修回日期: 2010-10-15
接受日期: 2010-10-18
在线出版日期: 2010-12-08
胃癌是常见恶性肿瘤, 也是导致肿瘤相关死亡的主要病种. Her2在Her/erbB家族的活化和信号转导中起重要作用, 在胃癌中的表达率为11.9%-23.7%, 与胃癌Lauren分型、肿瘤发生部位及较差的长期生存有关. 靶向Her2治疗方法包括单克隆抗体、小分子酪氨酸激酶抑制剂、siRNA等. ToGA研究是第一个基于Her2检测结果, 将Her2单克隆抗体曲妥珠单抗联合化疗一线用于晚期胃癌治疗的Ⅲ期临床研究, 并将晚期胃癌中位总生存期提升至史无前例的13.8 mo; 同时该研究也为胃癌分子靶向治疗带来诸多思考.
引文著录: 周尘飞, 张俊, 朱正纲. 靶向抑制Her2在胃癌治疗中的应用. 世界华人消化杂志 2010; 18(34): 3648-3655
Revised: October 15, 2010
Accepted: October 18, 2010
Published online: December 8, 2010
Gastric cancer is one of the most common malignancies and represents a major cause of cancer-related death in China. Her2 plays an important role in the activation of Her/ErbB family receptors and post-receptor signal transduction events. The rates of Her2 expression in gastric cancer range from 11.9% to 23.7%. Her2 expression correlates with Laruen's classification, location of primary tumor, and poor outcome in gastric cancer. Her2-targeted therapeutic strategies include the use of monoclonal antibodies and small molecule tyrosine kinase inhibitors. ToGA study is the first phase III randomized clinical trial to evaluate the efficacy and safety of trastuzumab, an anti-Her2 monoclonal antibody, in the first-line treatment of advanced and metastatic gastric cancer. Interestingly, trastuzumab could remarkably prolong the median overall survival of patients with gastric cancer to 13.8 mo. However, this trial also triggers much controversy over Her2-targeted therapies for gastric cancer.
- Citation: Zhou CF, Zhang J, Zhu ZG. Her2-targeted therapies for gastric cancer. Shijie Huaren Xiaohua Zazhi 2010; 18(34): 3648-3655
- URL: https://www.wjgnet.com/1009-3079/full/v18/i34/3648.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v18.i34.3648
胃癌发病率和死亡率均居全球恶性肿瘤前列, 总体5年生存率为10%-40%. 接受根治性切除的进展期胃癌患者, 中位总生存期约24 mo, 而姑息切除术后者仅为8.1 mo[1], 对于无法手术或发生转移的患者, 联合化疗所获得的中位总生存期仅为12 mo左右[2,3]. 晚近, 以分子靶向治疗为代表的新型治疗手段逐渐从实验室向临床应用转化. 就胃癌分子靶向治疗的靶点选择和药物开发而言, 目前研究较多者, 除抗肿瘤血管生成药物外, 还包括Her/erbB受体家族, 已有针对上述靶点的药物在Ⅱ、Ⅲ期临床研究阶段显示了良好疗效. 本文拟就Her2受体结构特点及在信号转导中的作用、胃癌Her2检测技术与评价标准、Her2分子靶向治疗药物应用于胃癌的证据和争论等热点问题综述如下.
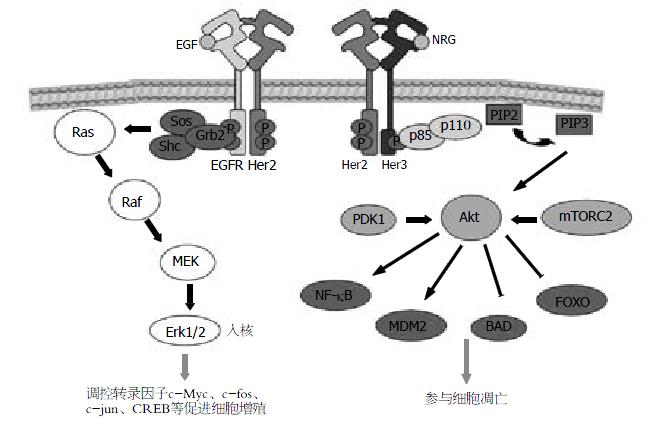
Her2/erbB2属Her/erbB家族, 其成员还包括EGFR/erbB1、Her3/erbB3及Her4/erbB4. Her2/erbB2编码基因位于染色体17q21.1, 与拓扑异构酶Ⅱα基因相邻, 表达相对分子质量185 000 Da的单链跨膜糖蛋白, 属Ⅰ型跨膜受体酪氨酸激酶, 胞外部分为糖基化N末端, 由4个细胞外结构域(extracellular domain, ECD)组成; 跨膜区为单一跨膜序列; 胞内部分则由酪氨酸激酶结构域及含有数个磷酸化位点的C末端共同构成[4-6].
Her/erbB家族ECD的4个ECDs, 依次命名为DomainⅠ、Ⅱ、Ⅲ和Ⅳ[7,8]. 依功能不同分为2组, DomainⅠ与DomainⅢ参与配体结合及二聚体控制. DomainⅡ与DomainⅣ参与受体二聚体形成, DomainⅡ上的指样突起通过锁定两受体间的二聚体环直接稳定二聚体. 在无配体结合的游离受体中, DomainⅣ与DomainⅡ的指样结构间存在分子间相互作用, 使受体处于类似"关"的自身抑制状态, 防止受体在无配体结合时的结构性二聚体化. 配体与受体的DomainⅠ/Ⅲ结合后, 使ECDs间的分子构象发生改变, 暴露出DomainⅡ的二聚体环, 继而介导二聚体形成.
与上述Her/erbB家族中的其他受体不同, Her2的ECDs空间位置类似配体结合后的活化状态, DomainⅠ/Ⅲ间的关系与结合配体的EGFR相似, 不存在DomainⅡ/Ⅳ间相互作用形成的自身抑制状态, 使DomainⅡ的指样结构暴露. DomainⅠ/Ⅲ间的接触面由疏水残基构成核心, 围绕以亲水基团, 结构较为稳定, 故目前尚未发现Her2的高亲和力配体. 通常Her2与其家族成员形成异二聚体发挥作用[9,10]. 在某些病理情况下, 过度表达的Her2可在配体缺失的情况下自身或与家族其他受体形成二聚体, 从而结构性激活下游信号. DomainⅣ中关键残基的不同(Her3的Gly562和His565, 在Her2被Pro和Phe取代)可能解释了Her2不存在DomainⅡ/Ⅳ相互作用的原因[7]. 由于Her2结构的特殊性, 使其在二聚体形成的过程中发挥重要作用.
受体二聚体化是Her/erbB家族活化的重要方式. 胞外信号诱导二聚体形成, 活化胞内段酪氨酸激酶, 使自身酪氨酸位点磷酸化, 并通过募集各种接头蛋白, 依次激活下游信号通路; Her/erbB家族信号通路参与了肿瘤细胞的增殖、分化、迁移、黏附、抗凋亡和细胞转化[11-13], 并可通过上调HIF-α诱导VEGF表达, 参与肿瘤血管生成[14].
Her2是Her/erbB受体家族中最主要的二聚体伙伴[9,15]. 配体诱导的Her/erbB受体二聚体化存在严格次序: 即在Her/erbB受体均存在的情况下, neu分化因子(neu differentiation factor, NDF)优先诱导Her3/4与Her2形成异二聚体. 仅在无Her2存在时, NDF活化的Her3/4才与EGFR形成异二聚体. 在EGF诱导的EGFR/Her3二聚体中, 充分活化Her3仍需Her2的存在[9]. 表明了Her2在配体依赖的二聚体化中的"中心"角色. Her2参与的信号转导中, 比较明确且重要的为Ras/MAPK通路[16]及PI3K/Akt通路[17](图1). 研究发现, 应用PI3K抑制剂阻断Her2阳性细胞Akt磷酸化, 可抑制肿瘤细胞生长, 细胞阻滞于G1期, 效果强于抑制MEK1/2[18]. 提示了Her2/Her3二聚体及其诱导的PI3K/Akt通路相对于Ras/MAPK通路, 在肿瘤发生和进展中起着更为重要的作用.
迄今, 对胃癌组织的Her2检测尚未像乳腺癌一样做到标准化, 评价标准亦未能达成共识. 胃癌中Her2的检测技术主要包括免疫组织化学染色(immunohistochemistry, IHC)、荧光原位杂交(fluorescence in situ hybridization, FISH)及显色原位杂交(chromogenic in situ hybridization, CISH). 早期的研究多沿袭了乳腺癌HercepTestTM-Her2的评分标准(表1), 所测胃癌标本中Her2过表达或扩增阳性率为11.9%-23.7%[20-27](表2), 其阳性表达与肠型胃癌和原发部位为胃食管连接部相关, 而与进展期胃癌、肿瘤大小、浆膜侵犯、Borrmann分型等无关.
| HercepTestTM-Her2评分标准 | 改良HercepTestTM-Her2评分标准[19] | ||
| 分值 | 标准 | 分值 | 标准 |
| 0 | 无染色或膜染色<10%肿瘤细胞 | 0 | 无染色或膜染色<10%肿瘤细胞 |
| 1+ | >10%肿瘤细胞, 膜部分弱染色 | 1+ | >10%肿瘤细胞, 膜弱染色或部分膜染色 |
| 2+ | >10%肿瘤细胞, 膜完全轻到中度染色 | 2+ | >10%肿瘤细胞, 膜完全或底侧面轻到中度染色 |
| 3+ | >10%肿瘤细胞, 膜完全高度染色 | 3+ | >10%肿瘤细胞, 膜完全或底侧面中到高度染色, 活检标本: IHC 3+或FISH + |
| 作者 | n | 技术 | Her2阳性率(%) | 胃癌分期 | 原发部位 | 浆膜侵犯 | Lauren分型 | 参考文献 |
| Yonemura等 | 260 | IHC细胞膜染色 | 11.90 | EGC 4.7% | - | 阳性16.9% | - | [20] |
| AGC 14.3% P<0.05 | 阴性7.4% P<0.05 | |||||||
| Nakajima等 | 128 | IHC细胞膜染色 | 16.40 | EGC 15% | - | 阳性21.7% | 肠型26.8% | [21] |
| AGC 16.70% | 阴性12.3% | 弥漫型3.5% P<0.001 | ||||||
| Gravalos等 | 166 | HercepTestTM | 13.00 | - | GC 9.5% | - | 肠型16.7% | [22] |
| FISH | GEJC 25% P = 0.01 | 弥漫型7% P = 0.276 | ||||||
| Ballestín等 | 181 | HercepTestTM | 13.50 | - | GC 10.90% | - | 肠型18.10% | [23] |
| FISH | GEJC 24.20% P = 0.084 | 弥漫型7% P = 0.186 | ||||||
| Tanner等 | 231 | CISH | 17.32 | - | GC 12% | - | 肠型21.50% | [24] |
| GEJC 24% | 弥漫型2% P = 0.005 | |||||||
| Park等 | 182 | HercepTestTM | 15.90(IHC) | EGC 3.33% | 阳性3.70% | 肠型7.69% | [25] | |
| FISH CISH | 3.85(FISH) | AGC 4.10% P>0.05 | 阴性3.96% P>0.05 | 弥漫型1.20% P = 0.05 | ||||
| León-Chong等 | 1 024 | HercepTestTM | 23.7(IHC) | - | GC 21% | - | 肠型36% | [26] |
| FISH | GEJC 36% | 弥漫型7% | ||||||
| Bang等 | 3 667 | 改良HercepTestTM | 22.1 | - | GC 20.9% | - | 肠型 32.0% | [27] |
| FISH | GEJC 33.2% P<0.001 | 弥漫型 6.1% P<0.001 |
Her2过度表达或扩增能否作为独立的预后判断因素仍存争议, 但较为一致的结果是, Her2过表达或扩增阳性胃癌患者的长期生存率差于Her2阴性患者. Park等[25]随访了182例胃癌术后患者, Her2过表达或扩增者5年生存率明显低于阴性者(34.5% vs 55.9%, P = 0.05; 21.4% vs 63.0%, P = 0.011).
Hofmann等[19]认为, 因胃癌组织中肿瘤细胞膜不完全染色(典型者为U型染色)较乳腺癌中更为常见, 加之胃癌异质性高于乳腺癌, 若完全照搬乳腺癌评分标准可能造成结果偏移, 故建议调整评分标准(表1). 晚近公布的大型Ⅲ期临床试验ToGA研究就采用了改良的HercepTestTM-Her2评分标准筛选受试者. HER2阳性率为22.1%, 与肠型胃癌和原发部位为胃食管连接部有关. 进一步的生存分析显示, 基于该评分标准的检测结果与临床预后的相关性良好, IHC 3+(无论FISH状态)或IHC 2+/FISH +者均可从曲妥珠单抗治疗中获益, 中位生存时间延长至16.0 mo(HR = 0.65, 95%CI: 0.51-0.83), 为Her2在胃癌中表达状态作为曲妥珠单抗疗效预测因素提供了依据[27]. 故该作者建议在胃癌Her2检测中应首先采用IHC方法并应用改良HercepTestTM-Her2评分标准, 仅在IHC 2+时方考虑联合FISH检测.
曲妥珠单抗(Trastuzumab; rhuMAb 4D5; HerceptinTM)是重组人源性IgG1单克隆抗体, 能高选择性、高亲和力地与人Her2胞外结构域DomainⅣ结合, 但结合后并不能完全阻止Her2与Her/erbB家族其他成员形成二聚体[28]. 其直接抗肿瘤效应包括: (1)阻断Her2/Her3非配体依赖的二聚体形成及结构性活化[29]; (2)阻断Her2同源二聚体解离; (3)阻断Her2胞外结构域解离[30]; (4)活化PTEN阻断Akt磷酸化. 曲妥珠单抗与Her2结合后, 可与表达Fc受体的免疫细胞结合产生抗体依赖的细胞毒效应(antibody-dependent cell-mediated cytotoxicity, ADCC)而间接发挥抗肿瘤作用[31].
在单用曲妥珠单抗时, Tanner等[24]发现, 曲妥珠单抗抑制高水平扩增Her2的胃癌细胞系N87和乳腺癌细胞系SKBR-3生长的效果相似. 曲妥珠单抗(5 mg/kg qw)能抑制接种N87细胞裸鼠皮下移植瘤生长. Matsui等[32]发现曲妥珠单抗能延长接种高表达Her2的MKN-45P胃癌细胞株并伴腹膜播散裸鼠的总生存时间.
联合化疗药物时, Naruse等[33]发现, 曲妥珠单抗(2.5 mg/L 72 h)与顺铂联合应用可使顺铂的IC50较顺铂单药组下降31%, 而在PBMC存在的情况下, 可进一步使顺铂的IC50下降超过70%. Kim等[34]也发现曲妥珠单抗联合顺铂可协同抑制SNU-216细胞系生长.
Gong等[35]分别应用浓度为10、50、100 mg/L的曲妥珠单抗连续5 d, 并在第1天加用阿霉素0.1 mg/L处理Her2表达强阳性(YCC-2)、中度阳性(NCI-N87)及弱阳性(YCC-3)胃癌细胞系, 在YCC-2及NCI-N87细胞中观察到明显生长抑制. Tokuda等[36]将Her2阳性胃癌细胞4-1ST的裸鼠移植瘤模型, 随机分为曲妥珠单抗(36 mg/kg)组, 氨柔比星(25 mg/kg)组和联合用药组, 联合用药组肿瘤明显缩小.
曲妥珠单抗与细胞毒药物协同抗肿瘤作用的原因可能为: (1)分别阻断细胞周期的不同时相, 曲妥珠单处理后的细胞株表现为G1/S阻滞. 顺铂和蒽环类药物均属于细胞周期非特异性抗肿瘤药物; 在阻断细胞周期上有互补效应; (2)在胃癌细胞系中Her2过表达或扩增, 多伴有拓扑异构酶Ⅱα共扩增[23], 蒽环类药物为拓扑异构酶Ⅱα抑制剂, 可与曲妥珠单抗发挥协同作用.
在两项Ⅱ期临床研究中[37,38], 曲妥珠单抗分别联合顺铂或多西他赛加顺铂一线治疗Her2阳性的转移或进展期胃癌, 起始剂量8 mg/kg, 维持剂量6 mg/kg, 每3 wk重复. 曲妥珠单抗联合顺铂组的17例可评估患者中, 1例完全缓解(complete response, CR)、5例部分缓解(partial response, PR)、3例疾病稳定(stable disease, SD), 总体反应率(response rate, RR)35%. 曲妥珠单抗联合顺铂与多西他赛组治疗了5例患者, 其中1例获CR、3例PR、1例SD. 均未观测到与曲妥珠单抗相关的不良反应, 初步验证了其安全性.
Bang等[27]报道了使用曲妥珠单抗一线治疗无法手术的局部晚期、复发和/或转移性Her2阳性胃癌患者的国际多中心随机对照Ⅲ期临床研究(ToGA研究)结果, 从3 803例晚期胃癌患者(包括胃食管交界处癌)中筛选入组594例Her2阳性者, 随机接受曲妥珠单抗(首剂8 mg/kg, 6 mg/kg维持, q3w)联合标准化疗方案(卡培他滨加顺铂或静脉注射5-FU加顺铂)或标准化疗; 共计6个周期, 曲妥珠单抗持续应用至疾病进展. 与单纯化疗组相比, 曲妥珠单抗联合标准化疗方案(卡培他滨或5-FU加顺铂)降低了Her2阳性晚期胃癌患者26%的死亡风险, 总生存期延长了2.7 mo(11.1 mo vs 13.8 mo, P = 0.0046; HR = 0.74; 95%CI: 0.60-0.91), 客观有效率从35%提高到47%(P = 0.0017). 初步证实了曲妥珠单抗联合顺铂+氟尿嘧啶类标准化疗方案可使Her2过表达的胃癌患者获益.
虽然ToGA所获的成绩令人振奋, 但又引发了诸多有待进一步研究的问题: (1)Her2过表达在胃癌患者中所占比例有限(22.1%), 即使是Her2过表达患者, 仍有超过50%患者未获治疗反应, 在细胞内信号转导过程中是否存在其他通路或通路之间的相互作用影响其疗效; (2)曲妥珠单抗治疗乳腺癌的经验中, 多数患者在一年左右出现耐药[39,40], 在胃癌治疗中长期应用是否也会产生耐药有待观察. 耐药机制中PI3K的活化突变, PIK3CA和PTEN低表达或缺失为主要研究方向, 应用PI3K抑制剂, 包括GDC-0941、Ly294002等可逆转PI3K突变和PTEN缺失所导致的对曲妥珠单抗的耐药[41]; (3)ToGA研究中对照组两药联合所获得的中位总生存期已接近REAL2研究中EOX三药联合所获的成绩(11.2 mo). 肠型胃癌相对预后较好, 而其Her2过表达发生率较高, 试验人群的选择是否对试验结果产生影响, 目前也没有明确的证据提示Her2过表达的肠型胃癌患者与Her2表达正常或阴性者或其他病理类型胃癌间的预后差别; (4)目前胃癌一线化疗尚无确定的标准方案, 因此, 曲妥珠单抗在联合其他药物时, 能否获得更好的疗效; 有待进一步研究.
帕妥珠单抗(Pertuzumab; rhuMAb 2C4; OmnitargTM)是重组人源性单抗, 结合于Her2胞外DomainⅡ[28], 直接阻断Her2与家族其他受体二聚体化, 从而抑制下游信号通路, 也可通过ADCC效应发挥抗肿瘤作用. 帕妥珠单抗抑制配体诱导的二聚体化而不依赖Her2表达水平[42], 是与曲妥珠单抗的主要不同点, 提示其可能受益的人群更广. 帕妥珠单抗在胃癌治疗中尚无相关临床证据, 但在其他实体肿瘤的探索性研究中已显示了一定疗效和较好的安全性[43-45]. 鉴于ToGA临床研究所取得的成绩, 帕妥珠单抗用于胃癌治疗值得进一步研究.
拉帕替尼(Lapatinib; GSK572016; TykerbTM)是口服小分子酪氨酸激酶抑制剂(Tyrosine kinase inhibitor, TKI), 可同时抑制EGFR和Her2的酪氨酸激酶结构域的ATP结合位点, 阻断酪氨酸磷酸化和下游信号通路, 导致细胞增殖减少和凋亡增加[46,47]. 同时, 拉帕替尼可在体内外减少p95Her2阳性表达细胞的生长和信号通路磷酸化, 后者虽无胞外结构域, 但具酪氨酸激酶活性, 有报道显示其参与了曲妥珠单抗耐药[48]. 目前拉帕替尼联合卡培他滨加奥沙利铂或卡培他滨加奥沙利铂治疗Her2阳性晚期胃、食管及胃食管连接部癌的大型双盲随机对照临床试验(LOGiC研究; NCT00680901)正在进行, 有望为靶向抑制Her2阳性胃癌的治疗提供新的临床证据.
吉非替尼(Gefitinib; ZD1839; IressaTM)是特异性阻断EGFR的TKI[49]. Hirata等[50]报道了过表达Her2非小细胞肺癌细胞系对吉非替尼的敏感性, 提出吉非替尼可能通过阻断Her2、Her3与受抑制的EGFR的二聚体结构解离, 减少Her2/Her3二聚体的形成而抑制肿瘤细胞生长. Yokoyama等[18]发现胃癌肝转移细胞系Her2过表达率高于胃原发病灶, 吉非替尼可抑制高度表达Her2、中度表达Her3、低表达EGFR的胃癌肝转移细胞系生长, 效果较曲妥珠单抗明显; 同时发现其能够抑制Her2过表达伴Her3表达细胞系中Akt的磷酸化并诱导凋亡, 提示该药物可能通过阻断Her2/Her3二聚体形成抑制PI3K/Akt通路.
RNA干扰(RNA interference)技术, 通过将shRNAs或siRNAs导入细胞内, 在dicer和Ago基因家族成员作用下, 形成RNA诱导沉默复合物(RNA-induced silencing complex, RISC), 起到降解mRNA、抑制翻译、沉默转录基因而减少相应蛋白表达的效应[51]. 体外将靶向Her2的siRNA转染到Her2扩增的胃癌细胞内, 可抑制细胞生长、促进凋亡[52]. 但因缺乏有效的体内稳定转染及表达机制, 其应用于人体尚有诸多问题有待解决.
此外, 各类新型TKI[53,54]、Her2疫苗[55,56]、Hsp90抑制剂[57]等均处于临床前试验阶段, 尽管某些药物已获一定结果(表3), 但尚无高证据级别的临床应用报告.
| 药物 | 研究类型 | 对象 | 用法 | Her2表达 | 效应 |
| Lapatinib | 体外[58] | SNU-216、NCI-N87 | 单药 | 高表达 | IC50 0.02 μm、0.01 μm |
| Phase II[59] | 46例晚期胃癌患者 | 1500 mg qd 口服 | NK | 3例 PR, 9例SD; 中位TTF 2 mo, 中位 OS 5 mo | |
| Gefitinib[18] | 体内 | 接种GLM-1、GLM-4裸鼠 | 150 mg/kg, 5次/wk 灌胃 | NK | 肿瘤体积减小, P<0.001、P<0.01 |
| 体内 | 接种GLM-1裸鼠 | 150 mg/kg, 5次/wk 灌胃 | NK | 肿瘤体积减小P<0.01; | |
| 联合曲妥珠单抗20 mg/kg, 2次/wk 腹腔 注射, 共6 wk | NK | 联合曲妥珠单抗 P<0.001 vs单药组 | |||
| siRNA | 体外[52] | OE-19 、MKN45 | Her2 siRNA no.1 | 扩增 | 细胞死亡35%、38%, 凋亡16%、24%, P<0.001 |
| Her2 siRNA no.2 | NK | 细胞死亡46%、38%, 凋亡18%、26%, P<0.001 | |||
| Her2疫苗 | Phase I[55] | 9例晚期胃癌患者 | 提呈Her2(p369)的DC, 1次/wk, 免疫4次 | 过表达 | 1例PR伴TM下降, 1例SD 2例TM下降 |
Her2对维持心肌细胞正常发育及功能起重要作用. 携带Her2无效等位基因的小鼠存在明显的心脏发育障碍, 并在胚胎期第11天即死亡[60]. G蛋白偶联受体激动剂对心肌细胞的激动作用需要Her2的参与[61]. 因此, 应用分子靶向药物阻断Her2信号通路后对心脏功能的影响值得关注.
各临床结果显示, 无论是单克隆抗体或小分子酪氨酸激酶抑制剂, 左心室射血分数下降的发生率在1.6%-5%左右[27,46,62,63], 且多为无症状性, 治疗停止后6 mo内多数患者可以恢复[64], 显示了较好的安全性. 但仍有少部分患者出现了Ⅲ-Ⅳ级(NYHA)的慢性心衰, 同时回顾性研究显示, 曾使用过较高累积剂量蒽环类药物的乳腺癌患者, 接受曲妥珠单药治疗后, 心脏毒性的发生率相对较高[64]. 联合蒽环类药物可增加药物对大鼠心室肌细胞的毒性[65]. 因此, 在应用抗Her2靶向治疗时, 详细的病史询问、体格检查及密切随访心电图、心彩超并即时调整剂量, 以避免严重心脏毒性的出现.
Her2在受体活化和肿瘤细胞信号转导中发挥重要作用, 参与包括胃癌在内的多种肿瘤的发生发展. Her2过表达和扩增与胃癌的Lauren分型(肠型胃癌)和肿瘤原发部位关系密切, 是胃癌患者预后不佳的判别因素之一. 应用改良的HercepTestTM-Her2评分标准检测胃癌组织中Her2表达, 有助于更好地筛选曲妥珠单抗的潜在获益人群. ToGA临床研究所取得的成绩无疑为靶向抑制Her2应用于胃癌治疗提供了有力证据, 但也带来更多有待于明确的问题. 胃癌的发生发展是一个多因素、多阶段的复杂过程, 单一靶向Her2在胃癌治疗中价值仍属有限. 鉴于此, 通过抑制Sp-1[68]在内的细胞核内转录因子, 在DNA或RNA水平阻断Her2表达或Her/erbB家族信号转导通路中的关键激酶, 可望能成为该领域新的研究方向.
胃癌是我国常见的恶性肿瘤, 晚期无法手术, 患者预后不良. ToGA临床研究应用曲妥珠单抗联合化疗治疗进展期无法手术或转移性胃癌, 使患者的生存期得到明显延长, 为分子靶向治疗应用于胃癌提供了重要的临床信息.
杜雅菊, 主任医师, 哈尔滨医科大学附属第二医院消化内科
通过抑制Sp-1在内的细胞核内转录因子, 在DNA或RNA水平阻断Her2表达或Her/erbB家族信号转导通路中的关键激酶有望能成为该领域新的研究方向.
Cho等的研究阐明了Her2受体的结构及其与曲妥珠单抗的结合部位. Bang等报道的ToGA临床研究结果显示Her2阳性胃癌患者可从联合曲妥珠单抗中获益, 中位总生存期较单用化疗组延长2.7 mo, 客观反应率提高至47%.
本文综述了Her2受体结构、功能、在胃癌中的检测及靶向Her2治疗的应用, 对指导进一步的研究和临床应用提供参考信息.
本文可读性较好, 为靶向抑制治疗胃癌临床应用提供参考资料.
编辑: 李军亮 电编:何基才
| 1. | Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005;241:27-39. [PubMed] |
| 2. | Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215-221. [PubMed] [DOI] |
| 3. | Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36-46. [PubMed] [DOI] |
| 4. | Roskoski R Jr. The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem Biophys Res Commun. 2004;319:1-11. [PubMed] [DOI] |
| 5. | Earp HS 3rd, Calvo BF, Sartor CI. The EGF receptor family--multiple roles in proliferation, differentiation, and neoplasia with an emphasis on HER4. Trans Am Clin Climatol Assoc. 2003;114:315-333; discussion 333-334. [PubMed] |
| 6. | Roepstorff K, Grøvdal L, Grandal M, Lerdrup M, van Deurs B. Endocytic downregulation of ErbB receptors: mechanisms and relevance in cancer. Histochem Cell Biol. 2008;129:563-578. [PubMed] [DOI] |
| 7. | Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW Jr, Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756-760. [PubMed] [DOI] |
| 8. | Landgraf R. HER2 therapy. HER2 (ERBB2): functional diversity from structurally conserved building blocks. Breast CancerRes. 2007;9:202. [PubMed] [DOI] |
| 9. | Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647-1655. [PubMed] [DOI] |
| 10. | Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100:8933-8938. [PubMed] [DOI] |
| 11. | Meric-Bernstam F, Hung MC. Advances in targeting human epidermal growth factor receptor-2 signaling for cancer therapy. Clin Cancer Res. 2006;12:6326-6330. [PubMed] [DOI] |
| 12. | Holbro T, Civenni G, Hynes NE. The ErbB receptors and their role in cancer progression. Exp Cell Res. 2003;284:99-110. [PubMed] [DOI] |
| 13. | Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120-6130. [PubMed] [DOI] |
| 14. | Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995-4004. [PubMed] [DOI] |
| 15. | Zhang Y, Opresko L, Shankaran H, Chrisler WB, Wiley HS, Resat H. HER/ErbB receptor interactions and signaling patterns in human mammary epithelial cells. BMC Cell Biol. 2009;10:78. [PubMed] [DOI] |
| 16. | McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263-1284. [PubMed] |
| 17. | Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127-150. [PubMed] [DOI] |
| 18. | Yokoyama H, Ikehara Y, Kodera Y, Ikehara S, Yatabe Y, Mochizuki Y, Koike M, Fujiwara M, Nakao A, Tatematsu M. Molecular basis for sensitivity and acquired resistance to gefitinib in HER2-overexpressing human gastric cancer cell lines derived from liver metastasis. Br J Cancer. 2006;95:1504-1513. [PubMed] [DOI] |
| 19. | Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797-805. [PubMed] [DOI] |
| 20. | Yonemura Y, Ninomiya I, Yamaguchi A, Fushida S, Kimura H, Ohoyama S, Miyazaki I, Endou Y, Tanaka M, Sasaki T. Evaluation of immunoreactivity for erbB-2 protein as a marker of poor short term prognosis in gastric cancer. Cancer Res. 1991;51:1034-1038. [PubMed] |
| 21. | Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, Matsuda M, Sakaguchi T, Hirao T, Nakano H. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85:1894-1902. [PubMed] |
| 22. | Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273-278. [PubMed] [DOI] |
| 23. | Park DI, Yun JW, Park JH, Oh SJ, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Yoo CH. HER-2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci. 2006;51:1371-1379. [PubMed] [DOI] |
| 24. | León-Chong J, Lordick F, Kang YK, Park SR, Bang YJ, Sawaki A, Van Cutsem E, Stoss O, Jordan BW, Feyereislova A. HER2 positivity in advanced gastric cancer is comparable to breast cancer. 2007;Abstract 15057. |
| 25. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [PubMed] [DOI] |
| 26. | Badache A, Hynes NE. A new therapeutic antibody masks ErbB2 to its partners. Cancer Cell. 2004;5:299-301. [PubMed] [DOI] |
| 27. | Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429-440. [PubMed] [DOI] |
| 28. | Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39-51. [PubMed] [DOI] |
| 29. | Varchetta S, Gibelli N, Oliviero B, Nardini E, Gennari R, Gatti G, Silva LS, Villani L, Tagliabue E, Ménard S. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res. 2007;67:11991-11999. [PubMed] [DOI] |
| 30. | Matsui Y, Inomata M, Tojigamori M, Sonoda K, Shiraishi N, Kitano S. Suppression of tumor growth in human gastric cancer with HER2 overexpression by an anti-HER2 antibody in a murine model. Int J Oncol. 2005;27:681-685. [PubMed] |
| 31. | Naruse I, Fukumoto H, Saijo N, Nishio K. Enhanced anti-tumor effect of trastuzumab in combination with cisplatin. Jpn J Cancer Res. 2002;93:574-581. [PubMed] |
| 32. | Kim SY, Kim HP, Kim YJ, Oh do Y, Im SA, Lee D, Jong HS, Kim TY, Bang YJ. Trastuzumab inhibits the growth of human gastric cancer cell lines with HER2 amplification synergistically with cisplatin. Int J Oncol. 2008;32:89-95. [PubMed] |
| 33. | Gong SJ, Jin CJ, Rha SY, Chung HC. Growth inhibitory effects of trastuzumab and chemotherapeutic drugs in gastric cancer cell lines. Cancer Lett. 2004;214:215-224. [PubMed] [DOI] |
| 34. | Tokuda Y, Umemura S, Saito Y, Suzuki Y. Enhanced anti-tumor effect of trastuzumab combined with less cardiotoxic anthracycline, amrubicin, in a Her2-positive human cancer xenograft in athymic mice. J Clin Oncol (ASCO Annual Meeting). 2008;26:14641. |
| 35. | Cortés-Funes H, Rivera F, Alés I, Márquez A, Velasco A, Colomer R, García-Carbonero R, Sastre J, Guerra J, Grávalos C. Phase II of trastuzumab and cisplatin in patients with advanced gastric cancer with HER2/neu overexpression/amplification. J Clin Oncol (ASCO Annual Meeting). 2007;25:4613. |
| 36. | Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523-1529. [PubMed] [DOI] |
| 37. | Nahta R, Esteva FJ. HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res. 2006;8:215. [PubMed] [DOI] |
| 38. | Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18:977-984. [PubMed] [DOI] |
| 39. | Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117-127. [PubMed] [DOI] |
| 40. | Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317-328. [PubMed] [DOI] |
| 41. | Yamamoto N, Yamada Y, Fujiwara Y, Yamada K, Fujisaka Y, Shimizu T, Tamura T. Phase I and pharmacokinetic study of HER2-targeted rhuMAb 2C4 (Pertuzumab, RO4368451) in Japanese patients with solid tumors. Jpn J Clin Oncol. 2009;39:260-266. [PubMed] [DOI] |
| 42. | Albanell J, Montagut C, Jones ET, Pronk L, Mellado B, Beech J, Gascon P, Zugmaier G, Brewster M, Saunders MP. A phase I study of the safety and pharmacokinetics of the combination of pertuzumab (rhuMab 2C4) and capecitabine in patients with advanced solid tumors. Clin Cancer Res. 2008;14:2726-2731. [PubMed] [DOI] |
| 43. | Baselga J, Gelmon KA, Verma S, Wardley A, Conte P, Miles D, Bianchi G, Cortes J, McNally VA, Ross GA. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138-1144. [PubMed] [DOI] |
| 44. | Medina PJ, Goodin S. Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases. Clin Ther. 2008;30:1426-1447. [PubMed] [DOI] |
| 45. | Vogel C, Chan A, Gril B, Kim SB, Kurebayashi J, Liu L, Lu YS, Moon H. Management of ErbB2-positive breast cancer: insights from preclinical and clinical studies with lapatinib. Jpn J Clin Oncol. 2010;40:999-1013. [PubMed] [DOI] |
| 46. | Scaltriti M, Rojo F, Ocaña A, Anido J, Guzman M, Cortes J, Di Cosimo S, Matias-Guiu X, Ramon y Cajal S, Arribas J. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628-638. [PubMed] [DOI] |
| 47. | Cohen MH, Williams GA, Sridhara R, Chen G, McGuinn WD Jr, Morse D, Abraham S, Rahman A, Liang C, Lostritto R, Baird A, Pazdur R. United States Food and Drug Administration Drug Approval summary: Gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 2004;10:1212-1218. [PubMed] [DOI] |
| 48. | Hirata A, Hosoi F, Miyagawa M, Ueda S, Naito S, Fujii T, Kuwano M, Ono M. HER2 overexpression increases sensitivity to gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, through inhibition of HER2/HER3 heterodimer formation in lung cancer cells. Cancer Res. 2005;65:4253-4260. [PubMed] [DOI] |
| 49. | Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494-498. [PubMed] [DOI] |
| 50. | Arrington AK, Dahlberg PS, Davydova J, Vickers SM, Yamamoto M. ERBB2 suppression decreases cell growth via apoptosis in gastrointestinal adenocarcinomas. Surgery. 2009;146:213-219. [PubMed] [DOI] |
| 51. | Traxler P, Allegrini PR, Brandt R, Brueggen J, Cozens R, Fabbro D, Grosios K, Lane HA, McSheehy P, Mestan J. AEE788: a dual family epidermal growth factor receptor/ErbB2 and vascular endothelial growth factor receptor tyrosine kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2004;64:4931-4941. [PubMed] [DOI] |
| 52. | Jani JP, Finn RS, Campbell M, Coleman KG, Connell RD, Currier N, Emerson EO, Floyd E, Harriman S, Kath JC. Discovery and pharmacologic characterization of CP-724,714, a selective ErbB2 tyrosine kinase inhibitor. Cancer Res. 2007;67:9887-9893. [PubMed] [DOI] |
| 53. | Kono K, Takahashi A, Sugai H, Fujii H, Choudhury AR, Kiessling R, Matsumoto Y. Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T-cell responses in patients with gastric cancer. Clin Cancer Res. 2002;8:3394-3400. [PubMed] |
| 54. | Foy TM, Fanger GR, Hand S, Gerard C, Bruck C, Cheever MA. Designing HER2 vaccines. Semin Oncol. 2002;29:53-61. [PubMed] |
| 55. | Chandarlapaty S, Scaltriti M, Angelini P, Ye Q, Guzman M, Hudis CA, Norton L, Solit DB, Arribas J, Baselga J. Inhibitors of HSP90 block p95-HER2 signaling in Trastuzumab-resistant tumors and suppress their growth. Oncogene. 2010;29:325-334. [PubMed] [DOI] |
| 56. | Kim JW, Kim HP, Im SA, Kang S, Hur HS, Yoon YK, Oh DY, Kim JH, Lee DS, Kim TY. The growth inhibitory effect of lapatinib, a dual inhibitor of EGFR and HER2 tyrosine kinase, in gastric cancer cell lines. Cancer Lett. 2008;272:296-306. [PubMed] [DOI] |
| 57. | Iqbal S, Goldman B, Lenz HJ, Fenoglio-Preiser CM, Blanke CD. S0413: A phase II SWOG study of GW572016 (lapatinib) as first line therapy in patients (pts) with advanced or metastatic gastric cancer. J Clin Oncol (ASCO Annual Meeting). 2007;25:4621. |
| 58. | Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394-398. [PubMed] [DOI] |
| 59. | Negro A, Brar BK, Gu Y, Peterson KL, Vale W, Lee KF. erbB2 is required for G protein-coupled receptor signaling in the heart. Proc Natl Acad Sci U S A. 2006;103:15889-15893. [PubMed] [DOI] |
| 60. | Fleming GF, Meropol NJ, Rosner GL, Hollis DR, Carson WE 3rd, Caligiuri M, Mortimer J, Tkaczuk K, Parihar R, Schilsky RL, Ratain MJ. A phase I trial of escalating doses of trastuzumab combined with daily subcutaneous interleukin 2: report of cancer and leukemia group B 9661. Clin Cancer Res. 2002;8:3718-3727. [PubMed] |
| 61. | Viani GA, Afonso SL, Stefano EJ, De Fendi LI, Soares FV. Adjuvant trastuzumab in the treatment of her-2-positive early breast cancer: a meta-analysis of published randomized trials. BMC Cancer. 2007;7:153. [PubMed] [DOI] |
| 62. | Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, Perren T, Passalacqua R, Bighin C, Klijn JG. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. 2007;25:3859-3865. [PubMed] [DOI] |
| 63. | Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM, Suter TM. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105:1551-1554. [PubMed] [DOI] |
| 64. | Al-Dasooqi N, Bowen JM, Gibson RJ, Sullivan T, Lees J, Keefe DM. Trastuzumab induces gastrointestinal side effects in HER2-overexpressing breast cancer patients. Invest New Drugs. 2009;27:173-178. [PubMed] [DOI] |
| 65. | Crown JP, Burris HA 3rd, Boyle F, Jones S, Koehler M, Newstat BO, Parikh R, Oliva C, Preston A, Byrne J, Chan S. Pooled analysis of diarrhea events in patients with cancer treated with lapatinib. Breast Cancer Res Treat. 2008;112:317-325. [PubMed] [DOI] |
| 66. | Zhang J, Ji J, Yuan F, Chen J, Yan M, Yu YY, Liu BY, Yin HR, Lin YZ, Zhu ZG. [Transcription factor Sp1 expression in gastric cancer and its prognostic value]. Zhonghua Zhongliu Zazhi. 2005;27:531-533. [PubMed] |