修回日期: 2010-04-29
接受日期: 2010-05-10
在线出版日期: 2010-06-18
目的: 探讨塞来昔布在慢性增生性胆管炎(chronic proliferative cholangitis, CPC)治疗中的应用价值.
方法: 健康♂SD大鼠30只, 随机分为3组: 假手术组(n = 10), CPC模型组(n = 10), 塞来昔布治疗组(n = 10). 模型组及治疗组经十二指肠乳头向胆总管逆行插入5-0尼龙缝合线, 直至肝门. 假手术组仅开腹后关腹. 术后第1天开始治疗组予塞来昔布50 mg/(kg·d)腹腔注射. 1 wk后处死动物. 通过HE染色、Masson染色、PAS染色及免疫组织化学评估塞来昔布能否对CPC中过度增殖的胆管黏膜上皮、黏膜下腺体、胆管壁胶原纤维产生抑制作用及其效果.
结果: 塞来昔布治疗组胆管黏膜上皮、黏膜下腺体、胆管壁胶原纤维的增殖较CPC模型组受到明显的抑制, 但略高于假手术组, 免疫组织化学示环氧化酶2(cyclooxygenase 2, COX-2)表达明显弱于CPC模型组(IA值: 8.62±0.19 vs 35.27±0.43, P<0.05), 与假手术组接近(IA值: 8.62±0.19 vs 8.41±0.13, P>0.05).
结论: 塞来昔布可通过对COX-2的表达抑制, 有效抑制胆管黏膜上皮、黏膜下腺体、管壁胶原纤维的过度增殖, 降低黏蛋白的高分泌, 从而有望控制CPC, 降低肝内胆管结石术后复发率.
引文著录: 陈文龙, 蒋力生, 李富宇. 塞来昔布在胆管炎中的抗增殖作用. 世界华人消化杂志 2010; 18(17): 1761-1766
Revised: April 29, 2010
Accepted: May 10, 2010
Published online: June 18, 2010
AIM: To investigate the application value of celecoxib in treating chronic proliferative cholangitis (CPC).
METHODS: Thirty healthy male Sprague-Dawley rats were randomly divided into three groups: sham-operation group (n = 10), CPC model group (n = 10), and celecoxib therapy group (n = 10). CPC was induced in rats by inserting a 5-0 nylon suture into the common bile duct up to the porta hepatis retrogradely through the vater papilla. Rats in the sham-operation group only underwent abdominal wall incision and suturing. Celecoxib [50 mg/(kg·d)] was injected into the abdominal cavity of each rat in the therapy group from day 1 after operation. All rats were executed 1 wk after operation. The anti-proliferation activity of celecoxib was evaluated by hematoxylin and eosin (HE) staining, periodic acid-Schiff (PAS) staining, Masson staining and immunohistochemistry staining of the biliary epithelial mucosa, submucosal gland and collagen fiber in the bile duct wall of CPC rats.
RESULTS: The proliferative degree of the biliary epithelial mucosa and submucosal gland as well as the fibrotic degree of the biliary wall in the celecoxib therapy group were obviously lower than those in the CPC group, but still higher than those in the sham-operation group. Immunohistochemistry analysis showed that the expression intensity of cyclooxygenase 2 (COX-2) in the celecoxib therapy group was obviously inferior to that in the CPC model group (IA: 8.62 ± 0.19 vs 35.27 ± 0.43, P < 0.05), but close to that in the sham-operation group (IA: 8.62 ± 0.19 vs 8.41 ± 0.13, P > 0.05).
CONCLUSION: By down-regulating COX-2 expression, celecoxib can effectively inhibit the hyperplasia of the biliary epithelial mucosa, submucosal gland, and collagen fiber and reduce the amount of mucous glycoprotein secreted by the submucosal gland, thus holding the promise for controlling CPC and reducing the recurrence of intrahepatic bile duct stones.
- Citation: Chen WL, Jiang LS, Li FY. Anti-proliferation activity of celecoxib in cholangitis. Shijie Huaren Xiaohua Zazhi 2010; 18(17): 1761-1766
- URL: https://www.wjgnet.com/1009-3079/full/v18/i17/1761.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v18.i17.1761
肝内胆管结石(intrahepatic bile duct stone)是亚太地区常见病, 近年来, 其相对发病率明显下降, 但这种患者的绝对数量仍然很多[1], 且有癌变可能[2-5], 其治疗一直十分棘手. 通过对肝内胆管结石的病理学研究发现, 75%-100%的亚太地区肝内胆管结石患者均是以反复发作的慢性增生性胆管炎(chronic proliferative cholangitis, CPC)为病理学基础[6,7], 根除CPC有助于降低肝内胆管结石术后复发率和胆道再狭窄率[8,9]. 塞来昔布为环氧化酶2(cyclooxygenase-2, COX-2)选择性抑制剂, 多项研究发现其有确切的抗增殖作用[10-14]. 我们拟用CPC动物模型来探讨塞来昔布能否对过度增殖的胆管黏膜上皮、黏膜下腺体及胆管胶原纤维产生抑制作用及其效果, 从而达到控制肝内胆管结石复发的目的.
健康SD大鼠30只, ♂, 体质量200-250 g, 由四川大学华西实验动物供应站提供. 塞来昔布为辉瑞制药有限公司产品. 苏木素和伊红均为自配. PAS染色试剂盒及Masson染色试剂盒(广州市俪科贸易有限公司). COX-2一抗(Bios公司, 货号bs-0732R). 二抗系统是DAKO公司的EnvisionTM(K5007)兔鼠双标检测系统.
1.2.1 造模: 将大鼠随机分为假手术组(10只), CPC模型组(10只), 塞来昔布治疗组(10只), 适应性饲养1 wk, 新眠灵0.5 mg/kg肌肉注射麻醉大鼠后, 仰卧固定, 腹部消毒, 正中切口入腹. 假手术组仅开腹后关腹. 对照组及治疗组参照Park等[15]介绍的方法, 建立动物模型. 治疗组于术后第1天开始予塞来昔布50 mg/(kg·d)腹腔注射.
1.2.2 标本采集: 术后1 wk处死动物, 分离切开大鼠的全部胆总管后, 置于100 g/L甲醛中固定, 常规行HE染色、PAS染色、Masson染色, 采用Envision双抗法, 行COX-2的免疫组织化学检测.
1.2.3 图像分析: 用美国Nikon&Spot图像采集系统采集图像, Image-proplus 4.5分析软件(美国Media Cybernetic公司)进行图像分析. COX-2阳性表达为细胞膜及细胞质可见棕黄色颗粒的阳性反应物. 计算方法: 切片在光镜下按统一放大倍数(400倍)进行分析, 五名操作者独立将每张切片按等距抽样原则随机摄取5个视野, 输入计算机作为测定视场. 选取积分吸光度值(IA)作为参数进行计算.
统计学处理 应用SPSS12.0软件进行分析处理, 计量资料以mean±SD表示, 各组间比较用方差分析(One-way ANOVA), 两两比较用LSD过程. P<0.05为差异显著标准.
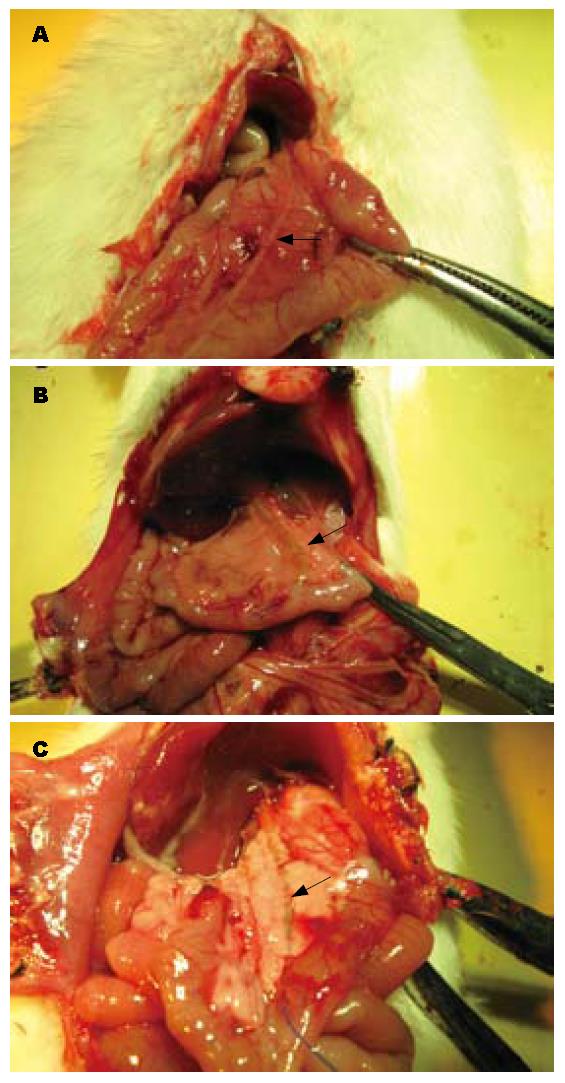
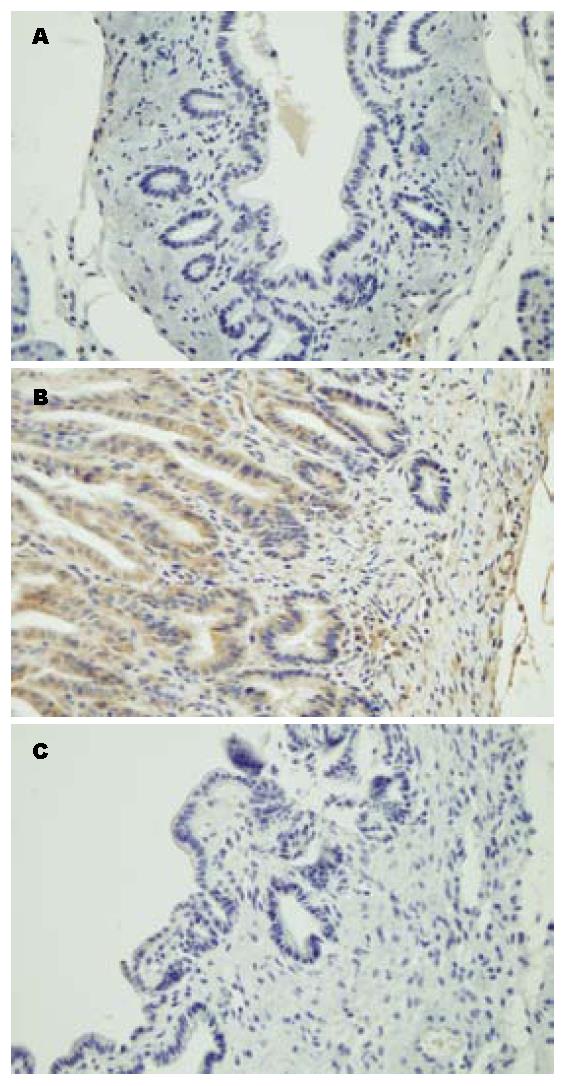
在标本采集时, 通过对各分组大鼠的胆管进行大体病理学观察发现, CPC模型组的胆管直径明显增粗, 慢性炎症反应十分明显, 管腔内可见浑浊胆汁(图1B). 塞来昔布治疗组的胆管直径及炎症较CPC模型组明显减轻(图1C), 但仍大于假手术组(图1A).
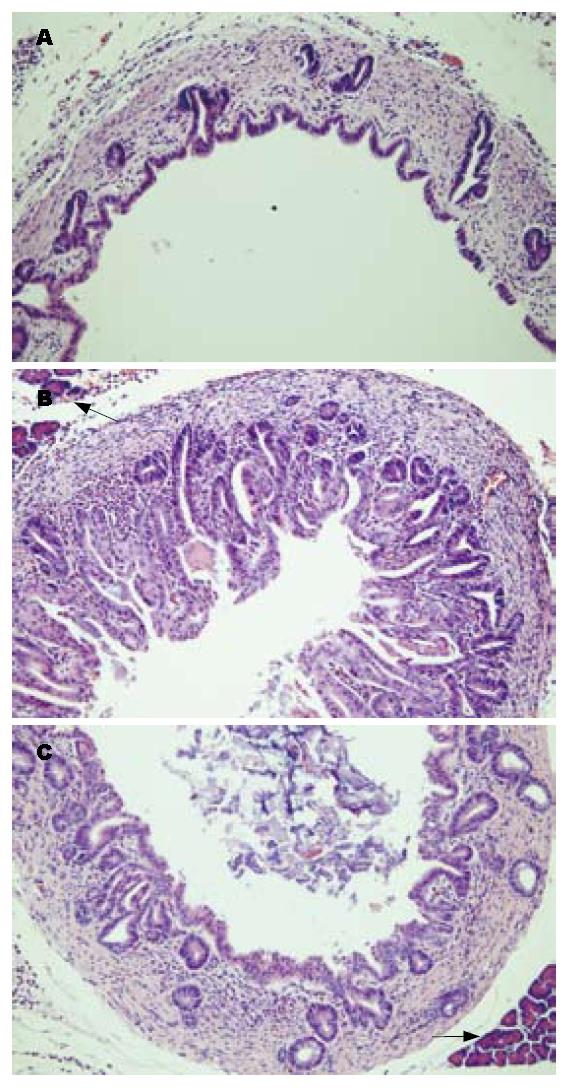
CPC模型组的胆管壁呈十分明显的慢性增生性改变, 胆管黏膜上皮呈乳头样增生, 黏膜下腺体也增生明显, 并被广泛增生的致密纤维结缔组织包裹(图2B). 塞来昔布治疗组的胆管黏膜上皮及黏膜下腺体、纤维结缔组织增生均较模型组减轻(图2C), 但与假手术组(图2A)相对, 仍有少数增生的黏膜下腺体.
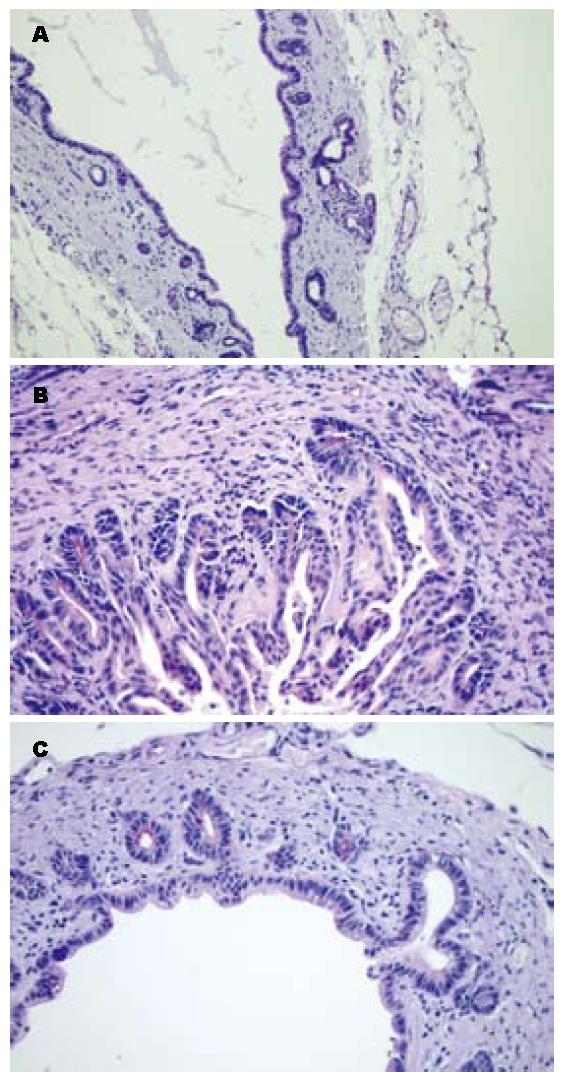
PAS染色下黏液糖蛋白阳性表达呈紫红色, CPC模型组胆管PAS染色明显增强, 阳性物质主要位于胆管的黏膜下腺体的细胞表面及部分细胞质和黏膜上皮细胞表面(图3B), 塞来昔布治疗组(图3C)和假手术组(图3A)的PAS染色则明显减弱, 但塞来昔布治疗组的阳染物质仍稍多于假手术组.
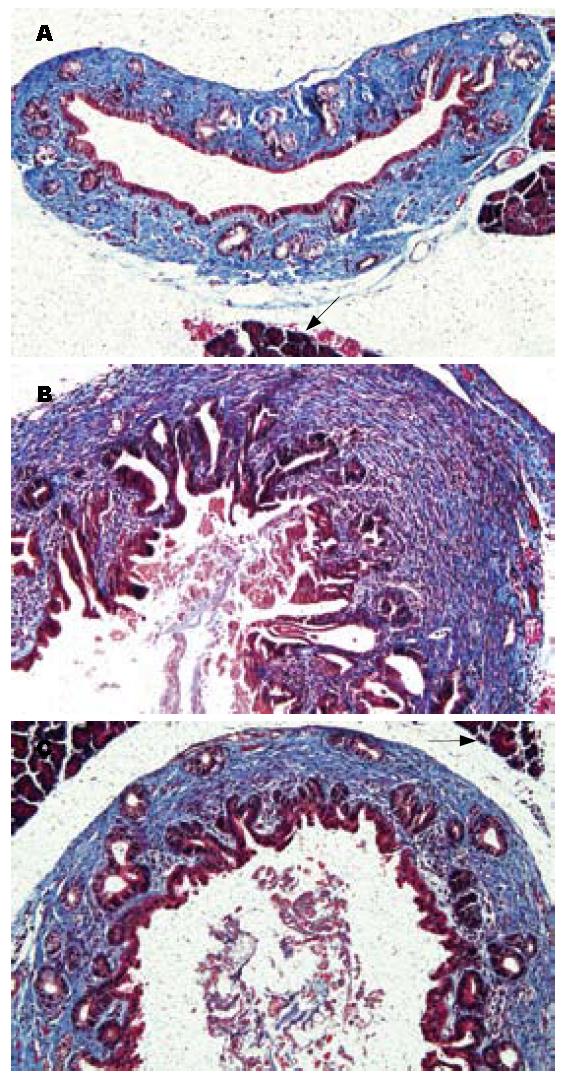
Masson染色下胶原纤维呈蓝色, CPC模型组动物胆管壁胶原纤维明显增生, 增生的黏膜下腺体也被周围的胶原纤维所包绕(图4B), 而塞来昔布治疗组(图4C)较假手术组(图4A)胶原纤维仅轻度增生.
COX-2阳性表达为细胞膜和细胞质出现棕黄色颗粒的阳性反应物. CPC模型组可见大量的COX-2阳染于增生的胆管黏膜上皮及黏膜下腺体的细胞膜和细胞质(图5B). 以积分吸光度(IA)作为参数来量化各组间的COX-2表达, 塞来昔布治疗组较CPC模型组COX-2阳染明显减弱(IA值: 8.62±0.19 vs 35.27±0.43, P<0.05)(图5C), 和假手术组接近(IA值: 8.62±0.19 vs 8.41±0.13, P>0.05)(图5A).
肝内胆管结石指左右肝管汇合部以上的胆管结石, 其病因与胆道细菌感染、寄生虫感染、饮食习惯、胆汁淤积、先天解剖异常、免疫因素等多种因素有关, 多为胆色素结石. 近年来, 肝内胆管结石发病率明显下降, 相对发病率下降至5%左右, 但患者的绝对数量仍然很多, 并有发展为胆管癌的可能, 治疗十分棘手[1-5]. 目前, 肝内胆管结石的治疗以外科治疗为主, 基本原则为"取尽结石, 解除梗阻, 去除病灶, 通畅引流". 主要术式有: 肝叶(肝段)切除术, 肝门部狭窄胆管切开整形、取石、胆肠内引流术, 及晚期的肝移植术[16]. 腹腔镜及纤维内镜的运用使患者痛苦减少, 治愈率提高[17-20]. 但对于结石的复发, 总体治疗效果欠佳, 术后4-10年的复发率可达4.2%-40%[6,21,22]. 究其原因, 主要是术后胆道狭窄的存在与再狭窄, 胆汁黏稠, 胆道感染未廓清等因素有关. 而这些因素, 主要是由于肝内胆管结石的基本病理改变CPC所致[7,23,24]. 因此, CPC成为预防肝内胆管结石复发的理想治疗靶点.
我国肝内胆管结石患者均是以反复发作的CPC为其病理学基础[6,7]. CPC主要表现为胆管黏膜上皮、管壁腺体及管壁、管周纤维组织的增生. 增生的组织导致管壁增厚、管腔狭窄、胆汁淤积. 增生的腺体分泌大量的黏液糖蛋白、酸性黏多糖等物质, 导致胆汁黏稠, 加重胆汁淤积, 促进成石[8,9]. 胆汁中黏液糖蛋白具有凝胶性、粘弹性及脂结合性, 其分泌的增加导致胆汁黏稠、淤积, 胆石形成[25]. 胆汁淤积导致胆汁中细菌繁殖, 通过β-G等途径, 进一步促进成石, 并导致胆管上皮特别是腺上皮的感染. 炎症感染进一步加重腺体的增殖, 增加黏液糖蛋白等的分泌, 如此反复. 增生的纤维组织将腺体包裹, 腺体周围小血管也因反复炎症刺激狭窄或闭塞, 药物难于到达局部, 感染不易廓清[26]. 一旦条件形成, 细菌再次大量繁殖, 最终导致肝内胆管结石的高复发率. 迁延不愈的胆管炎症, 致胆管上皮及腺上皮增生活跃, 发生不典型增生较多, 极易癌变. 能否应用塞来昔布对CPC抑制成为本研究的最初设想.
塞来昔布为COX-2选择性抑制剂. 环氧化酶是花生四烯酸转换为前列腺素类物质的关键限速酶, 包括两种同工酶: COX-1和COX-2. COX-1为人体固有酶, 生理情况下广泛分布于各种组织, 调节机体功能; COX-2是一种可诱导型酶, 在正常组织中水平很低, 在炎症、内毒素及致癌物等诱导下, 可异常表达, 促进前列腺素类物质的生成. 胆管上皮细胞受到结石、细菌、炎症等刺激后, 亦可使COX-2表达上调, 促进前列腺素E2(prostaglandin E2, PGE2)的生成[27,28]. PGE2生成增多, 可介导产生和分泌黏蛋白[29-31]. COX-2抑制剂通过对COX-2的选择性抑制, 减少前列腺素类物质的释放, 从而减少黏蛋白的生成, 抑制成石. 已有研究发现COX-2抑制剂NS-398可显著减少炎症介质PGE2的产生, 从而阻止黏蛋白的高分泌和高表达[32]. 作为与NS-398同为COX-2选择性抑制剂的塞来昔布, 多项研究表明其有确切的抗增殖作用[10-14]. 基于此, 本研究探讨了塞来昔布是否对CPC过度增殖的胆管黏膜上皮、黏膜下腺体及胆管壁胶原纤维和高分泌的黏液糖蛋白产生抑制作用.
结果显示, 通过塞来昔布的干预, CPC的黏膜上皮、黏膜下腺体和管壁纤维组织的增殖受到了明显的抑制. 外观上, 胆管直径减小, 胆管质地变软, 管壁炎症反应减轻; PAS阳性染色表达强度明显下降, 提示黏液糖蛋白这一成石初期主要的成核因素分泌下降; Masson染色下观察, 胆管壁胶原纤维的增殖亦受到明显抑制. 故塞来昔布对CPC的抗增殖及黏液糖蛋白的分泌抑制效果明显. 通过对COX-2表达的研究发现, CPC模型组COX-2的表达明显高于假手术组; 经塞来昔布干预后, COX-2的表达明显下降, 与假手术组相比无显著差异性. 据此我们推测, 塞来昔布是通过COX-2途径来发挥其作用的.
总之, 我们认为, 塞来昔布在CPC中具有确切的抗增殖作用, 亦能抑制黏液糖蛋白的高分泌, 对于肝内胆管结石术后复发率和胆管再狭窄率的降低, 有望产生较好的效果. 然而, 有资料表明, 塞来昔布有增加心血管病发病的危险[33]. 亦发现机制不明的塞来昔布介导的肝损害[34]. 但通过局部用药, 有望使这一不良反应降到最低. 权衡利弊, 塞来昔布以CPC为治疗靶点, 对于提高肝内胆管结石的远期疗效, 具有良好的潜在性应用前景.
肝内胆管结石病因复杂, 目前虽然有多种治疗措施, 但术后复发率高, 反复发作. 通过对肝内胆管结石的病理学研究发现, 其基本病理改变为慢性增生性胆管炎, 通过对慢性增生性胆管炎的控制, 有望改变这一状况.
房林, 副教授, 同济大学附属上海市第十人民医院普外科.
目前对肝内胆管结石的治疗方法主要集中在手术治疗和非手术治疗两方面, 但对于术后的高复发率及对慢性增生性胆管炎的控制, 尚无有效方法.
塞来昔布为环氧化酶2选择性抑制剂, 研究表明其对多种肿瘤细胞具有抗增殖作用, 本文通过动物实验研究了其是否对慢性增生性胆管炎过度增殖的黏膜上皮、黏膜下腺体及胆管壁胶原纤维和高分泌的黏液糖蛋白具有抑制作用.
塞来昔布以慢性增生性胆管炎为治疗靶点, 对提高肝内胆管结石的远期疗效具有良好的潜在性应用前景.
慢性增生性胆管炎: 表现为胆管黏膜上皮、管壁腺体及管壁、管周纤维组织的增生. 增生的组织导致管壁增厚、管腔狭窄、胆汁淤积. 增生的腺体分泌大量的黏液糖蛋白、酸性黏多糖等物质, 导致胆汁黏稠, 加重胆汁淤积, 促进成石.
本研究选题尚可, 可能有一定的临床应用价值, 但需进一步研究.
编辑: 曹丽鸥 电编: 吴鹏朕
| 1. | 黄 志强. 肝内胆管结石肝切除术的演变. 中国现代普通外科进展. 2009;12:1-2. |
| 2. | Zhou H, Wang H, Zhou D, Wang H, Wang Q, Zou S, Tu Q, Wu M, Hu H. Hepatitis B virus-associated intrahepatic cholangiocarcinoma and hepatocellular carcinoma may hold common disease process for carcinogenesis. Eur J Cancer. 2010;46:1056-1061. [PubMed] [DOI] |
| 3. | Cheon YK, Cho YD, Moon JH, Lee JS, Shim CS. Evaluation of long-term results and recurrent factors after operative and nonoperative treatment for hepatolithiasis. Surgery. 2009;146:843-853. [PubMed] [DOI] |
| 4. | Eun JR, Jang BI, Lee JY, Kim KO, Lee SH, Kim TN, Lee HJ. [Clinical characteristics of intrahepatic cholangiocarcinoma and prognostic factors in patients who received non-surgical treatment]. Korean J Gastroenterol. 2009;54:227-234. [PubMed] [DOI] |
| 5. | Han SL, Zhou HZ, Cheng J, Lan SH, Zhang PC, Chen ZJ, Zeng QQ. Diagnosis and surgical treatment of intrahepatic hepatolithiasis associated cholangiocarcinoma. Asian J Surg. 2009;32:1-6. [PubMed] [DOI] |
| 6. | Yoshida M, Yamamoto N, Nitta T, Uehara T, Terao R, Hatano E, Iimuro Y, Yamaoka Y. Suppression of proliferative cholangitis by E2F decoy oligodeoxynucleotide. J Surg Res. 2002;102:95-101. [PubMed] [DOI] |
| 7. | Li FY, Cheng NS, Mao H, Jiang LS, Cheng JQ, Li QS, Munireddy S. Significance of controlling chronic proliferative cholangitis in the treatment of hepatolithiasis. World J Surg. 2009;33:2155-2160. [PubMed] [DOI] |
| 8. | Terao R, Honda K, Hatano E, Uehara T, Yamamoto M, Yamaoka Y. Suppression of proliferative cholangitis in a rat model with direct adenovirus-mediated retinoblastoma gene transfer to the biliary tract. Hepatology. 1998;28:605-612. [PubMed] [DOI] |
| 9. | Li F, Cheng J, He S, Li N, Zhang M, Dong J, Jiang L, Cheng N, Xiong X. The practical value of applying chemical biliary duct embolization to chemical hepatectomy for treatment of hepatolithiasis. J Surg Res. 2005;127:131-138. [PubMed] [DOI] |
| 10. | Venkatesan P, Das S, Krishnan MM, Chakraborty C, Chaudhury K, Mandal M. Effect of AEE788 and/or Celecoxib on colon cancer cell morphology using advanced microscopic techniques. Micron. 2010;41:247-256. [PubMed] [DOI] |
| 11. | Amrite AC, Kompella UB. Celecoxib inhibits proliferation of retinal pigment epithelial and choroid-retinal endothelial cells by a cyclooxygenase-2-independent mechanism. J Pharmacol Exp Ther. 2008;324:749-758. [PubMed] [DOI] |
| 12. | Peluffo GD, Stillitani I, Rodríguez VA, Diament MJ, Klein SM. Reduction of tumor progression and paraneoplastic syndrome development in murine lung adenocarcinoma by nonsteroidal antiinflammatory drugs. Int J Cancer. 2004;110:825-830. [PubMed] [DOI] |
| 13. | Bozzo F, Bassignana A, Lazzarato L, Boschi D, Gasco A, Bocca C, Miglietta A. Novel nitro-oxy derivatives of celecoxib for the regulation of colon cancer cell growth. Chem Biol Interact. 2009;182:183-190. [PubMed] [DOI] |
| 15. | Park SM, Choi JW, Kim ST, Cho MC, Sung RH, Jang LC, Park JW, Lee SP, Park YH. Suppression of proliferative cholangitis in a rat model by local delivery of paclitaxel. J Hepatobiliary Pancreat Surg. 2003;10:176-182. [PubMed] [DOI] |
| 17. | Machado MA, Makdissi FF, Surjan RC, Teixeira AR, Sepúlveda A, Bacchella T, Machado MC. Laparoscopic right hemihepatectomy for hepatolithiasis. Surg Endosc. 2008;22:245. [PubMed] [DOI] |
| 18. | Cai X, Wang Y, Yu H, Liang X, Peng S. Laparoscopic hepatectomy for hepatolithiasis: a feasibility and safety study in 29 patients. Surg Endosc. 2007;21:1074-1078. [PubMed] [DOI] |
| 19. | Lee JK, Kim TK, Byun JH, Kim AY, Ha HK, Kim PN, Lee MG. Diagnosis of intrahepatic and common duct stones: combined unenhanced and contrast-enhanced helical CT in 1090 patients. Abdom Imaging. 2006;31:425-432. [PubMed] [DOI] |
| 20. | Mori T, Sugiyama M, Atomi Y. Gallstone disease: Management of intrahepatic stones. Best Pract Res Clin Gastroenterol. 2006;20:1117-1137. [PubMed] [DOI] |
| 21. | Lee TY, Chen YL, Chang HC, Chan CP, Kuo SJ. Outcomes of hepatectomy for hepatolithiasis. World J Surg. 2007;31:479-482. [PubMed] [DOI] |
| 22. | Uchiyama K, Onishi H, Tani M, Kinoshita H, Kawai M, Ueno M, Yamaue H. Long-term prognosis after treatment of patients with choledocholithiasis. Ann Surg. 2003;238:97-102. [PubMed] [DOI] |
| 23. | Nakanuma Y, Yamaguchi K, Ohta G, Terada T. Pathologic features of hepatolithiasis in Japan. Hum Pathol. 1988;19:1181-1186. [PubMed] [DOI] |
| 24. | Terada T, Nakanuma Y. Pathologic observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers: IV. Hyperplasia of intramural and extramural glands. Hum Pathol. 1992;23:483-490. [PubMed] [DOI] |
| 27. | Kuroki T, Tajima Y, Kanematsu T. Hepatolithiasis and intrahepatic cholangiocarcinoma: carcinogenesis based on molecular mechanisms. J Hepatobiliary Pancreat Surg. 2005;12:463-466. [PubMed] [DOI] |
| 28. | Shoda J, Ueda T, Kawamoto T, Todoroki T, Asano T, Sugimoto Y, Ichikawa A, Maruyama T, Nimura Y, Tanaka N. Prostaglandin E receptors in bile ducts of hepatolithiasis patients and the pathobiological significance for cholangitis. Clin Gastroenterol Hepatol. 2003;1:285-296. [PubMed] [DOI] |
| 29. | Phillipson M, Johansson ME, Henriksnäs J, Petersson J, Gendler SJ, Sandler S, Persson AE, Hansson GC, Holm L. The gastric mucus layers: constituents and regulation of accumulation. Am J Physiol Gastrointest Liver Physiol. 2008;295:G806-G812. [PubMed] [DOI] |
| 30. | Cho KN, Choi JY, Kim CH, Baek SJ, Chung KC, Moon UY, Kim KS, Lee WJ, Koo JS, Yoon JH. Prostaglandin E2 induces MUC8 gene expression via a mechanism involving ERK MAPK/RSK1/cAMP response element binding protein activation in human airway epithelial cells. J Biol Chem. 2005;280:6676-6681. [PubMed] [DOI] |
| 31. | Shimamoto C, Fujiwara S, Kato M, Ito S, Katsu K, Mori H, Nakahari T. Inhibition of ACh-stimulated exocytosis by NSAIDs in guinea pig antral mucous cells: autocrine regulation of mucin secretion by PGE2. Am J Physiol Gastrointest Liver Physiol. 2005;288:G39-G47. [DOI] |
| 32. | Kim HJ, Lee SK, Kim MH, Seo DW, Min YI. Cyclooxygenase-2 mediates mucin secretion from epithelial cells of lipopolysaccharide-treated canine gallbladder. Dig Dis Sci. 2003;48:726-732. [PubMed] [DOI] |
| 33. | White WB, Faich G, Whelton A, Maurath C, Ridge NJ, Verburg KM, Geis GS, Lefkowith JB. Comparison of thromboembolic events in patients treated with celecoxib, a cyclooxygenase-2 specific inhibitor, versus ibuprofen or diclofenac. Am J Cardiol. 2002;89:425-430. [PubMed] [DOI] |
| 34. | El Hajj II, Malik SM, Alwakeel HR, Shaikh OS, Sasatomi E, Kandil HM. Celecoxib-induced cholestatic liver failure requiring orthotopic liver transplantation. World J Gastroenterol. 2009;15:3937-3939. [PubMed] [DOI] |