修回日期: 2010-03-15
接受日期: 2010-03-23
在线出版日期: 2010-04-18
目的: 探讨粒细胞集落刺激因子(granulocyte colony-stimulating factor, G-CSF)促进肝大部切除小鼠肝再生的作用.
方法: 建立小鼠肝大部分切除(约70%)模型, 将肝切除小鼠随机分为3组. 单纯肝切除组: 肝切除后24 h, 腹腔注射生理盐水5 d; G-CSF+肝切除组: rhG-CSF 150 μg/(kg•d)腹腔注射5 d, 24 h后进行肝切除; 肝切除+G-CSF组: 肝切除术后24 h, rhG-CSF 150 μg/(kg•d)腹腔注射5 d. 于术后7 d取血清和肝组织, 用全自动生化分析仪检测血清肝功能指标, 并采用免疫组织化学方法观察肝内的增殖细胞核抗原(proliferating cell nuclear antigen, PCNA)的表达及BrdU阳性细胞.
结果: 肝切除+G-CSF组和G-CSF+肝切除组BrdU及PCNA表达及BrdU阳性细胞明显升高, 与对照组比较差异均具有统计学意义(PCNA: 74.08%±8.86%, 68.91%±9.64% vs 57.36%±13.37%, 均P<0.05); 肝组织病理检查发现G-CSF+肝切除组27%(3/11)小鼠肝组织出现了不同程度的炎症反应, 肝功生化学也显示血清ALT及AST升高, 且其差异有统计学意义(均P<0.05).
结论: G-CSF具有明显促进肝大部切除小鼠肝再生的作用, 但也容易诱发肝脏炎症反应.
引文著录: 何秀华, 李东良, 范敬静, 马明. 粒细胞集落刺激因子促进小鼠肝再生的作用. 世界华人消化杂志 2010; 18(11): 1115-1120
Revised: March 15, 2010
Accepted: March 23, 2010
Published online: April 18, 2010
AIM: To investigate the effect of granulocyte colony-stimulating factor (G-CSF) on liver regeneration after major hepatectomy in mice.
METHODS: Mice were subjected to 70% hepatectomy and randomly divided into three groups: control group (intraperitoneally injected with normal saline 24 h after hepatectomy, once a day for 5 d), G-CSF pretreatment group [intraperitoneally injected with recombinant human G-CSF (rhG-CSF) at 150 μg/kg body weight daily for 5 d and underwent hepatectomy 24 h later], and G-CSF treatment group (intraperitoneally injected with rhG-CSF 24 h after hepatectomy, at 150 μg/kg body weight daily for 5 d). Serum and hepatic tissue samples were harvested seven days after hepatectomy. Liver function parameters were measured using an automated biochemical analyzer. Immunohistochemistry was used to detect the expression of proliferating cell nuclear antigen (PCNA) and bromodeoxyuridine (BrdU)-positive cells.
RESULTS: The percentages of PCNA- and BrdU-positive cells in the G- CSF pretreatment and treatment groups were significantly higher than those in the control group (PCNA: 74.08% ± 8.86% and 68.91% ± 9.64% vs 57.36% ± 13.37%, respectively; both P < 0.05). Inflammatory response was noted in 27% (3/11) of mice in the G-CSF pretreatment group. Serum ALT and AST levels were significantly higher in the G-CSF pretreatment group than in the other two groups (both P < 0.05).
CONCLUSION: G-CSF promotes liver regeneration after major hepatectomy in mice, but G-CSF pretreatment induces infammatory response.
- Citation: He XH, Li DL, Fan JJ, Ma M. Granulocyte colony-stimulating factor promotes liver regeneration in mice. Shijie Huaren Xiaohua Zazhi 2010; 18(11): 1115-1120
- URL: https://www.wjgnet.com/1009-3079/full/v18/i11/1115.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v18.i11.1115
肝移植近年来发展迅速, 但供肝的严重短缺制约了临床肝移植的广泛开展[1-3], 活体肝移植为解决供肝来源的短缺开拓了新的途径[4,5]. 活体肝移植的关键性技术之一是使移植到受体的肝组织块以及供体剩余的肝组织能尽快再生, 以满足机体代谢所需有效的肝体积. 因此, 促进部分肝移植后肝再生的研究成为近年来器官移植领域一个新的研究热点. 有报道粒细胞集落刺激因子(granulocyte colony-stimulating factor, G-CSF)可能具有促进肝细胞再生的能力[6], 但其确切疗效及其促进肝再生的安全性尚不明确. 因此, 本研究建立肝大部切除小鼠模型, 通过检测反应肝细胞再生的增殖细胞核抗原(proliferating cell nuclear antigen, PCNA)和BrdU阳性细胞, 观察重组人粒细胞集落刺激因子(recombinant human granulocyte colony -stimulating factor, rhG-CSF)对肝再生的促进作用, 为G-CSF临床促进肝再生治疗提供理论依据.
清洁级BALB/C小鼠50只, ♂, 购自上海斯莱克实验动物有限责任公司(动物质量合格证scxh(沪)2007-0005), 饲养于中国人民解放军南京军区福州总医院比较医学中心IVC清洁级实验动物饲养系统. 饲养环境为恒温(25 ℃-27 ℃)恒湿(45%-50%), 自由摄取食物和水, 普通饲料喂养; 重组人粒细胞集落刺激因子由中国麒麟鲲鹏生物药业有限公司生产, 商品名为惠尔血, 批号20081151, 制剂标示量为每支150 μg; PCNA单克隆抗体(北京中杉公司); BrdU(美国 Sigma公司); 鼠抗BrdU单克隆抗体(北京中杉公司); SP免疫组织化学染色试剂盒 (北京中杉公司).
1.2.1 造模: 实验小鼠, 周龄6-8 wk, 体质量18-22 g, 1%戊巴比妥钠40 mg/kg腹腔注射麻醉, 仰卧位固定, 于剑突下0.5 cm腹中线剪开约3 cm长切口, 打开腹腔, 从肝蒂处结扎并切除肝左叶、中叶约70%肝体积. 观察创面无渗血后缝合关闭腹腔, 24 h未死亡, 活力及饮食基本恢复正常的34只肝大部切除小鼠用于实验研究.
1.2.2 分组: 肝切除组(n = 11), 肝大部切除后24 h, 腹腔注射生理盐水5 d; G-CSF+肝切除组(n = 11), rhG-CSF腹腔注射150 μg/(kg•d), 共5 d, 24 h后进行肝大部切除; 肝切除+G-CSF组(n = 12), 肝切除术后24 h, rhG-CSF腹腔注射150 μg/(kg•d), 共5 d.
1.2.3 观察指标: 术后第7天, 眼眶静脉采血, Olympus AU2700全自动生化分析仪检测丙氨酸氨基转移酶(ALT)、天冬氨酸氨基转移酶(AST)和血清白蛋白(ALB), 脱臼法处死动物, 于相同部位取适量肝组织, 40 g/L的中性甲醛液固定, 常规HE染色观察肝组织病理变化, 免疫组织化学观察PCNA的表达和BrdU阳性细胞. 取肝组织前2 h腹腔内注射BrdU 50 mg/kg, 以使之掺合入肝细胞DNA合成, 免疫组织化学操作按说明书进行, PCNA和BrdU的结果判定标准: 以细胞核呈界限清楚的棕色反应为阳性, 阳性结果分级按以下标准记录, 即每张切片随机观察5个高倍视野, 每个视野计100个细胞中的阳性细胞数, 取均值, 以百分数表示细胞增殖指数.
统计学处理 使用SPSS11.5统计软件处理实验数据, 多样本的均数的比较采用One-way ANOVA分析, 方差不齐采用H检验, P<0.05有统计学意义.
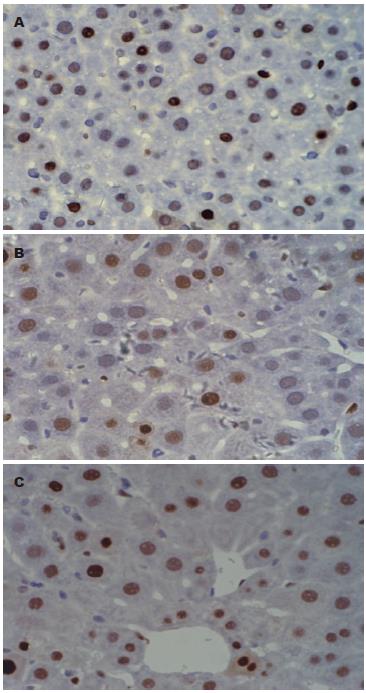
肝切除+G-CSF组与对照组比较差异有统计学意义(74.08%±8.86% vs 57.36%±13.37%, P = 0.001), G-CSF+肝切除组与对照组比较差异有统计学意义(68.91%±9.64% vs 57.36% ±13.37%, P = 0.017, 图1).
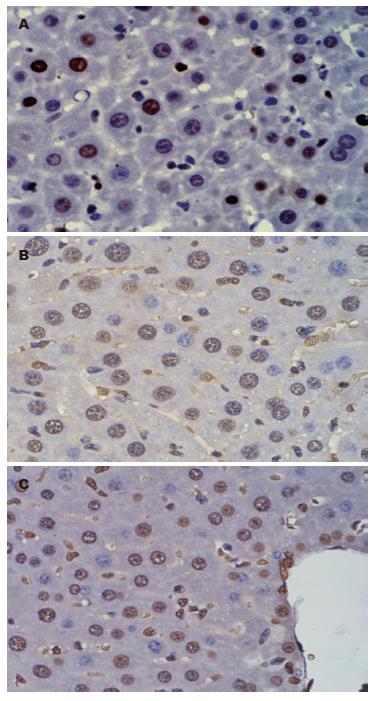
肝切除+G-CSF及G-CSF+肝切除组BrdU阳性细胞阳性率明显高于单纯肝切除组(图2).
G-CSF+肝切除组的ALT、AST有明显升高, 与单纯肝切除组及肝切除+G-CSF组比较差异具有统计学意义. 而肝切除+G-CSF与对照组相比差异无统计学意义(表1).
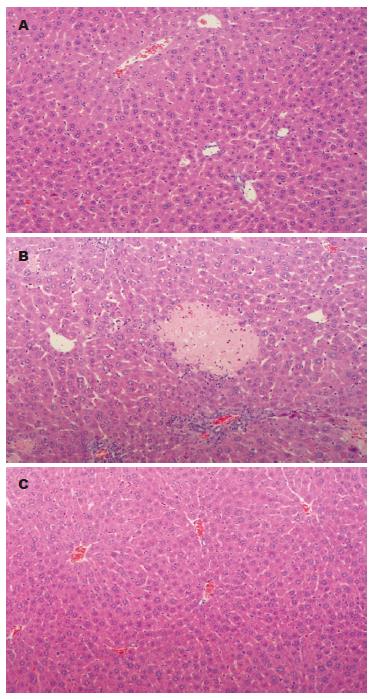
对肝组织病理学观察发现G-CSF+肝切除组3例小鼠出现肝脏局灶性坏死及汇管区炎性细胞浸润等炎症反应的组织学改变, 而单纯肝切除组及肝切除+G-CSF组未见类似病理改变(图3).
G-CSF于1980年由Burgess等首次克隆成功[7], 他是一种促粒细胞增殖的细胞因子, 主要应用于各种原因引起的粒细胞减少症[8-10]. G-CSF还可以诱导多潜能骨髓干细胞增殖, 并动员进入外周血循环[11,12], 使得自体骨髓移植成为可能[13]. 此外, G-CSF提高生存率及改善肝脏病理的作用已经在急性和慢性肝病的动物模型中得到了验证[14,15]. Theocharis等[16]制备大鼠暴发性肝衰竭和肝性脑病模型, 应用rhG-CSF组大鼠的生存率、肝组织病理变化、血清学指标及血氨水平均好于对照组, 认为可能与rhG-CSF改善肝再生有关. 近年来, G-CSF已经应用在了终末期肝病及急慢性肝衰竭患者中[17-19]. 但也有研究者对G-CSF促进肝再生作用提出质疑[20]. 基于上述研究, 本课题组将G-CSF用于研究对肝大部切除后剩余肝脏的再生作用.
PCNA是一种与细胞周期相关的增殖细胞核抗原, 存在并合成于核内, 静止细胞中PCNA含量很少, 增殖细胞和转化细胞中其含量发生明显变化, 其含量和表达强弱的变化与DNA合成及DNA复制的活跃程度一致. 实验结果显示无论术前还是术后给予G-CSF均比对照组PCNA表达增高, 说明肝再生更活跃, 而且术后给予G-CSF促进肝切除后剩余肝再生作用最明显. 5'-溴基-2'-脱氧尿苷(BrdU)是一种胸腺嘧啶脱氧核苷酸类似物, 在细胞核内参与有丝分裂前DNA合成, BrdU可以用于检测S期细胞, 通过评估分裂细胞摄取BrdU能反应细胞增殖情况. BrdU阳性细胞G-CSF组比对照组明显增多, 但术前术后给予未见明显差别. 上述的实验结果提示G-CSF促进部分肝切除后肝再生作用比较确定, 但作用机制仍未明确, 可能与rhG-CSF动员骨髓干细胞参与肝细胞再生有关, 也可能与rhG-CSF启动肝脏再生的内源性程序有关.
骨髓干细胞是重要的成体干细胞, 骨髓内主要有两类干细胞群体: 造血干细胞和间充质干细胞, 他们都具有定向分化或是横向分化为一些成体细胞的潜能, 包括神经细胞[21]、心肌细胞[22]、血管内皮细胞[23]、软骨细胞[24]以及肝实质细胞[25]等. 一般情况下, 骨髓干细胞进入到外周血循环中并迁移到损伤部位的数量极少, G-CSF是骨髓干细胞强有力的动员剂, 不仅可增加外周血干细胞数量, 也可能使干细胞迁移到肝损伤部位[26], 另外在肝脏损伤时也可能动员肝系相关的干细胞归巢入肝脏,参与肝再生[27]. 还有研究者表明G-CSF是本身直接促进肝再生, 而不是动员造血干细胞后促进肝再生[28]. 而也有学者表明是通过二者共同作用促进肝再生[29].
肝功能结果显示ALT及AST术前给予G-CSF均比术后给予及对照组明显升高, 差别有统计学意义, 并且普通病理结果发现术前给予G-CSF出现炎性细胞浸润及局灶坏死, 考虑腹腔注射G-CSF促进炎性细胞增殖和分化并诱导浸润肝脏, 促进其炎症反应, 所以术前给予G-CSF组炎症反应剧烈, 出现炎性细胞浸润, 局灶坏死, ALT及AST明显升高. 术后给予G-CSF, 在肝损伤前提下倾向于促进肝再生. 因此, G-CSF促进肝再生的作用仍需继续深入研究, 以探明其作用机制, 并对G-CSF的作用全面综合分析, G-CSF的使用剂量及给药时间仍需多组比较研究, 使得G-CSF用于肝再生领域, 充分发挥其优点而避其缺点.
在今后供肝源有限的情况下, 采用活体供肝, 由于活体供肝不可能替代全肝的功能. 那么, 采用G-CSF可以促进供体肝及受体肝[30]的再生, 但因其存在促进炎症反应, 我们应该继续深入研究其作用机制, 慎重用于临床.
肝移植近年来发展迅速, 但供肝的严重短缺制约了临床肝移植的广泛开展, 活体肝移植为解决供肝来源的短缺开拓了新的途径. 活体肝移植的关键性技术之一是使移植到受体的肝组织块以及供体剩余的肝组织能尽快再生, 以满足机体代谢所需有效的肝体积. 因此, 促进部分肝移植后肝再生的研究成为近年来器官移植领域一个新的研究热点.
潘兴华, 副主任医师, 中国人民解放军成都军区昆明总医院病理实验科.
提高部分肝移植物存活率的研究主要集中于提高手术技术和改善缺血再灌注损伤方面, 如何促进移植到受体的肝组织块以及供体剩余的肝组织的肝再生方面的相关报道较少.
本文利用小鼠肝大部分切除模型, 证实G-CSF可以促进肝大部切除小鼠肝再生的作用, 但也发现术前给予G-CSF容易诱发肝脏炎症反应.
本研究为今后临床部分肝移植术后更好地促进供体肝及受体肝的再生提供了实验依据. 但因G-CSF存在促进炎症反应的负面作用, 应该继续深入研究, 使得G-CSF应用于肝再生领域, 发挥其优点而避其缺点.
G-CSF: 粒细胞集落刺激因子, 作为一种促粒细胞增殖的细胞因子应用于临床, G-CSF还可以诱导多潜能骨髓干细胞增殖, 并动员进入外周血循环, 近年来研究发现G-CSF具有促进肝再生作用.
本文有一定创新性和科学价值.
编辑: 李军亮 电编:吴鹏朕
| 1. | Bardou-Jacquet E, Lorho R. [Liver transplantation: who should have it and when?]. Presse Med. 2009;38:1258-1265. [PubMed] |
| 2. | Li KK, Neuberger J. The management of patients awaiting liver transplantation. Nat Rev Gastroenterol Hepatol. 2009;6:648-659. [PubMed] |
| 3. | Ng KK, Lo CM. Liver transplantation in Asia: past, present and future. Ann Acad Med Singapore. 2009;38:322-332. [PubMed] |
| 4. | Müller SA, Mehrabi A, Schmied BM, Welsch T, Fonouni H, Engelmann G, Schemmer P, Weitz J, Schmidt J. Partial liver transplantation-living donor liver transplantation and split liver transplantation. Nephrol Dial Transplant. 2007;22 Suppl 8:viii13-viii22. [PubMed] |
| 5. | Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545-1559. [PubMed] |
| 6. | Theocharis SE, Margeli AP, Kittas CN. Effect of granulocyte colony-stimulating-factor administration on tissue regeneration due to thioacetamide-induced liver injury in rats. Dig Dis Sci. 1999;44:1990-1996. [PubMed] |
| 7. | Burgess AW, Metcalf D. The nature and action of granulocyte-macrophage colony stimulating factors. Blood. 1980;56:947-958. [PubMed] |
| 8. | Kelly S, Wheatley D. Prevention of febrile neutropenia: use of granulocyte colony-stimulating factors. Br J Cancer. 2009;101 Suppl 1:S6-S10. [PubMed] |
| 9. | Fioredda F, Calvillo M, Lanciotti M, Lanza T, Giunti L, Castagnola E, Lorenzi I, Tonelli R, Ghezzi P, Dufour C. Pegfilgrastim in children with severe congenital neutropenia. Pediatr Blood Cancer. 2010;54:465-467. [PubMed] |
| 10. | Ruiz LP, Aguila JD, Cedeño AM. [Granulocyte colony stimulating factor in the ambulatory treatment of neutropenia following chemotherapy]. Rev Panam Salud Publica. 2009;26:281-282. [PubMed] |
| 11. | Thomas J, Liu F, Link DC. Mechanisms of mobilization of hematopoietic progenitors with granulocyte colony-stimulating factor. Curr Opin Hematol. 2002;9:183-189. [PubMed] |
| 12. | Fischer JC, Frick M, Wassmuth R, Platz A, Punzel M, Wernet P. Superior mobilisation of haematopoietic progenitor cells with glycosylated G-CSF in male but not female unrelated stem cell donors. Br J Haematol. 2005;130:740-746. [PubMed] |
| 13. | Vose JM, Ho AD, Coiffier B, Corradini P, Khouri I, Sureda A, Van Besien K, Dipersio J. Advances in mobilization for the optimization of autologous stem cell transplantation. Leuk Lymphoma. 2009;50:1412-1421. [PubMed] |
| 15. | Wang JP, Sun DX, Li BS, Kang FB, Guo ZR, Li MR, Kang JW, Li WY. [Preventive and treatment effects of granulocyte colony stimulating factor on CCl4 induced chronic liver injury in mice]. Zhonghua Ganzangbing Zazhi. 2008;16:395-396. [PubMed] |
| 16. | Theocharis SE, Papadimitriou LJ, Retsou ZP, Margeli AP, Ninos SS, Papadimitriou JD. Granulocyte-colony stimulating factor administration ameliorates liver regeneration in animal model of fulminant hepatic failure and encephalopathy. Dig Dis Sci. 2003;48:1797-1803. [PubMed] |
| 17. | Gaia S, Smedile A, Omedè P, Olivero A, Sanavio F, Balzola F, Ottobrelli A, Abate ML, Marzano A, Rizzetto M. Feasibility and safety of G-CSF administration to induce bone marrow-derived cells mobilization in patients with end stage liver disease. J Hepatol. 2006;45:13-19. [PubMed] |
| 18. | Di Campli C, Zocco MA, Saulnier N, Grieco A, Rapaccini G, Addolorato G, Rumi C, Santoliquido A, Leone G, Gasbarrini G. Safety and efficacy profile of G-CSF therapy in patients with acute on chronic liver failure. Dig Liver Dis. 2007;39:1071-1076. [PubMed] |
| 19. | Lyra AC, Soares MB, da Silva LF, Braga EL, Oliveira SA, Fortes MF, Silva AG, Brustolim D, Genser B, Dos Santos RR. Infusion of autologous bone marrow mononuclear cells through hepatic artery results in a short-term improvement of liver function in patients with chronic liver disease: a pilot randomized controlled study. Eur J Gastroenterol Hepatol. 2010;22:33-42. [PubMed] |
| 20. | Caraceni P, Giannone F, Catani L, Talarico S, Pertosa AM, Domenicali M, Fogli M, Principe A, Trevisani F, Baccarani M. Effects of granulocyte colony stimulating-factor in a rat model of acute liver injury. Dig Liver Dis. 2007;39:943-951. [PubMed] |
| 21. | Alberti E, Los M, García R, Fraga JL, Serrano T, Hernández E, Klonisch T, Macías R, Martínez L, Castillo L. Prolonged survival and expression of neural markers by bone marrow-derived stem cells transplanted into brain lesions. Med Sci Monit. 2009;15:BR47-BR54. [PubMed] |
| 22. | Yoon J, Choi SC, Park CY, Choi JH, Kim YI, Shim WJ, Lim DS. Bone marrow-derived side population cells are capable of functional cardiomyogenic differentiation. Mol Cells. 2008;25:216-223. [PubMed] |
| 23. | Liu Z, Jiang Y, Hao H, Gupta K, Xu J, Chu L, McFalls E, Zweier J, Verfaillie C, Bache RJ. Endothelial nitric oxide synthase is dynamically expressed during bone marrow stem cell differentiation into endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H1760-H1765. [PubMed] |
| 24. | Hu J, Feng K, Liu X, Ma PX. Chondrogenic and osteogenic differentiations of human bone marrow-derived mesenchymal stem cells on a nanofibrous scaffold with designed pore network. Biomaterials. 2009;30:5061-5067. [PubMed] |
| 25. | Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756-763. [PubMed] |
| 26. | 张 智峰, 赵 钢, 赵 天宇, 朱 英. 重组人粒细胞集落刺激因子动员后自体外周血干细胞在大鼠纤维化肝脏内的发育. 中国组织工程研究与临床康复. 2008;12:569-572. |
| 27. | Oh SH, Witek RP, Bae SH, Zheng D, Jung Y, Piscaglia AC, Petersen BE. Bone marrow-derived hepatic oval cells differentiate into hepatocytes in 2-acetylaminofluorene/partial hepatectomy-induced liver regeneration. Gastroenterology. 2007;132:1077-1087. [PubMed] |
| 28. | Yannaki E, Athanasiou E, Xagorari A, Constantinou V, Batsis I, Kaloyannidis P, Proya E, Anagnostopoulos A, Fassas A. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp Hematol. 2005;33:108-119. [PubMed] |
| 29. | Piscaglia AC, Shupe TD, Oh SH, Gasbarrini A, Petersen BE. Granulocyte-colony stimulating factor promotes liver repair and induces oval cell migration and proliferation in rats. Gastroenterology. 2007;133:619-631. [PubMed] |