修回日期: 2010-06-13
接受日期: 2010-06-22
在线出版日期: 2010-07-28
目的: 探讨奥沙利铂和肿瘤坏死因子相关凋亡诱导配体(TRAIL)对胃癌BGC823细胞联合作用的机制.
方法: 不同浓度的TRAIL和/或奥沙利铂作用于人胃癌细胞系BGC823细胞, MTT检测细胞增殖能力. 流式细胞术PI染色检测细胞凋亡. TRAIL和/或奥沙利铂作用BGC823细胞后, 用抗CTX-B, 抗死亡受体4(DR4)和罗丹明标记的荧光二抗染色. 免疫荧光显微技术检测脂筏和DR4在细胞膜的分布.
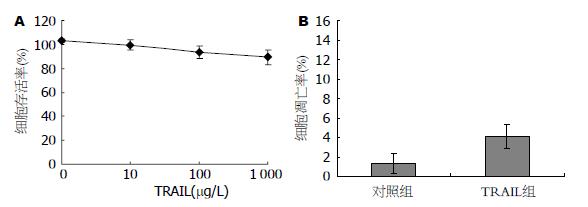
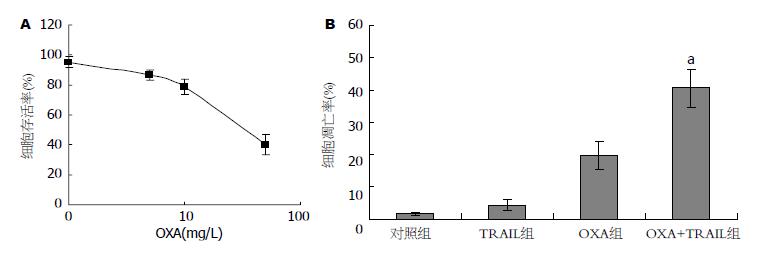
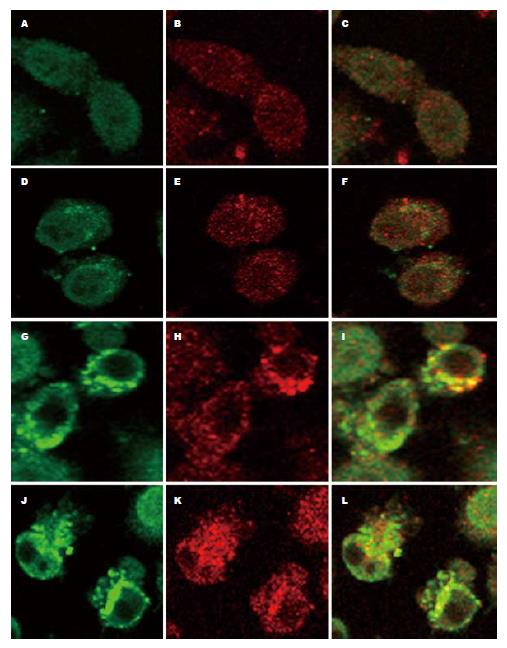
结果: 1-1 000 µg/L的TRAIL作用BGC823细胞24 h, 细胞增殖抑制率不超过20%. 100 µg/L的TRAIL对细胞的增殖抑制率在10%左右. 100 µg/L的TRAIL作用BGC823细胞24 h, 诱导4.12%±1.26%的细胞凋亡. 1-50 mg/L奥沙利铂处理BGC823细胞24 h, 抑制细胞增殖50%的药物浓度(IC50)为37.36 mg/L±8.12 mg/L. 与单药奥沙利铂相比, 奥沙利铂(38 mg/L, 24 h的IC50)联合TRAIL(100 µg/L)作用24 h, 细胞凋亡比率明显提高(19.83%±4.21% vs 40.42%±5.78%, P<0.05). 与对照组相比, 100 µg/L的TRAIL作用BGC823细胞24 h, 没有引起明显的脂筏聚集或DR4聚集. 奥沙利铂(38 mg/L)明显促进脂筏聚集和DR4聚集, 同时观察到DR4和脂筏的共定位. 奥沙利铂和TRAIL联合作用24 h, 同样观察到DR4定位在聚集的脂筏内.
结论: 奥沙利铂通过促进DR4在脂筏聚集增强TRAIL诱导的胃癌BGC823细胞凋亡.
引文著录: 徐玲, 刘静, 刘云鹏, 曲秀娟, 张晔, 侯科佐, 姜又红. 奥沙利铂对TRAIL诱导胃癌BGC823细胞凋亡的增强作用. 世界华人消化杂志 2010; 18(21): 2187-2191
Revised: June 13, 2010
Accepted: June 22, 2010
Published online: July 28, 2010
AIM: To determine whether there is a synergistic effect between oxaliplatin and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in inducing the apoptosis of gastric cancer BGC823 cells.
METHODS: After BGC823 cells were cultured and treated with TRAIL and/or oxaliplatin, cell proliferation was measured using MTT assay; cell apoptosis was determined by flow cytometry after propidium iodide staining; and the distribution of lipid rafts and death receptors 4 (DR4) on cell membrane was analyzed by immunofluorescence staining with anti-cholera toxin B subunit, anti-DR4 antibody and rhodamine-conjugated fluorescent secondary antibody.
RESULTS: After BGC823 cells were treated with 1-1 000 µg/L TRAIL for 24 h, the reduced rates of cell proliferation did not exceed 20%. Treatment with 100 µg/L TRAIL for 24 h induced about 10% inhibition of cell proliferation and 4.12% ± 1.26% cell apoptosis. After BGC823 cells were treated with 1-50 mg/L oxaliplatin for 24 h, it was found that the half maximal inhibitory concentration (IC50) was 37.36 mg/L ± 8.12 mg/L. Treatment with oxaliplatin (38 mg/L, IC50 dose) plus TRAIL resulted in a dramatic increase in cell apoptosis when compared to treatment with TRAIL alone (19.83% ± 4.21% vs 40.42% ± 5.78%, P < 0.05). TRAIL at a concentration of 100 µg/L did not induce obvious lipid raft aggregation or DR4 clustering. Oxaliplatin (38 mg/L) significantly promoted lipid raft aggregation and DR4 clustering and induced the co-localization of DR4 and lipid rafts. Treatment with oxaliplatin and TRAIL for 24 h also induced DR4 clustering into aggregated lipid rafts.
CONCLUSION: Oxaliplatin enhances TRAIL-induced BGC823 cell apoptosis by clustering DR4 into lipid rafts.
- Citation: Xu L, Liu J, Liu YP, Qu XJ, Zhang Y, Hou KZ, Jiang YH. Oxaliplatin enhances TRAIL-induced apoptosis of gastric cancer BGC823 cells. Shijie Huaren Xiaohua Zazhi 2010; 18(21): 2187-2191
- URL: https://www.wjgnet.com/1009-3079/full/v18/i21/2187.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v18.i21.2187
胃癌发病率和死亡率居我国消化系统恶性肿瘤之首, 多数患者在诊断时已经是晚期, 5年生存率只有10%-15%[1]. 尽管化疗药物的应用使晚期患者的生存期有所提高, 但中位总生存时间很难超过12 mo[2]. 肿瘤坏死因子相关凋亡诱导配体(tumor necrosis factor-related apoptosis-inducing ligand, TRAIL)是肿瘤坏死因子家族的成员之一. 在体外研究中, TRAIL诱导多种肿瘤细胞凋亡, 对正常细胞毒性很小[3]. 但是, 胃癌细胞对TRAIL相对不敏感[4-6]. 死亡受体4(death receptor 4, DR4)是TRAIL的激动型受体. TRAIL结合DR4后导致受体聚集, 并募集Fas相关蛋白死亡结构域(Fas-associated protein with death domain, FADD)和前caspase-8, 共同形成死亡诱导信号复合物(death-inducing signaling complex, DISC), DISC的形成促发下游效应物caspase活化, 从而诱导凋亡[7,8]. 最近的研究表明, 脂筏在启动死亡信号时发挥了重要作用, 脂筏为DR4的聚集提供了重要的膜平台[9,10]. Lacour等[11]报道, 化疗药顺铂能促进死亡受体CD95在脂筏内聚集, 增强CD95配体诱导的结肠癌细胞凋亡. 奥沙利铂作为第3代含铂药物, 在胃癌治疗中已经取得一定疗效[12,13]. 为提高胃癌对TRAIL的敏感性, 本研究将奥沙利铂和TRAIL联合作用胃癌BGC823细胞, 并探讨联合用药的机制.
重组人TRAIL购于美国Cytolab/Peprotech Asia公司. 奥沙利铂购自Sanofi-Aventis公司. RPMI 1640培养基购于Gibco公司. 胎牛血清购于天津血液病研究所. RNase A购于Amresco公司. 碘化丙啶(PI)、甲基偶氮唑蓝(MTT)和FITC-结合的霍乱弧菌来源的霍乱毒素B亚单位购于Sigma公司. 罗丹明结合的羊抗鼠IgG(H+L)和DR4的鼠单克隆抗体来自Santa Cruz公司.
1.2.1 细胞培养: 胃癌细胞株BGC823为本实验室常规传代培养, 生长于含有10%灭活胎牛血清、12 U/mL庆大霉素的RPMI 1640培养液中, 于37 ℃、50 mL/L CO2、饱和湿度的培养箱内培养, 0.25%胰酶消化液消化传代, 2-3 d传代1次.
1.2.2 MTT法进行细胞活力检测: 取对数生长期细胞接种于96孔板, 培养12 h后分别加入既定浓度的奥沙利铂和TRAIL, 每组设3个复孔, 每孔终体积为200 mL. 培养20 h后每孔加入MTT溶液(5 g/L)25 µL, 继续孵育4 h后吸去上清, 每孔加入200 µL DMSO, 振荡摇匀. 用酶标仪于570 nm波长条件下测定吸光度值.
1.2.3 细胞凋亡检测: 取对数生长期细胞接种于96孔板, 培养12 h后分别加入既定浓度的奥沙利铂和TRAIL. 24 h后分别收集对照组及处理组细胞, 750 mL/L乙醇固定4 h后, 加入10 µL的PI(20 mg/L), 避光孵育15 min, 用FACScan流式细胞仪进行检测, WinMDI软件进行分析.
1.2.4 免疫荧光显微技术: 取对数生长期细胞接种在Lab-Tek载波片. 在常温条件下, 3.3%多聚甲醛固定20 min, 再用0.2% Triton X-100透化处理3 min, 此后用5%牛血清白蛋白封闭30 min. 细胞用兔抗人FITC-结合的霍乱毒素B亚单位, 鼠抗人DR4处理1 h, 之后用罗丹明标记的羊抗鼠IgG(H+L)的荧光二抗处理45 min. 此后用固定液封闭, 共聚焦荧光显微镜下观察分析(FV1000S-SIM/IX81, Olympus).
统计学处理 所有数据均为3次独立实验结果, 以mean±SD表示. 采用SPSS13.0统计软件进行统计学分析. 两组之间比较采用t检验, P<0.05有统计学意义.
1-1 000 µg/L的TRAIL作用BGC823细胞24 h, 细胞增殖抑制率不超过20%. 100 µg/L的TRAIL对细胞的增殖抑制率在10%左右. TRAIL的浓度增加到1 000 µg/L, 并没能引起更明显的增殖抑制作用(图1A). 流式细胞仪分析显示, 100 µg/L的TRAIL作用BGC823细胞24 h, 诱导4.12%±1.26%的细胞凋亡(图1B). 这些结果表明, 胃癌BGC823细胞对TRAIL耐药.
1-50 mg/L奥沙利铂处理BGC823细胞24 h, MTT结果显示奥沙利铂以剂量依赖性方式抑制BGC823细胞增殖. 24 h抑制细胞增殖50%的药物浓度(IC50)为37.36 mg/L±8.12 mg/L(图2A). 奥沙利铂(38 mg/L, 24 h的IC50)作用BGC823细胞24 h, 细胞凋亡率为19.83%±4.21%. 奥沙利铂(38 mg/L)联合TRAIL(100 µg/L)作用24 h, 凋亡比率提高到40.42%±5.78%(图2B). 联合组和单药组相比有统计学差异(P<0.05). 这些结果表明, 奥沙利铂能提高胃癌BGC823细胞对TRAIL的敏感性.
与对照组相比, 100 µg/L的TRAIL作用BGC823细胞24 h, 没有引起明显的脂筏聚集或DR4聚集. 奥沙利铂(38 mg/L)明显促进脂筏聚集和DR4聚集, 同时观察到DR4和脂筏的共定位. 奥沙利铂和TRAIL联合作用24 h, 同样观察到DR4定位在聚集的脂筏内(图3). 这些结果表明, 奥沙利铂通过诱导胃癌细胞脂筏聚集促进了DR4的募集.
最近的研究表明, Jurket细胞对TRAIL敏感. 用100 µg/L的TRAIL处理Jurket细胞6 h, 凋亡细胞的比率超过50%[14]. 但胃癌细胞对TRAIL不敏感. Yang等[15]报道, 用300 µg/L的TRAIL处理胃癌SGC7901细胞24 h, 只引起11.8%的细胞凋亡. 在我们的研究中, 100 µg/L的TRAIL处理BGC823细胞24 h, 细胞凋亡不超过6%. 因此, 胃癌BGC823细胞对TRAIL不敏感. 有研究显示, 奥沙利铂增强了TRAIL诱导的结肠癌细胞凋亡, 特别是当细胞对TRAIL高度耐药[16,17]. 在我们的研究中, 奥沙利铂明显增强了TRAIL诱导的胃癌BGC823细胞凋亡.
近几年来的研究发现多种化疗药如顺铂, 紫杉醇和表柔比星都能和TRAIL协同杀伤肿瘤细胞[18-21]. 对化疗药和TRAIL的协同作用机制也在不断深入探讨. 最新研究表明, 脂筏参与了肿瘤坏死因子家族成员发挥生物学功能, 干扰脂筏形成则可抑制FasL诱导的细胞凋亡[22]. 有研究证实, 某些药物可以通过调控脂筏的聚集程度影响肿瘤细胞对肿瘤坏死因子家族成员的敏感性. Gajate等[23]报道, 依地福新和哌立福新通过促进CD95聚集在多发骨髓瘤细胞的脂筏中, 增强了细胞对CD95配体的敏感性. Psahoulia等[24]证实, 槲皮素也能促进DR4和DR5在结肠癌细胞脂筏内的聚集, 从而增强细胞对TRAIL的敏感性. 我们的结果表明, TRAIL单药没有引起明显的脂筏聚集和DR4聚集, 这可能是胃癌对TRAIL不敏感的原因之一. 我们的结果还显示, 奥沙利铂促进胃癌细胞DR4的聚集和脂筏的募集, 并且聚集的DR4和脂筏存在共定位. 这表明奥沙利铂通过促进DR4在脂筏聚集, 增强TRAIL和DR4的结合, 放大了凋亡信号通路, 诱导更多的胃癌细胞凋亡.
生物与靶向治疗是胃癌今后发展的方向, 如何提高靶向治疗的特异性和敏感性是本领域的重要科学问题. 奥沙利铂通过对脂筏的调控逆转了胃癌细胞对TRAIL的耐药, 脂筏有望成为肿瘤治疗的理想靶标. 本研究对明确胃癌细胞对TRAIL耐药的机制, 提高胃癌对TRAIL的敏感性具有重要的科学意义和潜在的临床价值.
感谢中国医科大学实验技术中心高建对本实验的指导和帮助.
TRAIL是肿瘤坏死因子家族的成员之一, 化疗药可增强肿瘤细胞对TRAIL诱导凋亡的敏感性. 本文探讨了奥沙利铂对TRAIL诱导胃癌细胞凋亡的影响及机制.
张俊, 副教授, 上海交通大学医学院附属瑞金医院外科.
胃癌对TRAIL耐药原因目前尚不明确, 需要深入探讨机制, 提高胃癌对TRAIL的敏感性.
Psahoulia等于2007年报道, 槲皮素通过促进DR4在结肠癌细胞脂筏内的聚集, 增强细胞对TRAIL的敏感性. 奥沙利铂在胃癌治疗中已经取得一定疗效.
本文将奥沙利铂和TRAIL联合作用胃癌细胞, 证实了奥沙利铂通过对脂筏的调控, 逆转了胃癌细胞对TRAIL的耐药.
生物治疗是胃癌今后发展的方向, 如何提高治疗的敏感性是本领域的重要问题. 本研究明确了脂筏在胃癌细胞对TRAIL耐药中的重要作用, 对提高胃癌对TRAIL的敏感性具有重要的科学意义和潜在的临床价值.
本文选题先进, 设计合理, 科学性强, 结果可靠, 学术价值较好.
编辑: 李军亮 电编:吴鹏朕
| 1. | Tsai JY, Safran H. Status of treatment for advanced gastric carcinoma. Curr Oncol Rep. 2003;5:210-218. [PubMed] [DOI] |
| 2. | Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903-2909. [PubMed] [DOI] |
| 3. | Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155-162. [PubMed] [DOI] |
| 4. | Nam SY, Jung GA, Hur GC, Chung HY, Kim WH, Seol DW, Lee BL. Upregulation of FLIP(S) by Akt, a possible inhibition mechanism of TRAIL-induced apoptosis in human gastric cancers. Cancer Sci. 2003;94:1066-1073. [PubMed] [DOI] |
| 5. | Park WS, Lee JH, Shin MS, Park JY, Kim HS, Kim YS, Park CH, Lee SK, Lee SH, Lee SN. Inactivating mutations of KILLER/DR5 gene in gastric cancers. Gastroenterology. 2001;121:1219-1225. [PubMed] [DOI] |
| 6. | Sheikh MS, Huang Y, Fernandez-Salas EA, El-Deiry WS, Friess H, Amundson S, Yin J, Meltzer SJ, Holbrook NJ, Fornace AJ Jr. The antiapoptotic decoy receptor TRID/TRAIL-R3 is a p53-regulated DNA damage-inducible gene that is overexpressed in primary tumors of the gastrointestinal tract. Oncogene. 1999;18:4153-4159. [PubMed] [DOI] |
| 7. | Falschlehner C, Emmerich CH, Gerlach B, Walczak H. TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol. 2007;39:1462-1475. [PubMed] [DOI] |
| 8. | Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26-35. [PubMed] [DOI] |
| 9. | Muppidi JR, Siegel RM. Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death. Nat Immunol. 2004;5:182-189. [PubMed] [DOI] |
| 10. | Scheel-Toellner D, Wang K, Singh R, Majeed S, Raza K, Curnow SJ, Salmon M, Lord JM. The death-inducing signalling complex is recruited to lipid rafts in Fas-induced apoptosis. Biochem Biophys Res Commun. 2002;297:876-879. [PubMed] [DOI] |
| 11. | Lacour S, Hammann A, Grazide S, Lagadic-Gossmann D, Athias A, Sergent O, Laurent G, Gambert P, Solary E, Dimanche-Boitrel MT. Cisplatin-induced CD95 redistribution into membrane lipid rafts of HT29 human colon cancer cells. Cancer Res. 2004;64:3593-3598. [PubMed] [DOI] |
| 12. | Sumpter K, Harper-Wynne C, Cunningham D, Rao S, Tebbutt N, Norman AR, Ward C, Iveson T, Nicolson M, Hickish T. Report of two protocol planned interim analyses in a randomised multicentre phase III study comparing capecitabine with fluorouracil and oxaliplatin with cisplatin in patients with advanced oesophagogastric cancer receiving ECF. Br J Cancer. 2005;92:1976-1983. [PubMed] [DOI] |
| 13. | Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2010;362:858-859. [PubMed] [DOI] |
| 14. | Weng C, Li Y, Xu D, Shi Y, Tang H. Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Jurkat leukemia T cells. J Biol Chem. 2005;280:10491-10500. [PubMed] [DOI] |
| 15. | Yang LQ, Fang DC, Wang RQ, Yang SM. Effect of NF-kappaB, survivin, Bcl-2 and Caspase3 on apoptosis of gastric cancer cells induced by tumor necrosis factor related apoptosis inducing ligand. World J Gastroenterol. 2004;10:22-25. [PubMed] |
| 16. | Toscano F, Fajoui ZE, Gay F, Lalaoui N, Parmentier B, Chayvialle JA, Scoazec JY, Micheau O, Abello J, Saurin JC. P53-mediated upregulation of DcR1 impairs oxaliplatin/TRAIL-induced synergistic anti-tumour potential in colon cancer cells. Oncogene. 2008;27:4161-4171. [PubMed] [DOI] |
| 17. | Galligan L, Longley DB, McEwan M, Wilson TR, McLaughlin K, Johnston PG. Chemotherapy and TRAIL-mediated colon cancer cell death: the roles of p53, TRAIL receptors, and c-FLIP. Mol Cancer Ther. 2005;4:2026-2036. [PubMed] [DOI] |
| 18. | Tsai WS, Yeow WS, Chua A, Reddy RM, Nguyen DM, Schrump DS, Nguyen DM. Enhancement of Apo2L/TRAIL-mediated cytotoxicity in esophageal cancer cells by cisplatin. Mol Cancer Ther. 2006;5:2977-2990. [PubMed] [DOI] |
| 19. | Asakuma J, Sumitomo M, Asano T, Asano T, Hayakawa M. Selective Akt inactivation and tumor necrosis actor-related apoptosis-inducing ligand sensitization of renal cancer cells by low concentrations of paclitaxel. Cancer Res. 2003;63:1365-1370. [PubMed] |
| 20. | Wu XX, Jin XH, Zeng Y, El Hamed AM, Kakehi Y. Low concentrations of doxorubicin sensitizes human solid cancer cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-receptor (R) 2-mediated apoptosis by inducing TRAIL-R2 expression. Cancer Sci. 2007;98:1969-1976. [PubMed] [DOI] |
| 21. | Woods DC, Alvarez C, Johnson AL. Cisplatin-mediated sensitivity to TRAIL-induced cell death in human granulosa tumor cells. Gynecol Oncol. 2008;108:632-640. [PubMed] [DOI] |
| 22. | Moretti S, Procopio A, Lazzarini R, Rippo MR, Testa R, Marra M, Tamagnone L, Catalano A. Semaphorin3A signaling controls Fas (CD95)-mediated apoptosis by promoting Fas translocation into lipid rafts. Blood. 2008;111:2290-2299. [PubMed] [DOI] |
| 23. | Gajate C, Mollinedo F. Edelfosine and perifosine induce selective apoptosis in multiple myeloma by recruitment of death receptors and downstream signaling molecules into lipid rafts. Blood. 2007;109:711-719. [PubMed] [DOI] |
| 24. | Psahoulia FH, Drosopoulos KG, Doubravska L, Andera L, Pintzas A. Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Mol Cancer Ther. 2007;6:2591-2599. [PubMed] [DOI] |