修回日期: 2010-06-10
接受日期: 2010-06-22
在线出版日期: 2010-07-18
目的: 分析8例肝胆恶性肿瘤皮肤转移的临床特点及治疗效果.
方法: 回顾分析我院2006-06/2009-06肝细胞癌2例、混合型肝细胞癌和胆管细胞癌1例及胆管癌5例皮肤转移的临床表现和治疗情况及预后.
结果: 1例肝细胞癌胆管癌栓并黄疸, 经皮经肝胆道引流后20 mo皮肤穿刺点种植性转移, 皮肤肿瘤切除后已经存活16 mo无复发; 1例肝细胞癌剖腹探查后9 mo切口皮下发现肿瘤,行肝脏左叶切除和切口肿瘤切除后存活8 mo; 1例混合型肝细胞癌和胆管细胞癌手术切除后4 mo多发皮肤转移结节病理检查为胆管细胞癌,化疗后存活3 mo; 2例胆管癌皮肤单发转移,切除后存后8 mo和10 mo; 3例胆管癌皮肤多发转移患者1例接受化疗存活6 mo, 2例放弃治疗,分别存活3 mo和4 mo.
结论: 为尽量避免肝胆肿瘤种植转移应改进穿刺和切口保护技术; 肝胆肿瘤单发皮肤转移可手术治疗并有较好效果, 多发皮肤转移应综合治疗, 提高生活质量.
引文著录: 刘博, 黄晓强, 王敬, 董家鸿, 黄志强. 肝胆恶性肿瘤皮肤转移8例. 世界华人消化杂志 2010; 18(20): 2166-2170
Revised: June 10, 2010
Accepted: June 22, 2010
Published online: July 18, 2010
AIM: To analyze the clinical characteristics and therapeutic outcomes of 8 cases of hepatobiliary malignancies with cutaneous metastases.
METHODS: From June 2006 to June 2009, eight patients with cutaneous metastases from hepatobiliary malignancies were treated at our hospital. By retrospectively reviewing the clinical data for these patients, the clinical characteristics, treatment and prognosis of this disease were summarized.
RESULTS: One patient with hepatocellular carcinoma (HCC) presenting as obstructive jaundice caused by bile duct tumor thrombi developed cutaneous metastasis at the port site 20 mo after percutaneous transhepatic biliary drainage (PTBD). The patient had survived 16 mo after resection of the port-site tumor and showed no recurrence. One HCC patient developed tumor at the incision site 9 mo after abdominal exploration. After the skin and liver tumors were excised, the patients survived 8 mo. One patient with combined HCC and cholangiocellular carcinoma developed multiple cutaneous metastases of cholangiocellular carcinoma and survived 8 mo. Two patients with cholangiocarcinoma and solitary nodular cutaneous metastasis received skin tumor excision and survived 8 and 10 mo, respectively. Of three patients with cholangiocarcinoma and multiple cutaneous metastases, one received chemotherapy and survived 6 mo, and the other 2 refused any treatment and survived 3 and 4 mo, respectively.
CONCLUSION: Puncture and operation procedures should be improved to avoid seeding metastasis. In patients with hepatobiliary malignancies, solitary nodular cutaneous metastasis has a better prognosis, while multiple cutaneous metastases have a worse prognosis.
- Citation: Liu B, Huang XQ, Wang J, Dong JH, Huang ZQ. Hepatobiliary malignancies with cutaneous metastases: an analysis of 8 cases. Shijie Huaren Xiaohua Zazhi 2010; 18(20): 2166-2170
- URL: https://www.wjgnet.com/1009-3079/full/v18/i20/2166.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v18.i20.2166
肝胆恶性肿瘤皮肤转移临床少见. 中国人民解放军总医院肝胆外科2006-06/2009-06收治肝胆恶性肿瘤皮肤转移患者8例, 我们对其临床资料和预后进行了分析.
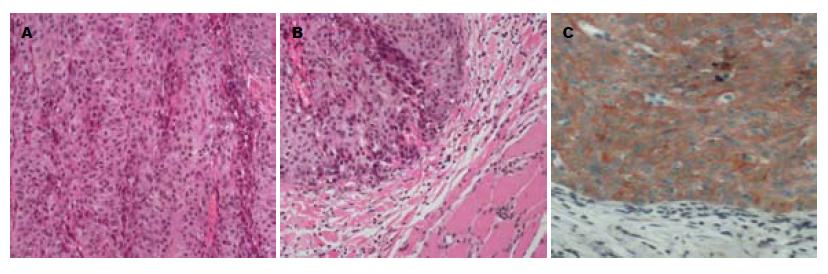
8例肝胆恶性肿瘤皮肤转移患者均为男性, 年龄34-73(平均59)岁. 原发肝胆肿瘤病理检查2例为肝细胞癌(hepatocellular carcinoma, HCC), 1例混合型HCC和胆管细胞癌, 5例胆管癌. HCC皮肤转移组织行Hepa-1染色, 胆管癌皮肤转移组织行特异性 CK19染色证实其来源.
每3 mo对所有8例肝胆恶性肿瘤皮肤转移患者进行定期随访, 详细记录患者存活情况和死亡时间.
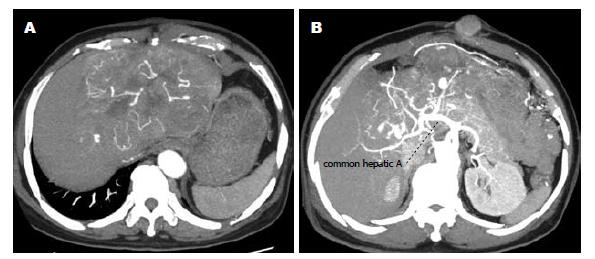
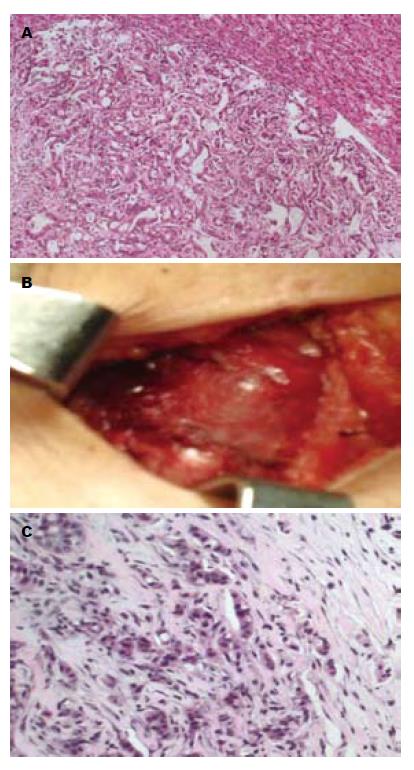
1例HCC胆管癌栓合并梗阻性黄疸, 等待肝移植期间行经皮经肝胆道引流(percutaneous transhepatic biliary drainage, PTBD), 术后20 mo皮肤穿刺点发现类圆形结节1 cm×1 cm(图1), 皮肤肿瘤切除后已经存活16 mo, 无复发; 1例HCC在当地医院剖腹探查因出血仅取病理9 mo后发现切口皮下肿瘤2.5 cm×2.5 cm(图2), 行肝脏左叶切除和切口肿瘤切除后存活8 mo; 1例为混合型HCC和胆管细胞癌, 行右后叶肝脏切除后4 mo发现多发皮肤转移, 皮肤结节细针穿刺病理检查为胆管细胞癌, 化疗后存活3 mo; 1例肝门部胆管癌行根治切除手术后2 mo发现双肺、头皮和上肢皮肤多发转移, 放弃治疗存活3 mo; 1例肝门部胆管癌就诊时发现颈部单发皮肤肿瘤2 cm×2 cm, 手术切除后存活10 mo; 1例肝门部胆管癌就诊时发现右大腿单发皮肤肿瘤3 cm×2 cm(图3), 行肝门部肿瘤放疗和皮肤转移瘤切除后存活8 mo; 1例远端肝外胆管癌就诊时CT检查发现腹壁多发皮肤肿瘤, 胆肠吻合及化疗后存活6 mo; 1例远端肝外胆管癌手术后6 mo出现肺转移和多发皮肤转移, 放弃治疗存活4 mo. 8例患者临床资料详见表1.
| 患者 | 性别 | 年龄(岁) | 原发灶治疗方式 | 病理类型 | 皮肤转移部位(单、多发) | 皮肤转移治疗方式 | t1 | t2 |
| 1 | 男 | 59 | 肝移植 | 中低分化HCC | PTBD穿刺点 | 手术切除 | 20 | >16 |
| 2 | 男 | 68 | 左叶肝脏切除 | 中分化HCC | 腹壁切口 | 手术切除 | 9 | 8 |
| 3 | 男 | 44 | 右后叶肝脏切除 | 混合型HCC胆管细胞癌 | 头皮、右下肢 | 化疗 | 4 | 3 |
| 4 | 男 | 65 | 肝门胆管癌根治 | 中分化胆管癌 | 头皮、左上肢 | 放弃 | 2 | 3 |
| 5 | 男 | 34 | 肝门胆管癌根治 | 低分化胆管癌 | 颈部, 单发 | 手术切除 | 0 | 10 |
| 6 | 男 | 73 | 肝门胆管癌活检、放疗 | 中分化胆管癌 | 右大腿, 单发 | 手术切除 0 | 8 | |
| 7 | 男 | 59 | 胆肠吻合 | 中低分化胆管癌 | 腹壁, 多发 | 化疗 | 0 | 6 |
| 8 | 男 | 67 | 胆管癌根治 | 中分化胆管癌 | 胸壁、腹壁 | 放弃 | 6 | 4 |
肿瘤细胞可以经过血液等自然途径转移到皮肤, 也可以经穿刺孔道和切口发生脱落细胞种植转移, 即医源性转移. 自然途径的皮肤转移是肿瘤的晚期表现[1-3], Krathen等[4]总结20 380例内脏恶性肿瘤发生皮肤转移的比例为5.3%, 多数生存期不足12 mo. Hu等[5]统计12 146例恶性肿瘤患者发生皮肤转移124例, 占1.02%. 其中1189例HCC有4例发生皮肤转移, 仅占0.34%, 说明在自然转移途径下肝癌发生皮肤转移并不常见[6-10]. 但随着肝癌有创检查和治疗的增加, 医源性的肝癌皮肤种植转移病例明显增多[11-16].Stigliano等[11]总结1983-2007年99篇文献报道179例HCC种植转移病例, 多为活检、无水酒精注射和射频消融后种植转移, 发生率分别为2.29%(0%-11%)、1.4%(1.15%-1.85%)和0.61%(0%-5.56%). 本组1例肝癌接受穿刺胆道引流后20 mo穿刺点发现皮肤种植转移, 文献报道多见胆管癌、胰腺癌、十二指肠癌和胆囊癌PTBD后穿刺种植[17-20], HCC经PTBD穿刺点种植转移实属罕见, 检索文献未见报道. 为了减少穿刺和引流引起种植转移的发生, 在肝胆肿瘤诊断治疗中要严格掌握有创检查治疗指征, 改良穿刺技术. 应尽量采取超声造影、强化CT和核磁共振等非侵袭性的诊断技术, 尽量避免穿刺活检, 穿刺进行PTBD和微波射频等治疗时应改进穿刺技术, 建议使用套管, 拔出穿刺针和引流管后局部皮肤和皮下应该更仔细地擦拭和消毒, 减少肿瘤细胞的穿刺口残留. Liu等[21]认为腹腔镜下或开腹活检和穿刺治疗肝癌可以明显减少种植转移的机会. 另1例肝癌患者手术后9 mo发现切口种植. 为减少切口种植, 肝癌切除术中应严格无瘤操作. 在手术过程中常易发生肝癌组织破裂细胞脱落, 成为种植转移的潜在危险. 使用切口保护膜可以有效保护切口, 术后蒸馏水冲洗腹腔对术后种植转移的发生有一定的预防作用. 临床医生在对肿瘤患者随访时应注意穿刺点和手术切口的检查, 尽早发现可能的皮肤种植肿瘤. 第1例患者腹壁穿刺点种植转移灶切除后存活已经超过16 mo, 局部无复发, 第2例切口种植患者手术后存活8 mo, 局部无复发, 死于肺转移. 说明种植转移及时发现仍可取得较好治疗效果.
1例混合HCC和胆管细胞癌与5例胆管癌为通过血液等自然途径发生皮肤转移, 说明胆管癌的浸润和转移具有其自身的特点, 胆管癌比HCC更易发生自然途径的皮肤转移, 这与Hu等[5]按照病理类型分析腺癌皮肤转移率最高占1.46%, HCC为0.34%, 两者相差4倍的特点相符. Singal等[22]统计136例胆管癌平均生存期27.3 mo; 本组6例自然途径转移的胆管癌自确诊到发现皮肤转移的时间为0-6 mo. 平均生存时间5.7 mo, 与文献报道平均生存7.5 mo基本一致[3-5], 证实胆管癌皮肤转移后生存期明显缩短[23-25]. 其中2例以胆管癌原发病临床就诊时即发现皮肤转移, 文献更有以皮肤转移结节和溃疡为胆管癌首发症状的报道[26], 提示我们在胆管癌患者就诊和随访时注意皮肤检查, 尽可能早地发现皮肤转移, 争取更早地治疗. 本组2例胆管癌皮肤单发转移患者, 手术切除后分别生存8 mo和10 mo; 而4例皮肤多发转移胆管癌患者有3例合并肺部转移失去手术机会, 接受化疗2例并未显现明显效果, 生存期均不超过6 mo, 胆管癌多发皮肤转移治疗更加困难, 预后更差.
如何更早地发现肝癌和胆管癌经自然途径的皮肤转移和预防穿刺手术引起的种植性皮肤转移, 进一步提高患者生存期和生存质量, 值得更深入研究.
恶性肿瘤皮肤转移可见于血液或淋巴管途径的自然转移, 也可以见于穿刺或手术后的种植转移. 自然途径的皮肤转移是肿瘤的晚期表现, 皮肤种植转移是肿瘤有创诊断和治疗的严重迟发并发症.
孙殿兴, 主任医师, 白求恩国际和平医院肝病科; 吴泰璜, 教授, 山东省立医院肝胆外科
肝胆恶性肿瘤皮肤转移的生物学和病理学行为特点值得深入研究.
Krathen等总结20 380例内脏恶性肿瘤发生皮肤转移的比例为5.3%. Hu等统计1986-2006年20年间12 146例恶性肿瘤患者发生皮肤转移124例, 占1.02%. 常见皮肤转移前五位肿瘤是乳腺癌、肺癌、口腔癌、大肠癌和胃癌. 按照病理类型分析腺癌皮肤转移率最高占1.46%, 鳞癌为0.69%, 肝细胞癌为0.34%, 移形细胞癌为0.23%.
本文分析了8例肝胆恶性肿瘤皮肤转移的临床资料, 并对国内外相关报道进行了较详细的综合归纳, 对指导临床有一定的意义.
编辑: 李军亮 电编:何基才
| 1. | Nashan D, Müller ML, Braun-Falco M, Reichenberger S, Szeimies RM, Bruckner-Tuderman L. Cutaneous metastases of visceral tumours: a review. J Cancer Res Clin Oncol. 2009;135:1-14. [PubMed] [DOI] |
| 2. | Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. [PubMed] [DOI] |
| 3. | Kleyn CE, Lai-Cheong JE, Bell HK. Cutaneous manifestations of internal malignancy: diagnosis and management. Am J Clin Dermatol. 2006;7:71-84. [PubMed] [DOI] |
| 4. | Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164-167. [PubMed] [DOI] |
| 5. | Hu SC, Chen GS, Lu YW, Wu CS, Lan CC. Cutaneous metastases from different internal malignancies: a clinical and prognostic appraisal. J Eur Acad Dermatol Venereol. 2008;22:735-740. [PubMed] [DOI] |
| 6. | Magaña M, Gomez LM. Skin metastasis from hepatocarcinoma. Am J Dermatopathol. 2009;31:502-505. [PubMed] [DOI] |
| 7. | Al-Mashat FM. Hepatocellular carcinoma with cutaneous metastasis. Saudi Med J. 2004;25:370-372. [PubMed] |
| 8. | Royer MC, Rush WL, Lupton GP. Hepatocellular carcinoma presenting as a precocious cutaneous metastasis. Am J Dermatopathol. 2008;30:77-80. [PubMed] [DOI] |
| 9. | Amador A, Monforte NG, Bejarano N, Martí J, Artigau E, Navarro S, Fuster J. Cutaneous metastasis from hepatocellular carcinoma as the first clinical sign. J Hepatobiliary Pancreat Surg. 2007;14:328-330. [PubMed] [DOI] |
| 10. | Nggada HA, Ajayi NA. Cutaneous metastasis from hepatocellular carcinoma: a rare presentation and review of the literature. Afr J Med Med Sci. 2006;35:181-182. [PubMed] |
| 11. | Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007;33:437-447. [PubMed] [DOI] |
| 12. | Perkins JD. Seeding risk following percutaneous approach to hepatocellular carcinoma. Liver Transpl. 2007;13:1603. [PubMed] [DOI] |
| 13. | Tung WC, Huang YJ, Leung SW, Kuo FY, Tung HD, Wang JH, Hung CH, Lee CM, Changchien CS, Yeh SA. Incidence of needle tract seeding and responses of soft tissue metastasis by hepatocellular carcinoma postradiotherapy. Liver Int. 2007;27:192-200. [PubMed] [DOI] |
| 14. | Chang S, Kim SH, Lim HK, Kim SH, Lee WJ, Choi D, Kim YS, Rhim H. Needle tract implantation after percutaneous interventional procedures in hepatocellular carcinomas: lessons learned from a 10-year experience. Korean J Radiol. 2008;9:268-274. [PubMed] [DOI] |
| 15. | Onodera H, Oikawa M, Abe M, Chida N, Kimura S, Satake K, Motojima T, Goto Y. Cutaneous seeding of hepatocellular carcinoma after fine-needle aspiration biopsy. J Ultrasound Med. 1987;6:273-275. [PubMed] |
| 16. | Koffi E, Moutardier V, Sauvanet A, Noun R, Fléjou JF, Belghiti J. Wound recurrence after resection of hepatocellular carcinoma. Liver Transpl Surg. 1996;2:301-303. [PubMed] [DOI] |
| 17. | Bloom RA, Gordon RL, Manny Y, Engelberg M. Seeding of cholangiocarcinoma along T-tube tracts. Gastrointest Radiol. 1984;9:167-169. [PubMed] [DOI] |
| 18. | Balzani A, Clerico R, Schwartz RA, Panetta S, Panetta C, Skroza N, Innocenzi D, Calvieri S. Cutaneous implantation metastasis of cholangiocarcinoma after percutaneous transhepatic biliary drainage. Acta Dermatovenerol Croat. 2005;13:118-121. [PubMed] |
| 19. | St Peter SD, Nguyen CC, Mulligan DC, Moss AA. Subcutaneous metastasis at a surgical drain site after the resection of pancreatic cancer. Int J Gastrointest Cancer. 2003;33:111-115. [PubMed] [DOI] |
| 20. | Yamakawa T, Itoh S, Hirosawa K, Miyoshi T, Katoh K, Iizumi S, Kawabata K. Seeding of gallbladder carcinoma along the tract after percutaneous transhepatic choledochoscopy. Am J Gastroenterol. 1983;78:649-651. [PubMed] |
| 21. | Liu SY, Lee KF, Lai PB. Needle track seeding: a real hazard after percutaneous radiofrequency ablation for colorectal liver metastasis. World J Gastroenterol. 2009;15:1653-1655. [PubMed] [DOI] |
| 22. | Singal AG, Rakoski MO, Salgia R, Pelletier S, Welling TH, Fontana RJ, Lok AS, Marrero JA. The clinical presentation and prognostic factors for intrahepatic and extrahepatic cholangiocarcinoma in a tertiary care centre. Aliment Pharmacol Ther. 2010;31:625-633. [PubMed] [DOI] |
| 23. | Lee BK, Seo YH, Lee NH, Joo SY, Ko HM. [Chol-angiocarcinoma with distant cutaneous metastases]. Korean J Gastroenterol. 2009;54:342-345. [PubMed] [DOI] |
| 24. | Lu CI, Wong WR, Hong HS. Distant cutaneous metastases of cholangiocarcinoma: report of two cases of a previously unreported condition. J Am Acad Dermatol. 2004;51:S108-S111. [PubMed] [DOI] |
| 25. | Yanagi T, Matsumura T, Yoshizaki N. Cholangio-carcinoma with skin metastases. J Am Acad Dermatol. 2007;56:S58-S60. [PubMed] [DOI] |
| 26. | Dogan G, Karincaoglu Y, Karincaoglu M, Aydin NE. Scalp ulcer as first sign of cholangiocarcinoma. Am J Clin Dermatol. 2006;7:387-389. [PubMed] [DOI] |