修回日期: 2010-08-22
接受日期: 2010-08-27
在线出版日期: 2010-09-18
目的: 探讨谷胱甘肽转硫酶(GSTs)基因多态性与浙江汉族溃疡性结肠炎(UC)易感性的关系.
方法: 选取浙江省温州医学院附属第二医院和温州市其他大型综合性医院UC患者252例, 对照组共578例, 系同期温州医学院附属第二医院体检中心的健康体检者. 采用聚合酶链反应技术检测GST(M1、T1、P1)基因多态性在UC患者和正常对照者之间的分布差异.
结果: UC患者中GSTM1(-)、GSTT1(-)和GSTP1(VaL/VaL)基因型频率明显高于正常对照组(69.45% vs 46.30%, P = 0.0003; 61.51% vs 51.39%, P = 0.007; 48.81% vs 34.61%, P<0.0001). 进一步根据UC临床症状分层分析, 在远端结肠炎患者中GSTT1(-)、GSTP1(VaL/VaL)基因型的分布频率高于广泛性结肠炎患者(P = 0.0001, P = 0.001); 而GSTM1(-)基因型与UC的病变部位无相关性(P = 0.108). 并且GST(M1、T1、P1)突变基因型与UC患者病情严重程度无关(均P>0.05).
结论: GST(M1、T1、P1)基因突变与浙江汉族UC明显相关.
引文著录: 吴昊, 郑波, 王建嶂, 裴继华, 姜丽娜, 薛战雄. 谷胱甘肽转硫酶基因多态性与浙江汉族溃疡性结肠炎易感性的相关性. 世界华人消化杂志 2010; 18(26): 2780-2784
Revised: August 22, 2010
Accepted: August 27, 2010
Published online: September 18, 2010
AIM: To investigate the association between the genetic polymorphisms of glutathione S-transferases (GSTs) genes and susceptibility to ulcerative colitis (UC) in Zhejiang Han population.
METHODS: A total of 252 patients with UC were collected from the Second Affiliated Hospital of Wenzhou Medical College and other large general hospitals in Wenzhou City. The control group was composed of 578 healthy volunteers. PCR was used to examine the prevalence of GST (M1, T1, P1) gene polymorphisms in these subjects.
RESULTS: The frequencies of GSTM1 (-), GSTT1 (-) and GSTP1 (VaL/VaL) were significantly higher in UC patients than in controls (69.45% vs 46.30%, P = 0.0003; 61.51% vs 51.39%, P = 0.007; 48.81% vs 34.61%, P < 0.0001). A further observation was made on the UC patients according to the clinical features. The frequencies of GSTT1 (-) and GSTP1 (VaL/VaL) genotypes were higher in patients with distal colitis than in those with extensive colitis (P = 0.0001 and 0.001). However, the same result was not observed for GSTM1 (-) genotype (P = 0.108). In addition, the variant genotypes of GST (M1, T1, P1) were not significantly linked to the severity of the disease (all P > 0.05).
CONCLUSION: The GST (M1, T1, P1) variant genotypes are obviously correlated with the development of UC in Zhejiang Han population.
- Citation: Wu H, Zheng B, Wang JZ, Pei JH, Jiang LN, Xue ZX. Relationship between genetic polymorphisms of glutathione S-transferase genes and susceptibility to ulcerative colitis in Zhejiang Han population. Shijie Huaren Xiaohua Zazhi 2010; 18(26): 2780-2784
- URL: https://www.wjgnet.com/1009-3079/full/v18/i26/2780.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v18.i26.2780
炎症性肠病(inflammatory bowel disease, IBD)主要包括溃疡性结肠炎(ulcerative colitis, UC)和克罗恩病(Crohn's disease, CD)两种临床亚型, 肠黏膜的慢性非特异性炎症为其病理组织学的共同表现. 目前认为UC的发病机制涉及遗传、环境、免疫、肠黏膜防御功能等因素的综合作用, 而遗传免疫因素对IBD的影响尤为肯定. 而且UC属于癌前病变, 其发生大肠癌的危险性与病变范围和疾病持续时间明确相关. 谷胱甘肽转硫酶(glutathione S-transferases, GSTs)是机体内重要的Ⅱ相代谢酶之一, 在肝脏、生殖腺及结肠中有较高表达. GSTs能催化Ⅰ相代谢过程产生的有毒致癌物与谷胱甘肽(glutathione, GSH)巯基(sulfhydryl group, -SH)共轭结合, 最终以亲水性结合物的形式排出体外, 从而实现机体对毒性致癌物的解毒作用. 现研究表明GSTs基因突变使机体发生多种肿瘤的易感性增加, 但至今鲜见在同一种族人群中同时研究GST(M1、T1、P1)3个基因位点多态性与UC之间相互关系的报道. 本研究收集252例浙江汉族UC患者和578例正常对照者, 同时检测GST(M1、T1、P1)基因多态性, 旨在探讨GSTs基因多态性与UC易感性的关系.
252例UC患者来自浙江省温州医学院附属第二医院和温州市其他大型综合性医院, 其中男140例, 女112例, 平均年龄(44.4±15.6)岁. 经临床、实验室、放射影像学、内镜及组织学检查结果综合确立UC诊断[1]. 根据病变范围将UC患者分为: 远端结肠炎组(病变不超过结肠脾曲)187例; 广泛结肠炎组(病变超过结肠脾曲)65例. 并根据病变严重程度将UC患者分为两组: (轻度+中度)组190例, 重度组62例. 对照组共578例, 系同期温州医学院附属第二医院体检中心的健康体检者, 其中男317例, 女261例, 平均年龄(45.6±17.2)岁. 全部研究对象均为无血缘关系的浙江汉族人.
1.2.1 基因组DNA提取: 取空腹静脉血5 mL, EDTA抗凝, 蛋白酶K消化, 苯酚/氯仿法抽提DNA, -20 ℃冰箱保存备用.
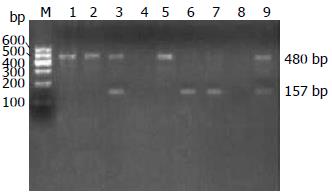
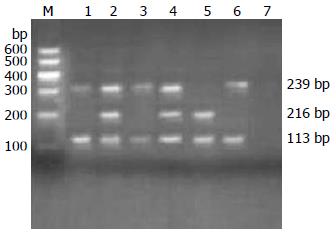
1.2.2 GST(M1、T1、P1)基因多态性检测: (1)采用PCR技术扩增GST(M1、T1、P1)目的基因. 参照文献设计引物[2,3], 引物由上海生物工程有限公司合成. 序列如下: GSTM1上游5'-GCTTCACGTGTTATGGAGGTTC-3'; 下游5'-GAGATGAAGTCCTCCAGATTT-3'. GSTT1上游5'-TCACCGGATCATGGCCAGCA-3'; 下游5'-TTCCTTACTTGGTCCTCACATCTC-3'. GSTP1上游5'-GTAGTTTGCACAAGGTCAAG-3'; 下游5'-ACAAACCTGAGGGGTAAG-3'. (2)PCR反应条件: GST(M1、T1)总反应体系50 µL, 含灭菌双蒸水30 µL, 上、下游引物(10 µmol/L)各1 µL, 10×Buffer 10 µL, MgCl2(25 mmol/L)2.5 µL, dNTPs(10 mmol/L)2 µL, Taq酶(1 U/µL)2.5 µL, 模板DNA(约40 ng/µL)1 µL. GSTP1总反应体系25 µL, 含灭菌双蒸水10.5 µL, 上、下游引物(10 pmol/µL)各1 µL, 10×Buffer 5 µL, MgCl2(25mmol/L)2.5 µL, dNTPs(10 mmol/L)1.5 µL, Taq酶(1 U/µL)2.5 µL, 模板DNA(约40 ng/µL)1 µL. 酶切体系: PCR反应产物10 µL(0.1-0.5 µg DNA), 去离子水18 µL, 10×Buffer 2 µL, BsmAⅠ限制性内切酶(MBI Fermentas, 美国)1-2 µL, 37 ℃水浴1-16 h. (3)GSTs基因型判定: GSTM1、GSTT1扩增产物用2.5%琼脂糖凝胶电泳检测, 长度分别为157、480 bp. 若出现157 bp片段和480 bp片段, 分别为GSTM1(+)和GSTT1(+), 而无相应的扩增产物者则为纯合子基因缺失, 分别为GSTM1(-)和GSTT1(-). GSTP1酶切产物用2.5%琼脂糖凝胶电泳检测. 若酶切产物含329、113 bp两个片段, 则为ILe/ILe基因型(野生型); 若酶切产物含329、216、113 bp 3个片段, 则为ILe/VaL基因型(杂合突变型); 若酶切产物含216、113 bp两片段, 则为VaL/VaL基因型(纯合子突变型).
统计学处理 计数资料采用χ2检验, 计算OR值和95%可信区间, P<0.05有显著性差异. 所有数据输入SPSS13.0统计软件包.
GSTM1、T1基因型如图1所示: 1、2、5, 9: GSTT1(+); 3、6、7: GSTM1(+); 4: GSTM1(-); 8: GSTT1(-). GSTP1基因型如图2所示: 1、3、6: ILe/ILe; 2、4: ILe/VaL; 5: VaL/VaL; 7: 空白.
GSTM1(-)、GSTT1(-)和突变的GSTP1(VaL/VaL)基因型频率在UC患者中明显高于正常对照组, 差异有统计学意义(P = 0.0003, OR = 2.216, 95%CI: 1.624-3.022; P = 0.007, OR = 1.512, 95%CI: 1.118-2.044; P<0.0001, P<0.0001, OR = 3.009, 95%CI: 1.650-5.490, 表1, 2).
| 分组 | n | GSTM1 | GSTT1 | ||
| (+) | (-) | (+) | (-) | ||
| 对照组 | 578 | 310(53.70) | 318(46.30) | 281(48.61) | 297(51.39) |
| UC组 | 252 | 77(30.55) | 175(69.45) | 97(38.49) | 155(61.51) |
| 临床特征 | |||||
| 远端结肠炎 | 187 | 52(27.81) | 135(72.19) | 53(28.34) | 134(71.66) |
| 广泛性结肠炎 | 65 | 25(38.46) | 40(61.54) | 44(67.69) | 21(32.31) |
| (轻+中)度 | 190 | 56(40.51) | 134(59.49) | 75(39.47) | 115(60.53) |
| 重度 | 62 | 21(33.33) | 41(66.67) | 22(35.48) | 40(64.52) |
| 分组 | n | GSTP1 | ||
| ILe/ILe | ILe/VaL | VaL/VaL | ||
| 对照组 | 578 | 234(40.48) | 144(24.91) | 200(34.61) |
| UC组 | 252 | 60(23.81) | 69(27.38) | 123(48.81) |
| 临床特征 | ||||
| 远端结肠炎 | 187 | 40(21.39) | 44(23.53) | 103(55.08) |
| 广泛性结肠炎 | 65 | 20(30.77) | 25(38.46) | 20(30.77) |
| (轻+中)度 | 190 | 43(22.63) | 57(30.0) | 90(47.37) |
| 重度 | 62 | 17(27.42) | 12(19.35) | 33(53.23) |
根据UC临床特征进一步分层分析发现, GSTT1(-)、GSTP1(VaL/VaL)基因型在远端结肠炎患者中的分布频率高于广泛结肠炎, 差异有统计学意义(P = 0.0001, OR = 5.297, 95%CI: 2.880-9.744; P = 0.001, OR = 2.759, 95%CI: 1.514-5.029); 而GSTM1(-)基因型与UC的病变部位无统计学关联(P = 0.108, OR = 0.616, 95%CI: 0.340-1.115). GSTM1(-)、GSTT1(-)和GSTP1(VaL/VaL)基因型与UC患者病情严重程度无关(均P>0.05, 表1, 2).
GSTs在人体中的解毒作用取决于酶活性的高低, 而GSTs的活性是由其基因多态性所决定的. 在不同人群中, GSTs基因多态性导致体内GSTs对毒性代谢产物的清除能力产生差异, 使人体对多种疾病的易感性发生改变, 特别是肿瘤[4]. 目前已证实GSTsα(GSTA)、GSTsμ(GSTM)、GSTsπ(GSTP)均具有遗传多态性[5]. 人类编码GSTs的基因容易产生缺失, 表现为GSTs空白基因型[GSTM1(-), GSTT1(-)], 个体如果携带这些空白基因型, 相应的GSTs蛋白不能在其肝脏中表达, 使个体对多种毒性物质的解毒能力减低, 导致各种毒物代谢相关性疾病发生的危险性增加.
GSTM1基因有3种等位基因: GSTM1A、GSTM1B、GSTM10(空白GSTM1等位基因), 由GSTM1A、GSTM1B等位基因编码的蛋白质只有单个氨基酸的差异, 且两者所表达的酶活性相似; 而GSTM10等位基因表达产物在体内无催化活性[6]. 研究发现不同种族之间, 空白GSTM1基因型频率为22%-62%[7]. 流行病学调查显示GSTM1基因突变与多种肿瘤的发生存在相关性, 如多发性皮肤肿瘤[8]、绝经后乳腺癌[9]、直结肠肿瘤[10]、头颈部肿瘤[11]等, 尤其是与膀胱癌和肺癌关系密切[12,13]. GSTM1基因表达产物主要在致癌性多环芳香族碳氢化合物和环氧化物的解毒方面具有重要作用[14].
GSTT1酶在人体内主要促进烟草中所含毒物的代谢, 如甲烷、氧化乙烯等. 研究发现空白GSTT1基因型在不同种族人群的分布频率中为12%-62%[15,16]. 空白GSTT1纯合子基因型携带者体内的所有组织缺乏GSTT1酶, 因此增加人体对某些疾病的易感性, 如少突神经胶质细胞瘤[17]、脑脊髓膜瘤和星型细胞瘤[18]以及大肠癌[19]和肺癌[14].
目前已经确定GSTP1基因位于11q13, 全长约3 kb, 由6个内含子和7个外显子组成. Watson等[20]研究报道白种人群中的GSTP1基因型突变率约为50%. 国外有关GSTP1基因型与疾病相关性的研究报道, 突变型GSTP1基因与急性白血病[21]、隐源性肝硬化[22]、卵巢癌[23]、子宫颈癌[24]等相关, 并且通过干预化疗药物在体内的代谢而影响患者的预后及存活率.
本研究发现在中国浙江正常汉族人群中, GST(M1、T1)空白基因型突变率和GSTP1(VaL/VaL)基因型携带率分别为46.30%、51.39%和34.61%, 与上述研究结果相近. 而GST(M1、T1)空白基因型频率和GSTP1(VaL/VaL)基因型携带率在汉族UC患者中则明显增高, 分别达到69.45%、61.51%和48.81%. 经统计分析, 发现GSTM1和GSTT1空白基因型以及突变的GSTP1基因型频率在UC患者中均明显增高. 进一步根据患者临床特征对UC组进行分层分析发现, GSTT1(-)及GSTP1(VaL/VaL)基因型在远端UC患者中的分布频率均显著高于广泛结肠炎患者, 而GSTM1(-)基因型与UC的病变部位无关. 并且GSTM1(-)和GSTT1(-)基因型与UC患者病情严重程度无关. 我们的研究结果显示携带GSTM1(-)、GSTT1(-)或GSTP1(VaL/VaL)基因型的个体其罹患UC的易感性增加, 这提示GSTs基因可能是浙江汉族人群UC的易感基因.
理论上, 由于人类编码GSTs的基因容易产生缺失, 当个体携带GSTs空白或突变基因型时, 相应的GSTs蛋白在人体内不能表达或表达降低, 使体内GSTs酶水平下降, 引起机体对毒性代谢产物的清除障碍, 毒性物质的过量蓄积, 加上其他诱发因素(如DNA损伤加重且修复功能降低), 共同诱发肠道异常免疫反应, 造成对结肠黏膜的不可逆损伤, 最终导致UC发生.
近年来UC在国内的发病率逐年升高, 由于病因不明, 临床治疗效果不佳, 患者病情常反复发作, 迁延不愈. 因此对UC发病机制的研究日益受到国内外学者的重视. GSTs是人体重要的毒物代谢酶之一, 此酶的活性高低将直接影响人体对多种毒性物质的清除. 而GSTs的活性是由其遗传基因多态性所决定的, 因此探讨GSTs基因多态性与UC患者易感性的关系, 将会对揭示UC的发病机制提供重要的遗传学依据.
姚登福, 教授, 南通大学附属医院分子医学中心; 白爱平, 副教授, 南昌大学第一附属医院消化内科
单核苷酸多态性(SNP)与UC易感性的研究一直是国内外学者研究的重点和热点. 至今有关GSTs基因多态性与疾病相关性的研究主要集中在肿瘤领域, 鲜见GSTs基因多态性与IBD易感性的研究报道.
本研究首次在浙江汉族人群和UC患者中同时检测GST(M1、T1、P1)3种SNP的分布差异, 并探讨GST基因多态性与UC亚临床特征的关系, 这将为揭示UC的遗传易感性提供一定的理论依据.
本文分析了GSTs基因多态性在浙江人群UC中的分布, 弥补了国内IBD研究在这一领域的空白, 同时对国内汉族人群IBD易感基因的研究产生深远影响.
本文设计合理, 科学性、创新性和可读性较好.
编辑: 李军亮 电编:何基才
| 2. | Bell DA, Taylor JA, Paulson DF, Robertson CN, Mohler JL, Lucier GW. Genetic risk and carcinogen exposure: a common inherited defect of the carcinogen-metabolism gene glutathione S-transferase M1 (GSTM1) that increases susceptibility to bladder cancer. J Natl Cancer Inst. 1993;85:1159-1164. [PubMed] [DOI] |
| 3. | Pemble S, Schroeder KR, Spencer SR, Meyer DJ, Hallier E, Bolt HM, Ketterer B, Taylor JB. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem J. 1994;300:271-276. [PubMed] |
| 4. | Curran JE, Weinstein SR, Griffiths LR. Polymorphisms of glutathione S-transferase genes (GSTM1, GSTP1 and GSTT1) and breast cancer susceptibility. Cancer Lett. 2000;153:113-120. [PubMed] [DOI] |
| 5. | Hirvonen A. Polymorphisms of xenobiotic-metabolizing enzymes and susceptibility to cancer. Environ Health Perspect. 1999;107 Suppl 1:37-47. [PubMed] [DOI] |
| 6. | Pinarbasi H, Silig Y, Gurelik M. Genetic polymorphisms of GSTs and their association with primary brain tumor incidence. Cancer Genet Cytogenet. 2005;156:144-149. [PubMed] [DOI] |
| 7. | Rebbeck TR. Molecular epidemiology of the human glutathione S-transferase genotypes GSTM1 and GSTT1 in cancer susceptibility. Cancer Epidemiol Biomarkers Prev. 1997;6:733-743. [PubMed] |
| 8. | Lear J, Heagerty A, Smith A, Bowers B, Jones P, Gilford J, Alldersea J, Fryer A, Strange R. Polymorphism in detoxifying enzymes and susceptibility to skin cancer. Photochem Photobiol. 1996;63:424-428. [PubMed] [DOI] |
| 9. | Helzlsouer KJ, Selmin O, Huang HY, Strickland PT, Hoffman S, Alberg AJ, Watson M, Comstock GW, Bell D. Association between glutathione S-transferase M1, P1, and T1 genetic polymorphisms and development of breast cancer. J Natl Cancer Inst. 1998;90:512-518. [PubMed] [DOI] |
| 10. | Zhong S, Wyllie AH, Barnes D, Wolf CR, Spurr NK. Relationship between the GSTM1 genetic polymorphism and susceptibility to bladder, breast and colon cancer. Carcinogenesis. 1993;14:1821-1824. [PubMed] [DOI] |
| 11. | Trizna Z, Clayman GL, Spitz MR, Briggs KL, Goepfert H. Glutathione s-transferase genotypes as risk factors for head and neck cancer. Am J Surg. 1995;170:499-501. [PubMed] [DOI] |
| 12. | Pinarbasi H, Silig Y, Cetinkaya O, Seyfikli Z, Pinarbasi E. Strong association between the GSTM1-null genotype and lung cancer in a Turkish population. Cancer Genet Cytogenet. 2003;146:125-129. [PubMed] [DOI] |
| 13. | D'errico A, Taioli E, Chen X, Vineis P. Genetic metabolic polymorphisms and the risk of cancer: a review of the literature. Biomarkers. 1996;1:149-173. [DOI] |
| 14. | Ryberg D, Skaug V, Hewer A, Phillips DH, Harries LW, Wolf CR, Ogreid D, Ulvik A, Vu P, Haugen A. Genotypes of glutathione transferase M1 and P1 and their significance for lung DNA adduct levels and cancer risk. Carcinogenesis. 1997;18:1285-1289. [PubMed] [DOI] |
| 15. | Nelson HH, Wiencke JK, Christiani DC, Cheng TJ, Zuo ZF, Schwartz BS, Lee BK, Spitz MR, Wang M, Xu X. Ethnic differences in the prevalence of the homozygous deleted genotype of glutathione S-transferase theta. Carcinogenesis. 1995;16:1243-1245. [PubMed] [DOI] |
| 16. | Lee EJ, Wong JY, Yeoh PN, Gong NH. Glutathione S transferase-theta (GSTT1) genetic polymorphism among Chinese, Malays and Indians in Singapore. Pharmacogenetics. 1995;5:332-334. [PubMed] [DOI] |
| 17. | Kelsey KT, Wrensch M, Zuo ZF, Miike R, Wiencke JK. A population-based case-control study of the CYP2D6 and GSTT1 polymorphisms and malignant brain tumors. Pharmacogenetics. 1997;7:463-468. [PubMed] [DOI] |
| 18. | Elexpuru-Camiruaga J, Buxton N, Kandula V, Dias PS, Campbell D, McIntosh J, Broome J, Jones P, Inskip A, Alldersea J. Susceptibility to astrocytoma and meningioma: influence of allelism at glutathione S-transferase (GSTT1 and GSTM1) and cytochrome P-450 (CYP2D6) loci. Cancer Res. 1995;55:4237-4239. [PubMed] |
| 19. | Deakin M, Elder J, Hendrickse C, Peckham D, Baldwin D, Pantin C, Wild N, Leopard P, Bell DA, Jones P. Glutathione S-transferase GSTT1 genotypes and susceptibility to cancer: studies of interactions with GSTM1 in lung, oral, gastric and colorectal cancers. Carcinogenesis. 1996;17:881-884. [PubMed] [DOI] |
| 20. | Watson MA, Stewart RK, Smith GB, Massey TE, Bell DA. Human glutathione S-transferase P1 polymorphisms: relationship to lung tissue enzyme activity and population frequency distribution. Carcinogenesis. 1998;19:275-280. [PubMed] [DOI] |
| 21. | Ye Z, Song H. Glutathione s-transferase polymorphisms (GSTM1, GSTP1 and GSTT1) and the risk of acute leukaemia: a systematic review and meta-analysis. Eur J Cancer. 2005;41:980-989. [PubMed] [DOI] |
| 22. | Ghobadloo SM, Yaghmaei B, Bakayev V, Goudarzi H, Noorinayer B, Rad FH, Samiy S, Aghabozorghi S, Zali MR. GSTP1, GSTM1, and GSTT1 genetic polymorphisms in patients with cryptogenic liver cirrhosis. J Gastrointest Surg. 2004;8:423-427. [PubMed] [DOI] |
| 23. | Beeghly A, Katsaros D, Chen H, Fracchioli S, Zhang Y, Massobrio M, Risch H, Jones B, Yu H. Glutathione S-transferase polymorphisms and ovarian cancer treatment and survival. Gynecol Oncol. 2006;100:330-337. [PubMed] [DOI] |
| 24. | Sobti RC, Kaur S, Kaur P, Singh J, Gupta I, Jain V, Nakahara A. Interaction of passive smoking with GST (GSTM1, GSTT1, and GSTP1) genotypes in the risk of cervical cancer in India. Cancer Genet Cytogenet. 2006;166:117-123. [PubMed] [DOI] |