修回日期: 2010-09-16
接受日期: 2010-09-27
在线出版日期: 2010-10-18
目的: 研究苦参碱对肝癌细胞株SMMC-7721凋亡的影响及其分子机制.
方法: 肝癌细胞分组为: 5-FU治疗组、苦参碱治疗组、5-FU联合苦参碱治疗组及对照组. 5-FU和苦参碱的给药浓度分别为半数肝癌细胞抑制浓度. 用流式细胞仪检测各组肝癌细胞凋亡率. 采用RT-PCR检测苦参碱对转录因子NF-κB p65、IκB、凋亡抑制基因Bcl-2和自噬相关基因Atg5和Beclin1表达的影响.
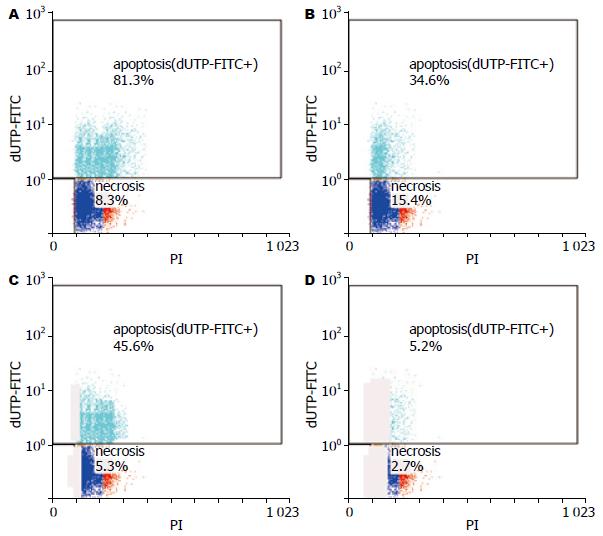
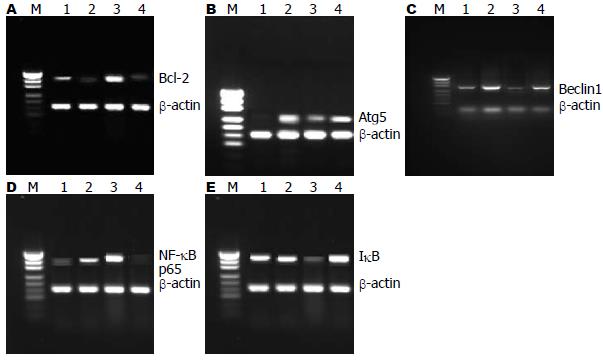
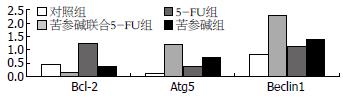
结果: 流式细胞仪检测结果提示, 苦参碱联合5-FU组的肝癌细胞凋亡率比5-FU治疗组、苦参碱治疗组及对照组高, 组间比较存在显著差异(81.3%±2.6% vs 34.6%±3.5%, 45.6%±2.4%, 5.2%±2.1%, 均P<0.05). RT-PCR检测结果显示, 与5-FU组相比, 苦参碱联合5-FU治疗组中NF-κB p65、Bcl-2表达下降(P<0.05), IκB、Atg5及Beclin1表达上调(P<0.05).
结论: 苦参碱可通过上调IκB、Atg5及Beclin1的表达和下调NF-κB p65、Bcl-2的表达, 促进肝癌细胞凋亡, 提高肝癌细胞的化疗敏感性.
引文著录: 查勇, 寸英丽, 黄云超. 苦参碱对肝癌细胞株SMMC-7721凋亡和自噬相关基因表达的影响. 世界华人消化杂志 2010; 18(29): 3078-3083
Revised: September 16, 2010
Accepted: September 27, 2010
Published online: October 18, 2010
AIM: To investigate the effect of matrine on cell apoptosis in human hepatocellular carcinoma cell line SMMC-7721 and to explore potential mechanisms involved.
METHODS: SMMC-7721 cells were divided into four groups: 5-fluorouracil group, matrine group, 5-FU plus matrine group, and control group. Matrine and 5-FU were administered at the half maximal inhibitory concentration. After 24 h, apoptosis was analyzed by flow cytometry. To identify genes involved in the antiapoptotic responses to 5-FU and matrine, the expression of NF-κB p65, IκB, Atg5, beclin1 and Bcl-2 was determined.
RESULTS: Increased sensitivity to 5-FU was observed in cells in the matrine plus 5-FU group as compared with those in the 5-FU group and control group (81.3% ± 2.6% vs 34.6% ± 3.5%, 45.6% ± 2.4%, 5.2% ± 2.1%, all P < 0.05). Compared to cells treated with 5-FU alone, cells exposed to a combination of matrine and 5-FU for 24 h had a significant reduction in the levels of NF-κB p65 and Bcl-2 (P < 0.05) and an increase in the levels of IκB, Atg5 and beclin1 (P < 0.05).
CONCLUSION: Matrine can enhance the sensitivity of SMMC-7721 cells to 5-FU by inducing increased expression of autophagy-related gene and decreased expression of anti-apoptotic genes.
- Citation: Zha Y, Cun YL, Huang YC. Matrine promotes cell apoptosis in human hepatocellular carcinoma cell line SMMC-7721. Shijie Huaren Xiaohua Zazhi 2010; 18(29): 3078-3083
- URL: https://www.wjgnet.com/1009-3079/full/v18/i29/3078.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v18.i29.3078
肿瘤细胞凋亡功能障碍与其化学治疗敏感性差存在密切的关系, 有研究表明[1-3], 5-氟尿嘧啶(5-fluorouracil, 5-FU)和阿霉素等化学药物可诱导肝癌细胞中NF-κB转录因子(nuclear factor-κB, NF-κB)激活, 而NF-κB转录因子激活可促进凋亡抑制因子(inhibitor of apoptosis protein, IAP)和B细胞淋巴瘤/白血病-2因子(B cell lymphoma/leukemia-2, Bcl-2)等的表达, NF-κB激活与肝癌细胞的凋亡耐受存在密切的关系[4-6].
苦参碱是中药苦参、山豆根和苦豆子的主要成分, 经临床证实苦参碱可抑制机体的炎症反应[7,8]. 亦有研究表明苦参碱可诱导肝癌细胞发生自噬和凋亡, 但苦参碱抗炎、抗肿瘤生长的分子机制尚未完全阐明[9-11]. 本实验中, 我们进一步观察苦参碱对肝癌细胞凋亡和化学治疗敏感性的影响, 分析其作用机制.
肝癌细胞株SM-7721(中科院上海细胞库), 高糖DMEM细胞培养基(Hyclone公司), 胎牛血清(Hyclone公司), 苦参碱(西安东华生物工程技术有限责任公司), 5-FU(上海海普药业有限公司), RNA提取试剂TRIzol(Invitrogen公司), RT-PCR引物(根据软件Oligo和Premier设计, 由上海生工生物工程公司合成, 表1), 一步法RT-PCR检测试剂盒(Invitrogen公司).
| 基因 | 引物序列 |
| β2-GM | 5'-TTAGCTCTCGCGCTACTCCTCTC-3' |
| 5'-GTCGGATTGATGAAACCCAGACACA-3' | |
| NF-κB p65 | 5'-GGGAAGGAACGCTGTCAGAG-3' |
| 5'-TAGCCTCAGGGTACTCCATCA-3' | |
| IκB | 5'-GATCCGCCAGGTGAAGGG-3' |
| 5'-GCAATTTCTGGCTGGTTGG-3' | |
| Bcl-2 | 5'-GTGGAGGAGCTCTTCAGGAG-3' |
| 5'-AGGCACCCAGGGTGATGATGCAA-3' | |
| Beclin1 | 5'-ACCAGATGCGTTATGCCC-3' |
| 5'-TTGATTGTGCCAAACTGTCC-3' | |
| Atg 5 | 5'-TATCATCCCACAGCCAACAG-3' |
| 5'-TCTTCTTAGGCCAAAGGTTTC-3' |
1.2.1 实验分组: 按照处理因素的不同将SM-7721肝癌细胞实验分组为: 5-FU治疗组、苦参碱治疗组、5-FU联合苦参碱治疗组和对照组. 参照文献[12,13], 本实验中我们采用选择5-FU和苦参碱的给药浓度为细胞半数抑制浓度, 分别为4.7×10-3 mol/L和5.3×10-6 mol/L, 用药处理时间为48 h.
1.2.2 细胞培养: SMMC-7721细胞采用高糖DMEM培养, 培养液中含100 mL/L的胎牛血清, 置入含50 mL/L CO2的培养箱中, 温度为37 ℃.
1.2.3 流式细胞仪检测肝癌细胞的凋亡: 应用流式细胞仪, 采用TUNEL法检测肝癌细胞的凋亡, 用0.25%-0.5%的胰酶消化6孔板中各实验组细胞, 收集放入EP管中. PBS洗涤细胞, 离心收集细胞, 40 mL/L多聚甲醛固定细胞, TUNEL法标记后, 进行流式细胞分析.
1.2.4 RT-PCR检测p65 siRNA干扰后相关基因的表达: 采用TRIzol法提取各组细胞总RNA, 总RNA紫外分光光度计测A260、A280值, 标本的总RNA的A260/A280值均在1.6-1.8. 按照Invitrogen公司RT-PCR试剂盒设定反应体系, RT-PCR反应条件: NF-κB p65的反应参数为94 ℃ 45 s, 55 ℃ 45 s, 72 ℃ 45 s, 32循环, 最后72 ℃ 10 min (扩增产物长为204 bp). IκB的反应参数为94 ℃ 45 s, 55 ℃ 45 s, 72 ℃ 45 s, 32循环, 最后72 ℃ 10 min(扩增产物长为304 bp). β2-GM的反应参数为94 ℃ 45 s, 55.5 ℃ 45 s, 72 ℃ 45 s, 32循环, 最后72 ℃ 10 min(扩增产物长为264 bp). Bcl-2的反应参数为94 ℃ 45 s, 45.5 ℃ 45 s, 72 ℃ 45 s, 32循环, 最后72 ℃ 10 min(扩增产物长为280 bp). Beclin1的反应参数为94 ℃ 45 s, 55 ℃ 45 s, 72 ℃ 45 s, 32循环, 最后72 ℃ 10 min(扩增产物长为320 bp). Atg5的反应参数为94 ℃ 45 s, 53.5 ℃ 45 s, 72 ℃ 45 s, 32循环, 最后72 ℃ 10 min(扩增产物长为332 bp).
RT-PCR产物电泳检测后, 紫外灯下观察, 用美国Gene-Genus凝胶电泳成像系统对DNA电泳条带进行光密度β扫描, 以目标基因与β-actin的密度作为目标基因表达水平的参数.
统计学处理 采用SPSS11.0统计软件, 计量资料用mean±SD表示, 两样本均数比较采用成组t检验, 计数资料比较采用χ2检验. P<0.05认为具有统计学意义.
苦参碱可促进肝癌细胞凋亡, 抑制坏死性死亡. 苦参碱联合5-FU组肝癌细胞凋亡率为81.3%±2.6%, 5-FU治疗组为34.6%±3.5%, 苦参碱治疗组为45.6%±2.4%, 对照组为5.2%±2.1%, 组间比较存在显著差异(P<0.05); 苦参碱联合5-FU组肝癌细胞坏死率为8.3%±2.2%, 5-FU治疗组为15.4%±2.5%, 苦参碱治疗组为5.3%±1.8%, 对照组为2.7%±1.5%(图1). 苦参碱联合5-FU治疗组与5-FU治疗组细胞坏死率比较, 组间存在显著差异(P<0.05); 苦参碱治疗组与对照组细胞坏死率比较, 组间存在显著差异(P<0.05).
肝癌细胞株SMMC-7721 NF-κB p65 mRNA高表达, IκB低表达, 5-FU可使NF-κB p65的表达进一步升高, 而苦参碱能有效的降低NF-κB p65的表达, 上调IκB的表达. 对照组、苦参碱联合5-FU治疗组、5-FU治疗组、苦参碱治疗组肝癌细胞中NF-κB p65 mRNA表达与β-actin的比分别为0.30±0.04、0.82±0.06、1.50±0.08和0.15±0.03; IκB mRNA表达与β-actin的比分别为0.72±0.06、0.84±0.03、0.23±0.02和1.16±0.05(图2).
一些研究表明苦参碱具有抑制肿瘤细胞增生、降低肝脏局部炎症反应的作用[7,8]. 在本实验中, 苦参碱可促进肝癌细胞凋亡, 抑制肝癌细胞坏死, 而5-FU治疗组有很大的一部分细胞通过坏死途径死亡. 苦参碱联合5-FU组肝癌细胞凋亡率为81.3%±2.6%, 5-FU治疗组为34.6%±3.5%, 苦参碱治疗组为45.6%±2.4%, 组间比较存在显著差异(P<0.05); 苦参碱联合5-FU组肝癌细胞坏死率为8.3%±2.2%, 5-FU治疗组为15.4%±2.5%, 苦参碱治疗组为5.3%±1.8%. 苦参碱联合5-FU组不仅提高了肝癌细胞的凋亡率, 而且降低了坏死细胞比率. 肿瘤细胞坏死率降低有利于减轻肿瘤局部的炎症反应, 增强其抗肿瘤效果[14,15].
现有研究表明NF-κB转录因子参与炎症和肿瘤增生等多种病理过程, 可调节肿瘤坏死因子-α(tumor necrosis factor-α, TNF-α)、白介素6(interleukin 6, IL-6)、Bcl-2、IAP等多种细胞因子的表达, 抑制肿瘤细胞凋亡, 促进肿瘤细胞增生[16-21]. NF-κB转录因子家族有5位成员, 他们是RelA(p65)、RelB、C-Rel、NF-κB1(p50)和NF-κB2(P52). NF-κB家族成员之间可形成同源和异源二聚体, 最常见的二聚体是P65/P50异源二聚体[22-24]. 在静止的细胞中, NF-κB通过与细胞质内抑制蛋白NF-κB(inhibitory proteins NF-κB, IκB)结合, 以P50-P65-IκB多聚体的无活性状态存在, 滞留于细胞质中. 当外界因素刺激时导致IκB激酶β亚单位激活, 从而启动细胞内的泛素化-蛋白酶系统, 使IκB蛋白降解, 活化的NF-κB进入细胞核发挥转录因子的作用, 促进多种细胞因子的表达[22,25]. 现有研究表明NF-κB转录因子激活和化疗耐药有关, 抑制NF-κB转录因子可促进肿瘤细胞凋亡[1,26-28]. 在本研究中, RT-PCR分析结果提示, 5-FU治疗可诱导肝癌细胞NF-κB p65的表达增加, IκB表达下降. 苦参碱可抑制NF-κB p65的表达, 上调IκB的表达. 因此, 在肝癌5-FU化疗时, 若联合苦参碱中药治疗, 可降低NF-κB转录因子的表达, 有助于逆转肝癌细胞化疗耐药, 提高肝癌化学治疗的效果.
有研究表明[29-31], NF-κB转录因子可调节凋亡拮抗基因Bcl-x和Bfl-l、肿瘤坏死因子受体相关因子(tumor necrosis factor receptor-associated factor, TRAF)、IAP等多种凋亡抑制因子的表达. NF-κB转录因子亦可参与肿瘤细胞自噬的调节[32]. 在本研究中我们进一步分析苦参碱对凋亡抑制基因Bcl-2和自噬相关基因Atg5、Beclin1表达的影响, 结果显示, 苦参碱治疗组、5-FU联合苦参碱治疗组中, Bcl-2表达下降, Atg5和Beclin1 mRNA表达均显著地升高, 与单5-FU治疗组相比, 差异具有显著性. 因此, 我们推测苦参碱可能通过抑制NF-κB转录因子, 下调凋亡抑制基因Bcl-2和上调自噬基因Atg5和Beclin1的表达而促进肿瘤细胞凋亡.
总之, 凋亡调节与肝癌患者的化学治疗敏感性存在密切的关系. 在本实验中, 联合苦参碱治疗, 肝癌细胞株对5-FU的化学治疗敏感性增加, 凋亡率升高, 同时, 伴有凋亡抑制基因Bcl-2表达下降和自噬相关基因Atg5、Beclin1的表达增加. 说明苦参碱有助于提高5-FU对原发性肝癌的治疗效果, 值得我们进一步研究.
肿瘤细胞凋亡功能障碍与其化学治疗敏感性差存在密切的关系, 5-氟尿嘧啶(5-FU)和阿霉素等化学药物可诱导肝癌细胞中NF-κB转录因子激活, 而NF-κB转录因子激活可促进凋亡抑制因子(IAP)和B细胞淋巴瘤/白血病-2因子(Bcl-2)等的表达, NF-κB激活与肝癌细胞的凋亡耐受存在密切的关系.
丁惠国, 主任医师, 首都医科大学附属北京佑安医院肝病消化科
经临床证实苦参碱可抑制机体的炎症反应. 亦有研究表明苦参碱可诱导肝癌细胞发生自噬和凋亡, 但苦参碱抗炎、抗肿瘤生长的分子机制尚未完全阐明.
有研究表明, NF-κB转录因子可调节凋亡拮抗基因Bcl-x和Bfl-l、肿瘤坏死因子受体相关因子(TRAF)、IAP等多种凋亡抑制因子的表达. NF-κB转录因子亦可参与肿瘤细胞自噬的调节.
苦参碱可通过上调IκB、Atg5及Beclin1的表达和下调NF-κB p65、Bcl-2的表达, 促进肝癌细胞凋亡, 提高肝癌细胞的化疗敏感性.
本文设计合理, 方法可靠, 具有一定的实用价值.
编辑: 李薇 电编:何基才
| 1. | Tapia MA, González-Navarrete I, Dalmases A, Bosch M, Rodriguez-Fanjul V, Rolfe M, Ross JS, Mezquita J, Mezquita C, Bachs O. Inhibition of the canonical IKK/NF kappa B pathway sensitizes human cancer cells to doxorubicin. Cell Cycle. 2007;6:2284-2292. [PubMed] [DOI] |
| 2. | Ueno S, Aoki D, Kubo F, Hiwatashi K, Matsushita K, Oyama T, Maruyama I, Aikou T. Roxithromycin inhibits constitutive activation of nuclear factor {kappa}B by diminishing oxidative stress in a rat model of hepatocellular carcinoma. Clin Cancer Res. 2005;11:5645-5650. [PubMed] [DOI] |
| 3. | Chung C, Park SG, Park YM, Joh JW, Jung G. Interferon-gamma sensitizes hepatitis B virus-expressing hepatocarcinoma cells to 5-fluorouracil through inhibition of hepatitis B virus-mediated nuclear factor-kappaB activation. Cancer Sci. 2007;98:1758-1766. [PubMed] [DOI] |
| 4. | Hsiang CY, Wu SL, Chen JC, Lo HY, Li CC, Chiang SY, Wu HC, Ho TY. Acetaldehyde induces matrix metalloproteinase-9 gene expression via nuclear factor-kappaB and activator protein 1 signaling pathways in human hepatocellular carcinoma cells: Association with the invasive potential. Toxicol Lett. 2007;171:78-86. [PubMed] [DOI] |
| 5. | Guo J, Verma UN, Gaynor RB, Frenkel EP, Becerra CR. Enhanced chemosensitivity to irinotecan by RNA interference-mediated down-regulation of the nuclear factor-kappaB p65 subunit. Clin Cancer Res. 2004;10:3333-3341. [PubMed] [DOI] |
| 6. | Konishi T, Sasaki S, Watanabe T, Kitayama J, Nagawa H. Overexpression of hRFI inhibits 5-fluorouracil-induced apoptosis in colorectal cancer cells via activation of NF-kappaB and upregulation of BCL-2 and BCL-XL. Oncogene. 2006;25:3160-3169. [PubMed] [DOI] |
| 7. | 吕 建芳, 范 恒, 沈 霖, 寿 折星, 庄 雄. 氧化苦参碱对实验性结肠炎大鼠肠黏膜细胞因子和核因子-κB p65表达的影响. 世界华人消化杂志. 2008;16:2289-2294. [DOI] |
| 14. | Domart MC, Esposti DD, Sebagh M, Olaya N, Harper F, Pierron G, Franc B, Tanabe KK, Debuire B, Azoulay D. Concurrent induction of necrosis, apoptosis, and autophagy in ischemic preconditioned human livers formerly treated by chemotherapy. J Hepatol. 2009;51:881-889. [PubMed] [DOI] |
| 15. | Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51-64. [PubMed] [DOI] |
| 16. | Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461-466. [PubMed] [DOI] |
| 17. | Yang LQ, Fang DC, Wang RQ, Yang SM. Effect of NF-kappaB, survivin, Bcl-2 and Caspase3 on apoptosis of gastric cancer cells induced by tumor necrosis factor related apoptosis inducing ligand. World J Gastroenterol. 2004;10:22-25. [PubMed] |
| 18. | Zhou W, Jiang ZW, Tian J, Jiang J, Li N, Li JS. Role of NF-kappaB and cytokine in experimental cancer cachexia. World J Gastroenterol. 2003;9:1567-1570. [PubMed] |
| 19. | Li TJ, Jia LP, Gao XL, Huang AL. Gene therapy that inhibits NF-kappaB results in apoptosis of human hepatocarcinoma by recombinant adenovirus. World J Gastroenterol. 2006;12:5287-5292. [PubMed] |
| 20. | Saitou Y, Shiraki K, Yamanaka T, Miyashita K, Inoue T, Yamanaka Y, Yamaguchi Y, Enokimura N, Yamamoto N, Itou K. Augmentation of tumor necrosis factor family-induced apoptosis by E3330 in human hepatocellular carcinoma cell lines via inhibition of NF kappa B. World J Gastroenterol. 2005;11:6258-6261. [PubMed] |
| 21. | Guo LL, Xiao S, Guo Y. Activation of transcription factors NF-kappaB and AP-1 and their relations with apoptosis associated-proteins in hepatocellular carcinoma. World J Gastroenterol. 2005;11:3860-3865. [PubMed] |
| 22. | Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47:921-928. [PubMed] |
| 24. | Majdalawieh A, Ro HS. Regulation of IkappaBalpha function and NF-kappaB signaling: AEBP1 is a novel proinflammatory mediator in macrophages. Mediators Inflamm. 2010;2010:823821. [PubMed] |
| 25. | Luqman S, Pezzuto JM. NFkappaB: a promising target for natural products in cancer chemoprevention. Phytother Res. 2010;24:949-963. [PubMed] |
| 26. | Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer. 2010;10:561-574. [PubMed] [DOI] |
| 27. | Kasinski AL, Slack FJ. Potential microRNA therapies targeting Ras, NFkappaB and p53 signaling. Curr Opin Mol Ther. 2010;12:147-157. [PubMed] |
| 28. | Sakamoto K, Maeda S. Targeting NF-kappaB for colorectal cancer. Expert Opin Ther Targets. 2010;14:593-601. [PubMed] [DOI] |
| 29. | Wang CY, Guttridge DC, Mayo MW, Baldwin AS Jr. NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol. 1999;19:5923-5929. [PubMed] |
| 30. | Zheng C, Kabaleeswaran V, Wang Y, Cheng G, Wu H. Crystal structures of the TRAF2: cIAP2 and the TRAF1: TRAF2: cIAP2 complexes: affinity, specificity, and regulation. Mol Cell. 2010;38:101-113. [PubMed] [DOI] |
| 31. | Manna SK, Babajan B, Raghavendra PB, Raviprakash N, Sureshkumar C. Inhibiting TRAF2-mediated activation of NF-kappaB facilitates induction of AP-1. J Biol Chem. 2010;285:11617-11627. [PubMed] [DOI] |
| 32. | Djavaheri-Mergny M, Amelotti M, Mathieu J, Besançon F, Bauvy C, Souquère S, Pierron G, Codogno P. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J Biol Chem. 2006;281:30373-30382. [PubMed] [DOI] |