修回日期: 2009-12-14
接受日期: 2009-12-21
在线出版日期: 2010-01-28
目的: 探讨在加入TGF-β1的条件下, Smad7过度表达对肝星形细胞-T6(HSC-T6)α1(Ⅲ)前胶原mRNA水平的作用.
方法: 体外培养HSC-T6细胞, 分为正常对照组、TGF-β1对照组、空载质粒组、Smad7质粒组、TGF-β1+空载质粒组和TGF-β1+Smad7质粒组. 通过Fugene6介导Smad7质粒转染, 继续培养48 h. 采用Real time-PCR、RT-PCR方法检测Smad7和α1(Ⅲ)前胶原mRNA水平.
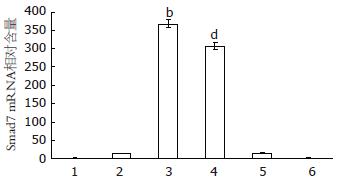
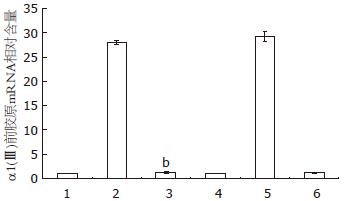
结果: TGF-β1+Smad7质粒组、Smad7质粒组Smad7 mRNA水平较正常对照组、空载质粒组显著增高(t = 59.43、59.41、54.27及54.25, 均t>t0.01, 均P<0.01). Smad7质粒组α1(Ⅲ)前胶原基因mRNA表达与正常对照组、空载质粒组相比无明显差异(t = 1.25与1.27, 均t<t0.05, 均P>0.05). 但TGF-β1+Smad7质粒组α1(Ⅲ)前胶原mRNA水平较TGF-β1+空载质粒组和TGF-β1对照组明显降低(t = 103.87与45.70, 均t>t0.01, 均P<0.01).
结论: Smad7可显著抑制TGF-β1对HSC-T6α1(Ⅲ)前胶原mRNA表达的促进作用,而对HSC-T6α1(Ⅲ)前胶原mRNA表达无明显影响.
引文著录: 徐林芳, 刘海林. Smad7抑制TGF-β1对肝星状细胞α1(Ⅲ)前胶原基因表达的促进作用. 世界华人消化杂志 2010; 18(3): 280-283
Revised: December 14, 2009
Accepted: December 21, 2009
Published online: January 28, 2010
AIM: To investigate the effects of Smad7 overexpression on transforming growth factor-β1 (TGF-β1)-stimulated procollagen α1 (III) expression in cultured hepatic stellate cells (HSC-T6).
METHODS: Cultured HSC-T6 cells were divided into six groups: normal control group, TGF-β1 group, vehicle plasmid group, Smad7 plasmid group, TGF-β1 plus vehicle plasmid group, and TGF-β1 plus Smad7 plasmid group. Smad7 plasmid and vehicle plasmid were transfected into HSC-T6 cells with FuGENE 6 Reagent. Forty-eight hours after transfection, the expression level of procollagen α1 (III) mRNA was determined by reverse transcription-polymerase chain reaction (RT-PCR) and real-time RT-PCR.
RESULTS: Compared with the normal control group and the vehicle plasmid group, the expression levels of Smad7 mRNA increased significantly in the Smad7 plasmid group and the TGF-β1 plus Smad7 plasmid group (t = 59.43, 59.41, 54.27 and 54.25, respectively; all t > t0.01 and all P < 0.01). Although there were no significant differences in the expression levels of procollagen α1 (III) mRNA between the Smad7 plasmid group and the normal control group or the vehicle plasmid group (t = 1.25 and 1.27, respectively; both t < t0.05 and both P > 0.05), the expression level of procollagen α1 (III) mRNA in the TGF-β1 plus Smad7 plasmid group was significantly lower than those in the TGF-β1 group and the TGF-β1 plus vehicle plasmid group (t = 103.8 and 45.7, respectively; both t > t0.01 and both P < 0.01).
CONCLUSION: Overexpression of Smad7 inhibits the expression of procollagen α1 (III) stimulated by exogenous TGF-β1 in cultured hepatic stellate cells.
- Citation: Xu LF, Liu HL. Smad7 overexpression inhibits TGF-β1-stimulated procollagen α1 (III) expression in cultured hepatic stellate cells. Shijie Huaren Xiaohua Zazhi 2010; 18(3): 280-283
- URL: https://www.wjgnet.com/1009-3079/full/v18/i3/280.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v18.i3.280
肝星状细胞(hepatic stellate cell, HSC)是肝纤维化形成过程中细胞外基质(extracellular matrix, ECM)的主要来源. 活化的HSC合成大量ECM, 包括Ⅰ、Ⅲ型胶原, 在肝内过度沉积[1,2]. 作为促肝纤维化细胞因子, TGF-β1能激活HSC促进ECM合成, 并抑制其降解[3,4]. 而Smad7可抑制TGF-β1的信号转导[5,6]. 我们的前期研究表明转染Smad7质粒可显著减少HSCα1(Ⅰ)前胶原mRNA表达, 但对α1(Ⅲ)前胶原mRNA水平无明显影响[7]. 究竟是Smad7对α1(Ⅲ)前胶原基因表达无调控作用, 还是需要在特定条件下才能发挥作用, 值得进一步深入研究. 肝纤维化时TGF-β1表达显著增加[8-10]. 因此, 本文应用Real time-PCR定量检测了加入TGF-β1后, Smad7质粒转染对HSC-T6细胞α1(Ⅲ)前胶原mRNA表达的作用.
HSC-T6细胞由上海中医药大学肝病研究所提供; pcDNA3.0-人Smad7重组质粒和pcDNA3.0空载质粒为本院组织工程实验室惠赠; Fugene6转染试剂、TRIzol RNA提取试剂盒、TGF-β1分别购自Roche公司、Sigma公司和英国Peprotech公司; 各目的基因引物由上海生工生物工程公司合成.
将浓度为1×108细胞/L的HSC-T6细胞悬液接种于6孔培养板, 每孔2 mL, 37 ℃, 50 g/L CO2、饱和湿度培养24 h, 随机分为6组: (1)正常对照组; (2)TGF-β1对照组; (3)Smad7质粒组; (4)TGF-β1+Smad7质粒组; (5)空载质粒组; (6)TGF-β1+空载质粒组. 每组3复孔. pcDNA3.0-人Smad7重组质粒或空载体pcDNA3.0质粒用配制的脂质体Fugene6转染液介导转染. TGF-β1的终浓度为10 μg/L, 正常对照组加入同体积的50 g/L FSC DMEM培养基. 继续培养48 h后, 收集细胞, 用TRIzol试剂盒提取细胞总RNA. 取RNA 2 µg按照RT试剂盒(Sigma公司)说明书进行逆转录. 应用Mx3000P实时荧光定量PCR仪(美国Stratagene公司)分别检测Smad7, αl(Ⅲ)前胶原mRNA水平. 引物序列如下: Smad7: 5'-CAACTGCAGACTGTCCAGATG-3'(上游), 5'-CTGCTGCATAAACTCGTGGTC-3'(下游); α1(Ⅲ)前胶原: 5'-GTACAGCTGGCCTTCCTCAG-3'(上游), 5'-GGCCTTGCGTGTTTGATATT-3' (下游); 反应体系为: ddH2O 10.125 μL, cDNA 1 μL, 2×SYBR Green QPCR master mix 12.5 μL, ROX passivereference 0.375 μL, Forwardprimer (10 μmol/L) 0.5 μL, Reverse primer(10 μmol/L) 0.5 μL, 总体积25 μL. 每个样品设3个复孔, 并设置无模板对照. 各目的基因反应条件为: Smad7: 95 ℃预变性10 min, 95 ℃, 2 min, 60 ℃, 30 s, 72 ℃, 1.5 min, 共40个循环; αl(Ⅲ)前胶原: 95 ℃预变性10 min, 1个循环; 接着94 ℃, 30 s, 55 ℃ 45 s, 72 ℃, 30 s, 共40个循环. 循环结束后继续进行建立熔解曲线的反应, 最后用专用软件自动分析结果. mRNA水平用相对含量图表示. 同时采用RT-PCR测定各目的基因mRNA水平, 其中Smad7和αl(Ⅲ)前胶原均为扩增28个循环, 引物和其他反应条件同上. PCR产物经12 g/L琼脂糖凝胶电泳, 用天能凝胶分析系统图像分析. 计算目的基因条带灰度值, 与内参β-actin条带灰度值的比值, 作为该目的基因表达的相对值.
统计学处理 数据用mean±SD表示, 多组比较用方差分析, 两组间比较用t检验. P<0.05为有统计学意义.
TGF-β1+Smad7质粒组、Smad7质粒组Smad7 mRNA水平较其他4组(正常对照组、空载质粒组、TGF-β1对照组、TGF-β1+空载质粒对照组)显著升高(t = 59.43, 59.41, 57.15, 57.13, 54.27, 54.25, 44.66, 51.70, 均P<0.01, 图1). 与正常对照组和空载质粒组相比, TGF-β1对照组、TGF-β1+空载质粒对照组Smad7 mRNA表达亦有增加(t = 37.26, 36.99, 54.26, 53.87, 均P<0.01). RT-PCR的结果与Real time-PCR一致. 表明Smad7质粒有效转染至HSC-T6细胞; TGF-β1可增加Smad7 mRNA表达, 但远低于Smad7质粒转染组.
与正常对照组和空载质粒组相比, Smad7质粒组αl(Ⅲ)前胶原mRNA水平无明显差异(t = 1.25, 1.27, P>0.05).但TGF-β1+Smad7质粒组αl(Ⅲ)前胶原mRNA水平较TGF-β1组和TGF-β1+空载质粒组显著降低(t = 103.87, 45.70, 均P<0.01, 图2). RT-PCR结果与Real time-PCR一致.
TGF-β1能激活HSC, 促进Ⅰ、Ⅲ、Ⅳ胶原和纤维连接蛋白、蛋白多糖等细胞外基质的合成. 同时, 使金属蛋白酶组织抑制因子(tissue inhibitors of metalloproteinase, TIMP)表达增加, 减少胶原的降解, 导致ECM大量沉积, 加速肝纤维化的发展[11]. TGF-β1首先通过与其Ⅱ型受体结合使之激活, 活化的Ⅱ型受体蛋白激酶使Ⅰ型受体(TβRⅠ)磷酸化, 后者再作用于Smad2、Smad3, 并与Smad2、Smad3和Smad4形成异源寡聚体复合物, 由胞质转入核内. 通过与特定的序列结合, 启动基因转录, 包括胶原基因、纤溶酶原激活物抑制因子-1基因、Smad7基因等发挥生物学效应[12-14]. Smad7是TGF-β1/Smad信号转导通路中的主要抑制性调控蛋白[5,15], 可与Smad2、Smad3竞争性结合TβRⅠ, 抑制TGF-β1/Smads的信号转导; 另外, Smad7还可与smurfl/2结合, 通过泛素化途径降解TβRⅠ. TGF-β1与Smad7之间存在负反馈调节. 本文中, TGF-β1组、TGF-β1+空载质粒组Smad7 mRNA表达较正常对照组和空载质粒组增高, 即是这种负反馈调节的反应. 但仅靠自身的负反馈调节尚不足于抑制TGF-β1对α1(Ⅲ)前胶原mRNA表达的促进作用. 因此, TGF-β1组、TGF-β1+空载质粒组的α1(Ⅲ)前胶原mRNA水平显著高于正常对照组和空载质粒组.
与正常对照组和空载质粒组相比, Smad7质粒组Smad7 mRNA水平显著升高, 而αl(Ⅲ)前胶原mRNA水平无明显差异. 说明Smad7对HSC-T6的α1(Ⅲ)前胶原mRNA表达并无直接作用, 与我们的前期研究结果一致[7]. TGF-β1+Smad7质粒组αl(Ⅲ)前胶原mRNA水平显著低于TGF-β1组和TGF-β1+空载质粒组, 表明Smad7过度表达可抑制TGF-β1对α1(Ⅲ)前胶原mRNA表达的促进作用.
总之, Smad7表达上调本身并不能直接抑制HSC-T6细胞α1(Ⅲ)前胶原基因转录, 但可抑制TGF-β1对α1(Ⅲ)前胶原基因表达的促进作用. 由于在肝纤维化发展过程中, TGF-β1显著增加, 所以Smad7过度表达可以发挥抗纤维化作用.
肝纤维化时TGF-β1的表达显著增加, 对肝纤维化的发生发展具有重要作用. Smad7是TGF-β/Smad信号转导通路中的主要抑制性调控蛋白, 其对α1(Ⅲ)前胶原mRNA表达的影响及作用机制尚未完全明了.
高润平, 教授, 吉林大学第一医院肝病科; 高泽立, 副教授, 上海交通大学医学院附属第九人民医院周浦分院消化科.
本研究采用体外培养肝星状细胞系(HSC-T6), 探讨在加入TGF-β1条件下Smad7过量表达对α1(Ⅲ)前胶原mRNA水平的影响, 有助于进一步了解Smad7的抗纤维化机制.
本研究表明, Smad7过量表达可显著抑制TGF-β1对HSC-T6细胞α1(Ⅲ)前胶原mRNA表达的促进作用. 为Smad7用于抗纤维化治疗提供了实验依据.
本文研究了目前肝病治疗领域的热点之一-肝纤维化的防治机制, 密切联系临床, 值得参考.
编辑: 李军亮 电编:吴鹏朕
| 1. | Chen YW, Li DG, Wu JX, Chen YW, Lu HM. Tetrandrine inhibits activation of rat hepatic stellate cells stimulated by transforming growth factor-beta in vitro via up-regulation of Smad 7. J Ethnopharmacol. 2005;100:299-305. [PubMed] [DOI] |
| 2. | Kharbanda KK, Rogers DD 2nd, Wyatt TA, Sorrell MF, Tuma DJ. Transforming growth factor-beta induces contraction of activated hepatic stellate cells. J Hepatol. 2004;41:60-66. [PubMed] [DOI] |
| 3. | Gressner AM, Weiskirchen R. The tightrope of therapeutic suppression of active transforming growth factor-beta: high enough to fall deeply? J Hepatol. 2003;39:856-859. [PubMed] [DOI] |
| 4. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [PubMed] [DOI] |
| 5. | Ten Dijke P, Goumans MJ, Itoh F, Itoh S. Regulation of cell proliferation by Smad proteins. J Cell Physiol. 2002;191:1-16. [PubMed] [DOI] |
| 6. | Hayashi H, Abdollah S, Qiu Y, Cai J, Xu YY, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA Jr, Wrana JL, Falb D. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell. 1997;89:1165-1173. [PubMed] [DOI] |
| 8. | Sun Y, Xuan S, Xin Y, Lu W, Chu L. [Relationship between serum TGF beta-1 with chronic hepatitis B involving in liver cell function and liver biopsy fibrosis]. Zhonghua Ganzangbing Zazhi. 2002;10:221-222. [PubMed] |
| 11. | Li JH, Huang XR, Zhu HJ, Johnson R, Lan HY. Role of TGF-beta signaling in extracellular matrix production under high glucose conditions. Kidney Int. 2003;63:2010-2019. [PubMed] [DOI] |
| 12. | Vozenin-Brotons MC, Sivan V, Gault N, Renard C, Geffrotin C, Delanian S, Lefaix JL, Martin M. Antifibrotic action of Cu/Zn SOD is mediated by TGF-beta1 repression and phenotypic reversion of myofibroblasts. Free Radic Biol Med. 2001;30:30-42. [PubMed] [DOI] |
| 13. | Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465-471. [PubMed] |
| 14. | Breitkopf K, Haas S, Wiercinska E, Singer MV, Dooley S. Anti-TGF-beta strategies for the treatment of chronic liver disease. Alcohol Clin Exp Res. 2005;29:121S-131S. [PubMed] [DOI] |
| 15. | Schiffer M, von Gersdorff G, Bitzer M, Susztak K, Böttinger EP. Smad proteins and transforming growth factor-beta signaling. Kidney Int Suppl. 2000;77:S45-S52. [PubMed] [DOI] |