TGF-β1 expression and attenuates hepatocyte apoptosis in biliary-obstructed rats. Shijie Huaren Xiaohua Zazhi 2011; 19(17): 1773-1779
- URL: https://www.wjgnet.com/1009-3079/full/v19/i17/1773.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v19.i17.1773
梗阻性黄疸是外科常见的临床问题, 此类患者往往并发不同程度的肝功能不全甚至出现肝功能衰竭[1-3]. 既往研究表明, 肝细胞凋亡在梗阻性黄疸肝脏损伤过程中起着重要作用, 是肝细胞死亡的主要方式之一, 而肝组织转化生长因子β1(transforming growth factor-β1, TGF-β1)对肝细胞凋亡有着明显的促进作用[4-8]. 最近研究发现, 白介素-10(interleukin-10, IL-10)可减少外源性内毒素所致肝损伤过程中的肝细胞凋亡[9]. 此外, 也有研究发现, 外源性IL-10可通过抑制肝组织TGF-β1的表达而改善肝纤维化[10]. 本实验旨在通过研究外源性IL-10对梗阻性黄疸大鼠肝组织TGF-β1表达、肝细胞凋亡率的影响, 探讨IL-10对梗阻性黄疸大鼠肝细胞凋亡的作用及其分子生物学机制.
Wistar♂大鼠, 体质量200-240 g, 中国医科大学附属盛京医院实验动物中心提供; 重组大鼠IL-10购自美国PeproTech公司; 末端脱氧核甘酸介导生物素标记(TUNEL)凋亡试剂盒购自德国Roche Diagnostics公司; 大鼠TGF-β1多克隆抗体购自美国Santa Cruz公司; 免疫组织化学SP染色试剂盒购自北京中杉金桥生物技术有限公司; 总RNA抽提试剂盒、逆转录试剂盒及PCR扩增试剂盒均购自TaKaRa公司; 内参β-actin及TGF-β1引物由北京奥科生物技术有限公司设计合成; 荧光定量仪: LightCycler定量PCR扩增仪及配套分析软件Roche LightCycler Run 5.32(德国Roche Diagnostics公司).
1.2.1 分组: Wistar♂大鼠30只随机分为假手术组(SO组)12只、梗阻性黄疸组(OJ组)12只和IL-10组6只. SO组和OJ组再随机分设术后3 d和术后7 d两个亚组, 每个亚组6只.
1.2.2 模型制备和取材: 参照Erguder等报道的方法建立梗阻性黄疸模型, 各组大鼠均用100 g/L水合氯醛(按3 μL/g)腹腔内注射麻醉, 于肝十二指肠韧带内辨认胆总管, 距离十二指肠2.0 cm处游离胆总管约0.5 cm, SO组手术到此为止, OJ组和IL-10组3/0丝线双重结扎并于结间切断胆总管[11,12]. IL-10组大鼠术后第1天开始每天腹腔内注射IL-10(4 μg/kg), 于术后7 d取材. SO组和OJ组大鼠术后对应时间点腹腔内注射同等剂量的生理盐水, 分别于术后3 d和7 d取材. 上述各组大鼠均腹主动脉采血, 离心取上层血清后置于-80 ℃冰箱保存待行肝功能检测; 快速夹取肝左叶部分组织置于-80 ℃冰箱保存待行肝组织TGF-β1 mRNA检测; 另取肝左叶部分组织置于40 g/L多聚甲醛中固定待行肝脏免疫组织化学及肝细胞凋亡检测.
1.2.3 肝功能指标检测: 使用全自动生化分析仪测定不同时点血清TBIL、DBIL、ALT和AST水平.
1.2.4 实时荧光定量PCR法检测肝组织TGF-β1 mRNA表达水平: (1)总RNA抽取: 按总RNA抽提试剂盒说明书操作步骤提取肝组织总RNA, 紫外分光光度计测定纯度并定量; (2)逆转录合成cDNA: 反应体系为20.0 μL: 5×PrimeScriptTM Buffer 4.0 μL, Random 6 mers 1.0 μL, PrimeScriptTM RT Enzyme Mix Ⅰ 1.0 μL, Oligo dT Primer 1.0 μL, 总RNA 1.0 μL, 加去RNA酶水至总体积为20.0 μL. 37 ℃水浴15 min后, 85 ℃水浴5 s灭活内转录酶. 产物置于-20 ℃冰箱内冻存备用; (3)PCR扩增: 内参基因β-actin: 上游引物5'-GGAGATTACTGCCCTGGCTCCTA-3', 下游引物5'-GACTCATCGTACTCCTGCCTGCTG-3', 扩增产物长度150 bp. 目的基因TGF-β1: 上游引物5'-AGGGCTTTCGCTTCAGTGCT-3', 下游引物5'-CCATGAGGAGCAGGAAGGGT-3', 扩增产物长度141 bp. 反应体系为20.0 μL: 上下游引物各0.4 μL, SYBR Premix Ex TaqTM 10.0 μL, dH2O 7.2 μL, cDNA模板2.0 μL. 反应条件: 95 ℃预变性10 s, 60 ℃退火20 s, 72 ℃延伸20 s, 共45个循环, 在退火阶段收集特异性荧光信号. 结果计算: ΔΔCt = (实验组目的基因Ct均值-实验组内参基因Ct均值)-(对照组目的基因Ct均值-对照组内参基因Ct均值), 然后取2-ΔΔCt即代表被测样品初始TGF-β1 mRNA的含量[13].
1.2.5 免疫组织化学法检测肝组织TGF-β1表达: 石蜡切片常规脱蜡至水, 3% H2O2溶液消除内源性过氧化物酶活性, 0.1 mol/L枸橼酸盐缓冲液微波修复抗原, 正常山羊血清封闭非特异性抗原后, 滴加兔抗大鼠TGF-β1多抗(1:100), 二抗, DAB显色, 苏木素复染封片. PBS代替一抗作对照, 检测肝组织TGF-β1表达情况. 每例切片随机计数5个400倍视野, 以平均每100个细胞中含阳性细胞个数作为其平均阳性率.
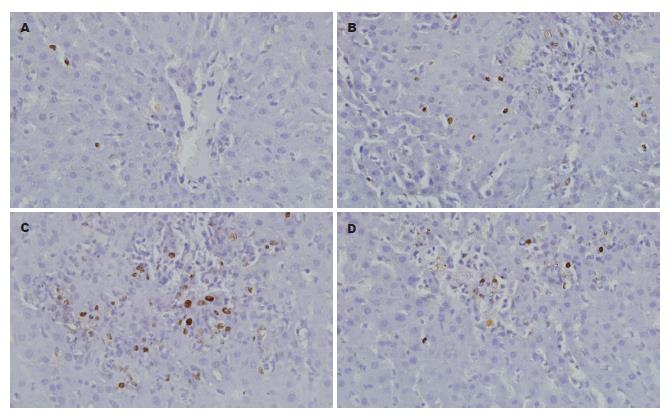
1.2.6 肝细胞凋亡检测: 采用TUNEL法检测大鼠肝细胞凋亡状态. 主要步骤: (1)石蜡切片常规脱蜡水化, 浓度为20 μg/mL蛋白酶K消化37 ℃×15 min; (2)加50 μL TUNEL反应混合液于标本上, 于暗湿盒中反应37 ℃×60 min; (3)加50 μL converter-POD于标本上, 于暗湿盒中反应37 ℃×30 min; (4)DAB显色, 苏木素复染. 每例切片随机计数5个400倍视野, 以平均每100个细胞中含凋亡细胞个数作为凋亡指数(apoptosis index, AI).
统计学处理 所有实验数据均以mean±SD表示, 组间比较采用SPSS17.0统计软件进行单因素方差分析(One-way ANOVA), P<0.05为差异有统计学意义.
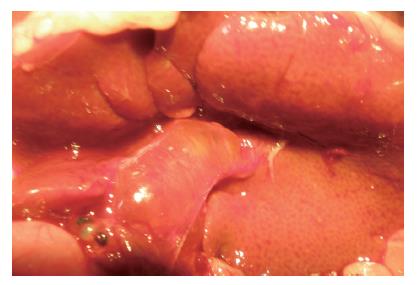
OJ组和IL-10组大鼠胆总管结扎术后1 d出现尿色加深, 术后2 d出现皮肤及巩膜黄染, 大便颜色变浅. 取材时可见肝脏呈淤胆状, 颜色由暗红转为棕黄色; 结扎处近肝脏侧胆总管明显增粗, 直径由正常的1 mm增至3-5 mm不等. SO组大鼠无上述梗阻性黄疸表现(图1).
SO组术后各时点血清中胆红素和转氨酶水平均较低. OJ组随着胆总管结扎时间的延长, 术后血清中胆红素和转氨酶水平逐渐升高且均高于SO组, 差异有统计学意义(P<0.05); IL-10组转氨酶水平明显低于OJ组, 差异有统计学意义(P<0.05), 但胆红素水平差异无统计学意义(P>0.05, 表1).
| 分组 | ALT(U/L) | AST(U/L) | TBIL(µmol/L) | DBIL(µmol/L) | ||||
| 3 d | 7 d | 3 d | 7 d | 3 d | 7 d | 3 d | 7 d | |
| SO组 | 47.67± 12.79 | 44.50± 9.97 | 75.17± 11.96 | 76.50± 12.39 | 5.36± 1.51 | 6.44± 1.93 | 3.69± 0.98 | 4.78± 1.42 |
| OJ组 | 91.83±21.47a | 178.83± 46.25a | 208.67± 32.36a | 461.17± 88.48a | 72.46±11.35a | 109.28± 22.68a | 61.16± 13.85a | 95.79± 19.00a |
| IL-10组 | 94.17± 20.02c | 257.83± 56.53c | 103.13± 13.77c | 88.10± 7.74c | ||||
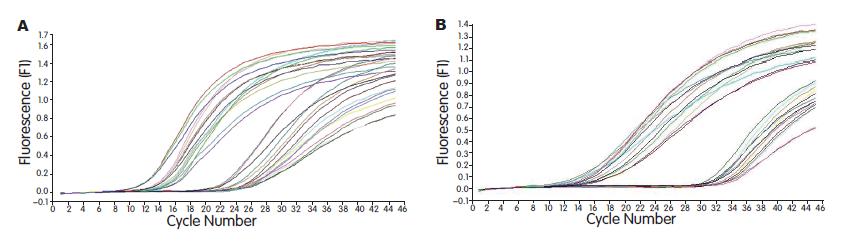
SO组术后各时点肝组织中TGF-β1 mRNA表达水平均较低. OJ组随着胆总管结扎时间的延长, 术后肝组织中TGF-β1 mRNA表达水平逐渐升高且均高于SO组, 差异有统计学意义(P<0.05); IL-10组肝组织中TGF-β1 mRNA水平明显低于OJ组, 差异有统计学意义(P<0.05, 表2, 图2).
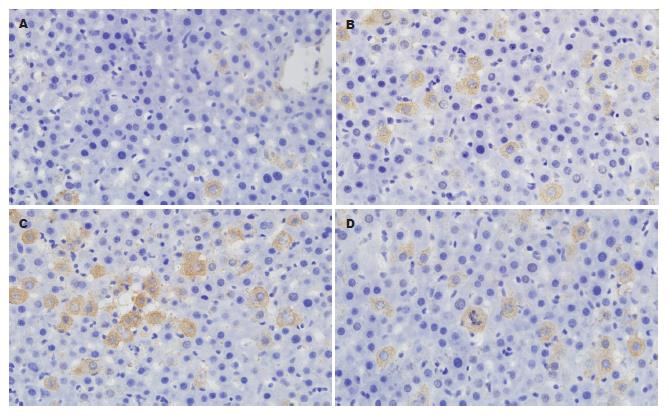
阳性染色呈棕黄色颗粒, 定位于细胞质. SO组术后各时点肝组织均可见少量的肝细胞呈阳性表达. OJ组随着胆总管结扎时间的延长, 术后肝组织中阳性细胞表达率逐渐升高且均高于SO组, 差异有统计学意义(P<0.05); IL-10组肝组织中阳性细胞表达率明显低于OJ组, 差异有统计学意义(P<0.05, 表2, 图3).
肝功能衰竭是梗阻性黄疸患者的重要死亡原因, 随着细胞凋亡测定方法的改进, 越来越多的证据表明, 肝细胞凋亡在梗阻性黄疸肝功能衰竭中起着重要的促进作用. 以肝细胞凋亡为靶点的治疗方向日益成为肝胆外科研究领域中的热点[14-18]. 本实验研究结果提示, 大鼠在胆总管结扎3 d后, 即出现血清中ALT和AST明显升高, 肝组织TGF-β1表达量和肝细胞凋亡率大量增加; 至术后第7天血清生化酶指标进一步升高, 肝组织TGF-β1表达量和肝细胞凋亡率同步增加. 由此可见, 梗阻性黄疸早期, 肝脏即出现大量的细胞凋亡, 肝功能进行性受损[7,19]. 因此, 及早即给予相应的治疗措施, 可望取得更理想的治疗效果.
IL-10是一种Th2细胞产生的免疫调节性细胞因子, 在多种实验性肝损伤模型中发现, IL-10可明显抑制多种炎症介质的表达而减轻肝损伤[9,10,20-22]. 最近研究发现, IL-10可通过抑制肝组织TGF-β1的表达而减少由内毒素引起的肝细胞凋亡[9]. Shi等[10]也发现外源性IL-10可通过抑制肝组织TGF-β1的表达而改善肝纤维化. 此外, 谷俊朝等[22]研究还发现外源性IL-10可以抑制大鼠急性出血坏死性胰腺炎肝细胞凋亡. 在本实验中, 我们研究发现梗阻性黄疸大鼠应用IL-10治疗后, 与OJ组相比, 肝组织TGF-β1的表达量明显下降, 肝细胞凋亡率与肝功能均显著改善. 上述结果表明, IL-10可以抑制梗阻性黄疸肝细胞凋亡进而减轻肝功能损害, 这可能与IL-10抑制肝组织TGF-β1的表达有关. 在实验过程中, 我们考虑到IL-10药物代谢半衰期较短[23,24]以及胆总管结扎术后早期腹腔内大量炎性液体渗出可能会影响IL-10的吸收情况, 因此, 在IL-10治疗组未设置术后3 d取材时间点.
正常肝脏中, TGF-β1主要由肝窦内皮细胞、库普弗细胞和肝星形细胞(hepatic stellate cell, HSC)表达[25]. 当发生梗阻性黄疸时, 肝内HSC大量增殖活化, 并通过自分泌及旁分泌方式致肝组织TGF-β1大量表达, 此时, 肝组织中TGF-β1主要由活化的HSC表达[26,27]. 翁山耕等[28,29]研究发现, 外源性IL-10可以抑制库普弗细胞诱导的HSC增殖活化. 此外, Shi等[30]研究表明, 外源性IL-10可以抑制体外培养活化的HSC表达TGF-β1. 但IL-10是否通过抑制HSC的增殖活化进而减少肝组织TGF-β1的表达, 尚需进一步证实. 同时, 由于细胞因子的调节极其复杂, 其他细胞因子是否也参与IL-10抗梗阻性黄疸肝细胞凋亡的作用, 也值得深入探讨.
总之, 本研究中我们观察到梗阻性黄疸引起肝组织TGF-β1大量表达致肝细胞凋亡增加使肝功能受损, 应用IL-10治疗后, 肝细胞凋亡和受损肝功能均得到显著的改善, 这可能与IL-10降低梗阻性黄疸肝组织TGF-β1的表达有关.
梗阻性黄疸是外科常见的临床问题, 此类患者往往并发不同程度的肝功能不全甚至出现肝功能衰竭. 随着细胞凋亡测定方法的改进, 越来越多的证据表明, 肝细胞凋亡在梗阻性黄疸肝功能衰竭中起着重要的促进作用.
崔云甫, 教授, 哈尔滨医科大学第二附属医院普外一科
梗阻性黄疸受损肝脏治疗中, 以肝细胞凋亡为靶点的治疗日益成为肝胆外科研究领域中的热点.
Zhong等研究表明, 白介素-10可通过抑制肝组织TGF-β1的表达而减少由外源性内毒素所致肝损伤过程中的肝细胞凋亡.
本实验通过建立梗阻性黄疸大鼠肝细胞凋亡模型, 阐述白介素-10可通过降低肝组织TGF-β1的表达, 抑制梗阻性黄疸肝细胞凋亡并改善受损肝功能.
本研究发现白介素-10可通过降低肝组织转化生长因子β1的表达抑制肝细胞凋亡并改善受损肝功能, 对以肝细胞凋亡为靶点治疗梗阻性黄疸受损肝脏具有一定的临床意义.
本文选题较好, 实验设计较合理, 对以肝细胞凋亡为靶点治疗的梗阻性黄疸有一定的临床意义.
编辑: 李薇 电编:何基才
| 1. | Lalisang TJ, Sjamsuhidajat R, Siregar NC, Taher A. Profile of hepatocyte apoptosis and bile lakes before and after bile duct decompression in severe obstructive jaundice patients. Hepatobiliary Pancreat Dis Int. 2010;9:520-523. [PubMed] |
| 2. | Crespo S, Raimondo M. The long term use of covered metal stents in managing malignant biliary obstruction. Are we changing outcomes? Dig Liver Dis. 2010;42:765-766. [PubMed] [DOI] |
| 3. | Gümüş M, Celebi F, Böyük A, Gürsan N, Akçay F. Dehydroepiandrosterone ameliorates hepatocellular damage in obstructive jaundice. Cell Biochem Funct. 2010;28:515-520. [PubMed] [DOI] |
| 4. | Dirlik M, Canbaz H, Düşmez Apa D, Cağlikülekçi M, Yaylak F, Balli E, Tamer L, Kanik A, Aydin S. The monitoring of progress in apoptosis of liver cells in bile duct-ligated rats. Turk J Gastroenterol. 2009;20:247-256. [PubMed] |
| 5. | Lee TY, Lee KC, Chang HH. Modulation of the cannabinoid receptors by andrographolide attenuates hepatic apoptosis following bile duct ligation in rats with fibrosis. Apoptosis. 2010;15:904-914. [PubMed] [DOI] |
| 6. | Wang DS, Dou KF, Li KZ, Gao ZQ, Song ZS, Liu ZC. Hepatocellular apoptosis after hepatectomy in obstructive jaundice in rats. World J Gastroenterol. 2003;9:2737-2741. [PubMed] |
| 8. | Fujiwara Y, Shimada M, Yamashita Y, Adachi E, Shirabe K, Takenaka K, Sugimachi K. Cytokine characteristics of jaundice in mouse liver. Cytokine. 2001;13:188-191. [PubMed] [DOI] |
| 9. | Zhong J, Deaciuc IV, Burikhanov R, de Villiers WJ. Lipopolysaccharide-induced liver apoptosis is increased in interleukin-10 knockout mice. Biochim Biophys Acta. 2006;1762:468-477. [PubMed] |
| 10. | Shi MN, Huang YH, Zheng WD, Zhang LJ, Chen ZX, Wang XZ. Relationship between transforming growth factor beta1 and anti-fibrotic effect of interleukin-10. World J Gastroenterol. 2006;12:2357-2362. [PubMed] |
| 11. | Erguder BI, Kilicoglu SS, Namuslu M, Kilicoglu B, Devrim E, Kismet K, Durak I. Honey prevents hepatic damage induced by obstruction of the common bile duct. World J Gastroenterol. 2008;14:3729-3732. [PubMed] [DOI] |
| 13. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [PubMed] |
| 14. | Huang CY, Sheen-Chen SM, Ho HT, Tang RP, Eng HL. Antithrombin-III attenuates hepatocyte apoptosis in bile duct ligated rat: a striking cellular change. Surg Innov. 2010;17:132-135. [PubMed] |
| 15. | Pi-Chieh Wang K, Lee LM, Lin TJ, Sheen-Chen SM, Lin JW, Chiu WT, Wang CC, Hung KS. Gene transfer of IGF1 attenuates hepatocellular apoptosis after bile duct ligation. J Surg Res. 2011;167:237-244. [PubMed] [DOI] |
| 16. | Margaritis VG, Filos KS, Michalaki MA, Scopa CD, Spiliopoulou I, Nikolopoulou VN, Vagianos CE. Effect of oral glutamine administration on bacterial tanslocation, endotoxemia, liver and ileal morphology, and apoptosis in rats with obstructive jaundice. World J Surg. 2005;29:1329-1334. [PubMed] [DOI] |
| 18. | Sheen-Chen SM, Ho HT, Chen WJ, Eng HL. Effect of ZVAD-fmk on hepatocyte apoptosis after bile duct ligation in rat. World J Gastroenterol. 2005;11:2330-2333. [PubMed] |
| 19. | Grasl-Kraupp B, Rossmanith W, Ruttkay-Nedecky B, Müllauer L, Kammerer B, Bursch W, Schulte-Hermann R. Levels of transforming growth factor beta and transforming growth factor beta receptors in rat liver during growth, regression by apoptosis and neoplasia. Hepatology. 1998;28:717-726. [PubMed] [DOI] |
| 20. | Li JQ, Qi HZ, He ZJ, Hu W, Si ZZ, Li YN, Li DB. Cytoprotective effects of human interleukin-10 gene transfer against necrosis and apoptosis induced by hepatic cold ischemia/reperfusion injury. J Surg Res. 2009;157:e71-e78. [PubMed] [DOI] |
| 21. | Dinant S, Veteläinen RL, Florquin S, van Vliet AK, van Gulik TM. IL-10 attenuates hepatic I/R injury and promotes hepatocyte proliferation. J Surg Res. 2007;141:176-182. [PubMed] [DOI] |
| 22. | 谷 俊朝, 王 宇, 张 忠涛, 薛 建国, 李 建设, 周 延忠. 外源性白介素-10对急性出血坏死性胰腺炎大鼠肝脏Bcl-2、Bax表达和肝细胞凋亡的影响. 中华肝胆外科杂志. 2004;10:260-262. |
| 23. | Wissing KM, Morelon E, Legendre C, De Pauw L, LeBeaut A, Grint P, Maniscalki M, Ickx B, Vereerstraeten P, Chatenoud L. A pilot trial of recombinant human interleukin-10 in kidney transplant recipients receiving OKT3 induction therapy. Transplantation. 1997;64:999-1006. [PubMed] [DOI] |
| 24. | Donckier V, Loi P, Closset J, Nagy N, Quertinmont E, Le Moine O, Devière J, Goldman M, Gelin M, Gianello P. Preconditioning of donors with interleukin-10 reduces hepatic ischemia-reperfusion injury after liver transplantation in pigs. Transplantation. 2003;75:902-904. [PubMed] [DOI] |
| 25. | Bissell DM, Wang SS, Jarnagin WR, Roll FJ. Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest. 1995;96:447-455. [PubMed] [DOI] |
| 26. | Makino H, Shimizu H, Ito H, Kimura F, Ambiru S, Togawa A, Ohtsuka M, Yoshidome H, Kato A, Yoshitomi H. Changes in growth factor and cytokine expression in biliary obstructed rat liver and their relationship with delayed liver regeneration after partial hepatectomy. World J Gastroenterol. 2006;12:2053-2059. [PubMed] |
| 27. | De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA, Schwabe RF. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007;132:1937-1946. [PubMed] [DOI] |
| 29. | Zhang X, Yu WP, Gao L, Wei KB, Ju JL, Xu JZ. Effects of lipopolysaccharides stimulated Kupffer cells on activation of rat hepatic stellate cells. World J Gastroenterol. 2004;10:610-613. [PubMed] |
| 30. | Shi MN, Zheng WD, Zhang LJ, Chen ZX, Wang XZ. Effect of IL-10 on the expression of HSC growth factors in hepatic fibrosis rat. World J Gastroenterol. 2005;11:4788-4793. [PubMed] |