修回日期: 2009-08-24
接受日期: 2009-08-31
在线出版日期: 2009-09-18
目的: 探讨大鼠骨髓间充质细胞向肝细胞样细胞的诱导分化, 观察诱导分化细胞的形态和分子生物学特性, 及其对急性肝损伤大鼠的治疗作用.
方法: 分离、培养并纯化大鼠骨髓间充质细胞, 培养基中加入肝细胞生长因子(HGF)、纤维生长因子-4(FGF-4)及上皮细胞生长因子(EGF), 分别于7、14、21、28 d观察细胞形态变化, 采用RT-PCR方法检测细胞白蛋白(ALB)、甲种胎儿球蛋白(AFP)、细胞角蛋白-18(CK-18)的mRNA表达, 将诱导分化28 d的细胞经DAPI染料染色后, 经门静脉注入同种异体大鼠体内, 经24、48、72及168 h分批处死大鼠, 观察大鼠肝组织内荧光细胞的分布. 大鼠腹腔注射硫代乙酰胺制作急性肝衰竭模型, 将1×106及5×106个诱导分化细胞经门静脉注入大鼠体内, 经48、168 h采血查肝功, 168 h处死大鼠, 取肝组织病理学检查.
结果: 大鼠间充质细胞经上述3种细胞因子诱导后, 形态和生物学行为方面都发生向肝细胞样细胞方面的转化, 经门静脉注入大鼠体内后, 有大量该种细胞在肝组织内分布, 24 h荧光细胞最多, 随时间延长逐渐减少, 可持续7 d. 骨髓间充质细胞的诱导分化细胞经门静脉注入肝损伤模型大鼠体内后, 大鼠肝功能有所好转, 注入1×106细胞大鼠ALT由注入前238.0±113.5 U/L, 48 h后降至189±68.4 U/L, 168 h后降至149.0±54.2 U/L, TBIL由注入前2.9±1.6 µmol/L, 48 h至3.0±1.4 µmol/L, 168 h至1.3±0.3 µmol/L; 注入5×106个细胞者(ALT)由注入前238.0±113.5 U/L, 48 h后降至169.7±46.0 U/L, 168 h后降至103.7±46.0 U/L, TBIL由注入前2.9±1.6 µmol/L, 48 h至2.9±1.3 µmol/L, 168 h至0.9±0.3 µmol/L.
结论: 大鼠骨髓间充质细胞可在某些细胞因子的诱导作用下发生类肝细胞样转化, 将转化细胞经门静脉植入同种异体大鼠体内后可在肝组织内存活并对大鼠肝损伤有一定修复作用.
引文著录: 邢全台, 孙启龙, 李栋, 邢培祥, 马瑞萍, 陈丰哲, 邵美英, 姚永远, 关彦彦. 骨髓间充质干细胞向肝细胞样细胞的诱导分化. 世界华人消化杂志 2009; 17(26): 2654-2661
Revised: August 24, 2009
Accepted: August 31, 2009
Published online: September 18, 2009
AIM: To induce the differentiation of rat bone marrow mesenchymal stem cells towards hepatocyte-like cells, observe the morphology and molecular biological feature of differentiated cells and investigate their therapeutic effects against hepatic injury.
METHODS: Rat bone marrow mesenchymal stem cells were separated, cultured and purified. Hepatocyte growth factor (HGF), fibroblast growth factor-4 (FGF-4) and epidermal growth factor (EGF) were then added to culture medium to induce differentiation. The expression of albumin, alpha-fetoprotein (AFP) and cytokeratin 18 (CK 18) mRNAs was detected using reverse transcription-polymerase chain reaction (RT-PCR) on days 7, 14, 21 and 28 after induction. On day 28, the differentiated cells were stained with DAPI (4', 6-diamidino-2-phenylindole) and injected to rats via the portal vein. These rats were sacrificed 24, 48, 72 and 168 hours after injection, respectively. The liver samples were taken to examine fluorescent distribution in the liver under microscopy. Acute hepatic failure (AHF) was induced in rats by intraperitoneal injection of thioacetamide. Differentiated cells at a density of 1 × 106 or 5 × 106 cells/L were injected to AHF rats via the portal vein. Blood samples were taken from these rats to perform liver function tests 48 and 168 hours after injection. The rats were executed 168 hours after injection to take liver samples for histological examination.
RESULTS: After induction with HGF, FGF-4 and EGF, bone marrow mesenchymal stem cells differentiated towards hepatocyte-like cells. The differentiated cells exhibited the morphology and biological behavior of hepatocyte-like cells. After differentiated cells stained with DAPI were injected into rats via the portal vein, many stained cells were distributed in the liver, and some of them could live for more than 7 days. When the differentiated cells were injected into AHF rats via the portal vein, hepatic injury in these rats was improved. In AHF rats injected with differentiated cells at a density of 1 × 106 cells/L, serum alanine aminotransferase (ALT) changed from 238.0 ± 113.5 U/L at baseline to 189 ± 68.4 U/L at 48 hours and 149.0 ± 54.2 U/L at 168 hours, and serum total bilirubin (TBIL) from 2.9 ± 1.6 µmol/L at baseline to 3.0 ± 1.4 µmol/L at 48 hours and 1.3 ± 0.3 µmol/L at 168 hours. In AHF rats injected with differentiated cells at a density of 5 × 106 cells/L, serum ALT changed from 238.0 ± 113.5 U/L at baseline to 169.7 ± 46.0 U/L at 48 hours and 103.7 ± 46.0 U/L at 168 hours, and serum TBIL from 2.9 ± 1.6 µmol/L to 2.9 ± 1.3 µmol/L at 48 hours and 0.9 ± 0.3 µmol/L at 168 hours.
CONCLUSION: Rat bone marrow mesenchymal stem cells can differentiate towards hepatocyte-like cells in the presence of inducing factors. Differentiated cells can live in the liver and improve hepatic injury in rats with AHF.
- Citation: Xing QT, Sun QL, Li D, Xing PX, Ma RP, Chen FZ, Shao MY, Yao YY, Guan YY. Differentiation of bone marrow mesenchymal stem cells towards hepatocyte-like cells in vitro. Shijie Huaren Xiaohua Zazhi 2009; 17(26): 2654-2661
- URL: https://www.wjgnet.com/1009-3079/full/v17/i26/2654.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v17.i26.2654
许多慢性肝脏疾病最终会导致肝衰竭, 此时, 肝脏移植是最佳方案. 然而, 受到供体来源短缺、可能发生的移植排斥反应等限制, 肝移植只能应用于很少一部分肝衰竭患者. 近几十年来, 人们一直在寻找新的治疗方法, 包括人工肝和肝细胞移植等, 干细胞研究则是另一个焦点. 已有许多研究表明[1-10], 干细胞在一定诱发因素作用下, 可能向某些特定的细胞系转化. 而骨髓中含有较多的干细胞, 因此近年对骨髓干细胞分化潜能的研究也较多. 我们对骨髓间充质细胞向肝细胞方向诱导分化的可能性及诱导分化细胞形态功能特性进行了探讨, 并观察诱导分化细胞的形态和分子生物学特性及其对急性肝损伤大鼠的治疗作用.
Wistar大鼠(共50只, ♀, 体质量250±20 g, 清洁级, 随机分为A、B、C、D、E 5组, A、B、C、D用于诱导细胞对肝损伤作用的研究, E组用于移植诱导细胞在大鼠肝脏分布和生存研究), 购自山东大学实验动物中心, 自由饮水, 分笼饲养; DMEM低糖培养基(Invitrogen公司), 胎牛血清(PeproTech公司), 大鼠专用淋巴细胞分离液(Percoll公司), 肝细胞生长因子(hepatic-growth factor, HGF), 成纤维细胞生长因子-4(fibro-growth factor, FGF-4), 上皮细胞生长因子(epidermic-growth factor, EGF), 以上3种因子均购于Peprotech公司, 4, 6-二脒-2苯基吲哚(4', 6'-diamidino-2-phenylindole dihydrochloride, DAPI), 购自Biotium公司, 硫代乙酰胺(天津化学试剂有限公司产品), CO2细胞培养箱(Heraeus公司), 荧光显微镜(Olympus公司), 离心机(上海安亭科学仪器厂), 流式细胞仪(BD Biosciences公司), 全自动生化分析仪(日本日立), ABI实时定量PCR分析仪(美国应用生物系统公司).
1.2.1 干细胞培养、诱导及标记: 无菌取大鼠股骨, 用PBS自骨骺冲出骨髓, 细胞悬液用大鼠专用淋巴细胞分离液分得单个核细胞, 以5×108个/L悬浮于低糖培养基(DMEM+160 mL/L胎牛血清+L-谷氨酰胺+青、链霉素各100 kU/L)中, 置50 mL/L CO2细胞培养箱内常规培养, 待贴壁细胞达瓶底面积70%以上时进行传代; 取传至5-6代、处于对数生长期的骨髓间充质细胞, 加入诱导分化培养基(含HGF、FGF4、EGF终浓度均为20 mg/L, 3 d换液1次, 于处理后7、14、21和28 d倒置光学显微镜直接观察细胞形态变化; 将细胞置于终浓度500 mg/L的DAPI溶液中, 37℃孵育30 min后洗涤3次染色备用.
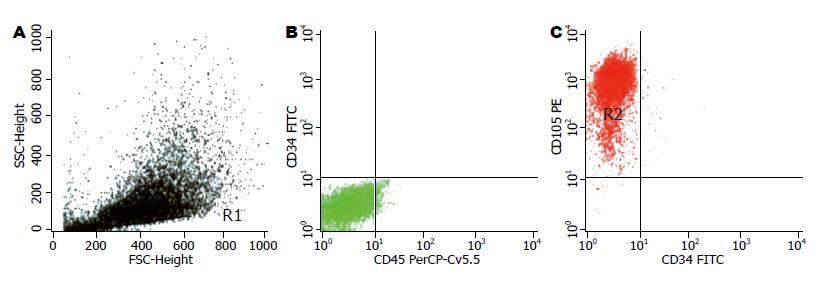
1.2.2 细胞表型检测: 取传至5-6代的细胞2×106, 流式细胞仪检测其表型CD34、CD45、CD105.
1.2.3 RT-PCR方法检测细胞ALB、AFP、CK-18 mRNA的表达: TRIzol试剂提取总RNA, RT-PCR反应按试剂盒说明, PCR反应总体积为25 μL, 扩增程序为95℃ 30 s, 65℃ 45 s, 72℃ 45 s, 5循环; 95℃ 30 s, 60℃ 45 s, 72℃ 45 s, 30循环; 95℃ 30 s, 55℃ 45 s, 72℃ 45 s, 4循环; 最后72℃延长10 min, 实时PCR定量分析仪测定, 设β-actin内参照表达为1. 引物设计参照文献[11], 引物序列见表1.
| 引物 | 序列 |
| ALB | 正义 5'-ATACACCCAGAAAGCACCTC-3' |
| 反义 5'-CACGAATTGTGCGAATG-3' | |
| AFP | 正义5'-AACAGCAGAGTGCTGCAAAC-3' |
| 反义 5'-AGGTTTCGTCCCTCAGAAAG-3' | |
| CK-18 | 正义5'-GGACCTCAGCAAGATCATGGC-3' |
| 反义 5'-CCACGATCTTACGGGTAGTTG-3' |
1.2.4 E组大鼠体内植入染色细胞: 无菌打开大鼠腹腔, 将0.5×106个染色细胞经门静脉注入, 分别在注入细胞的24、48、72及168 h处死大鼠, 取出肝脏, 迅速进行冰冻切片, 荧光显微镜观察肝组织内的荧光分布.
1.2.5 急性肝损伤造模: 参照文献[12], 分别将5%硫代乙酰胺溶液按300 mg/kg予A、B、C 3组大鼠腹腔内注射, 24 h重复1次, 观察大鼠活动、饮食、毛发等变化.
1.2.6 诱导细胞注入: 手术打开上述3组大鼠腹腔, 找出门静脉, A组门静脉注入生理盐水1 mL, B组注入1 mL浓度1×106/L诱导分化细胞, C组注入1 mL浓度5×106诱导细胞, 注入前1 h及术后48、168 h分别经尾静脉采血0.5 mL, D组未经任何处理, 为空白对照组. 全自动生化分析仪测定血清ALT、AST、TBIL、ALB. 168 h后处死大鼠, 取出肝脏组织, 100 g/L甲醛固定, HE染色切片镜检.
统计学处理 采用SSPS13.0软件, 计量资料采用t检验.
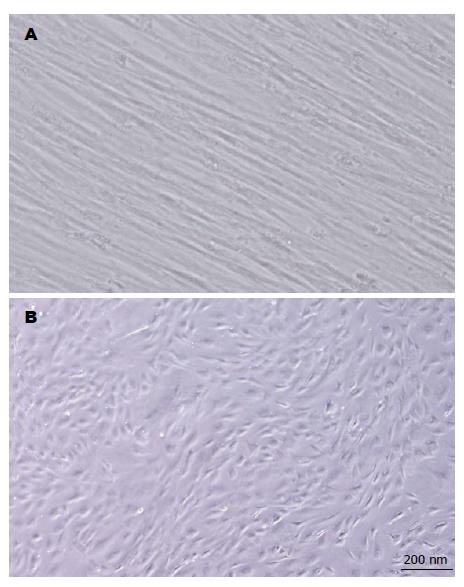
一般传4代后, 细胞形态均匀一致, 呈条索状, 或纺锤形, 分布均匀, 排列紧密, 方向一致(图1A).
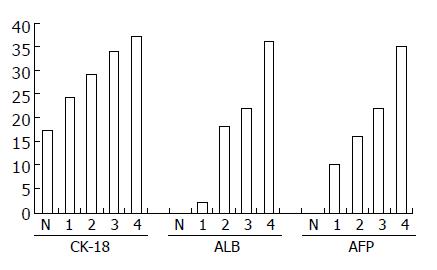
随诱导分化时间的延长, 细胞逐渐变粗短, 成多角形或不规则形(图1B). ALB、CK18及AFP mRNA的表达随诱导分化时间的延长而增加(图3).
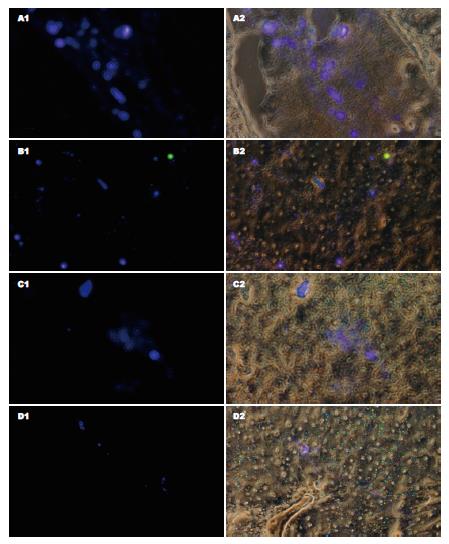
门静脉注入细胞24 h, 可见较多荧光细胞在肝组织内分布, 48 h荧光细胞主要分布于肝索, 72 h后, 荧光细胞减少, 168 h仍可见少量荧光细胞散在分布(图4).
重复腹腔内注射5%硫代乙酰胺溶液按300 mg/kg 2次, 可见大鼠活动减少, 饮食减少, 毛发蓬松, 无光泽, 血清ALT、AST、TBIL明显升高, ALB显著下降(表2). 门静脉骨髓间充质细胞诱导分化细胞后血清ALT、AST、TBIL在48 h有所下降, 其中注入较多细胞者(C组)下降较明显, 168 h后这种变化更加明显. 白蛋白在注入细胞48 h后B、C 2组与A组均无明显差异, 而在168 h后, C组ALB有显著上升, B、C 2组之间存在明显差异(表3-4).
| 检测项目 | 注射前 | 注射后 | P值 |
| ALT(U/L) | 35.2±7.9 | 238.0±113.5 | 0.000 |
| AST(U/L) | 67.2±11.9 | 343.7±56.0 | 0.000 |
| TBIL(μmol/L) | 0.5±0.3 | 2.9±1.6 | 0.001 |
| ALB(g/L) | 46.8±13.8 | 27.6±16.7 | 0.003 |
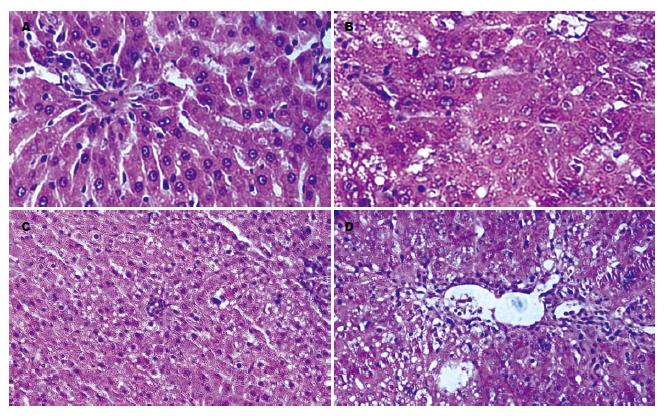
显示注入骨髓间充质细胞之诱导分化细胞后大鼠肝组织病理损害有所减轻(图5). 正常肝组织细胞排列整齐, 结构清晰, 胞质内无空泡, 间质无白细胞浸润(图5A), 经硫代乙酰胺注射的大鼠肝组织结构紊乱、细胞边界不清、胞质内可见空泡、间质内可见白细胞浸润(图5B), 经门静脉注入5×106诱导细胞后168 h大鼠肝组织结构(图5C)较注入生理盐水168 h后的大鼠肝组织结构(图5D)清晰, 胞内空泡数量少, 白细胞浸润程度轻.
我们采用大鼠骨髓为研究对象, 根据骨髓间充质细胞贴壁生长的特点, 经过4-5次传代即可得到形态较一致, 呈梭形或纺锤形的细胞, 符合间充质细胞的形态特点. 细胞表型检测, CD34与CD45基本无表达, 而95%的细胞CD105阳性表达, 与文献[13]报道一致, 可以认为所培养细胞为骨髓间充质干细胞. 其多能干细胞, 具有潜在的分化能力, 在适当条件下, 可能分化为肝细胞样细胞[7,14-17]、软骨样细胞[18-20]、神经细胞样细胞[2,21-22]、心肌细胞样细胞[10,23-24]、胰岛细胞样细胞[4,25-26]、雪旺细胞样细胞[27]等. 我们在培养基内加入HGF、FGF-4和EGF, 促使骨髓间充质干细胞向肝细胞样细胞分化, 经过2-4 wk后, 细胞长度变短, 形态由梭形和纺锤形变为多边形和不规则形, 细胞内颗粒增加, 细胞开始表达ALB、AFP、CK-18 mRNA并随时间延长, 表达强度逐渐增加, 提示细胞具备了肝细胞的部分功能.
观察干细胞或其诱导细胞在移植后的动态变化, 有助于我们评估干细胞或其诱导细胞移植治疗器官功能衰竭的可行性, 找到合适的细胞移植方法和途径, 是细胞移植成功并发挥治疗作用的重要前提. 活体跟踪这些移植细胞的手段还较少, 用氧化铁标记细胞, MRI观测标记细胞的分布及代谢[28-29], 或放射性核素标记细胞, PET观测标记细胞行踪都得到一定经验[30], 腺病毒介导的绿色荧光蛋白或β-半乳糖苷酶表达, 或DAPI细胞核染色观测细胞分布和生物学变化也在探索中[31-32], 为观察诱导分化细胞在移植入动物体内的动态变化, 我们对细胞先进性染色标记, 实验中我们将诱导细胞经荧光标记后经门静脉注入大鼠体内, 观察到荧光细胞在肝组织内的分布, 可持续1 wk, 但1 wk后的荧光细胞分布数量较前减少, 说明随时间延长, 移植的诱导细胞逐渐死亡并被清除, 其死亡原因是移植排斥反应抑或染色剂对细胞毒性作用, 尚未知.
对于骨髓干细胞能否诱导分化为肝细胞样细胞, 体外培养得到的答案是肯定的[7,17,33], 在动物体内移植骨髓干细胞的研究中, 也得到了一些有意义的发现, 如骨髓干细胞移植可减轻肝脏损伤[34], 骨髓干细胞移植能够减轻移植排斥反应[35], 而在移植细胞的体内分布和存活方面的研究相对较少. 为观察经诱导分化的骨髓干细胞治疗急性肝损伤的可能性, 我们经2次腹腔内注入硫代乙酰胺后制作了大鼠急性肝损伤模型, 并由门静脉注入不同数量的诱导分化细胞, 经过48 h后, 大鼠肝衰竭指标出现不同程度的改善, 部分指标与对照组(生理盐水)相比有显著差异, 另有些指标改善, 但差异不具显著性. 说明诱导细胞均可在肝组织存活一定时间并发挥类肝细胞的功能, 该作用与植入大鼠体内的细胞数量有相关性.
肝衰竭是慢性肝病的主要致死原因, 肝移植是最有效的治疗手段, 然而, 肝脏来源有限, 且受到排异反应的困扰, 因此其临床应用受到限制, 迫切需要找到一种替代性器官来源. 干细胞培养是近年迅速发展起来的一项技术, 研究表明, 骨髓中含有大量间充质干细胞, 这些细胞具有多种分化潜能, 在合适的条件下, 可向相应的细胞系分化.
潘兴华, 副主任医师, 中国人民解放军成都军区昆明总医院病理实验科
何忠杰 et al报道: 骨髓多能干细胞具有潜在分化能力, 在适当条件下, 可能分化为肝细胞样细胞、软骨样细胞、神经细胞样细胞、心肌细胞样细胞、胰岛细胞样细胞、雪旺细胞样细胞等.
本研究应用荧光染色方法观察了骨髓干细胞诱导分化细胞通过门静脉注入大鼠体内后在大鼠肝脏内的分布和存活时间, 并分析了门静脉注入骨髓干细胞诱导分化细胞对大鼠急性肝损伤的修复作用.
本研究对进一步探讨骨髓间充质干细胞向肝细胞样细胞的诱导分化, 对人类肝衰竭的治疗有一定的借鉴意义.
DAPI: 是一种荧光染料, 脂溶性, 能够穿透细胞膜深入细胞核, 染色较牢固, 是观测细胞在体内分布和存在的重要手段, 缺点是不能观测细胞动态.
本文的主要创新点是观察了骨髓间充质干细胞来源的肝样细胞在损伤肝组织中的存活和分布.
编辑: 李军亮 电编:何基才
| 1. | Hu J, Feng K, Liu X, Ma PX. Chondrogenic and osteogenic differentiations of human bone marrow-derived mesenchymal stem cells on a nanofibrous scaffold with designed pore network. Biomaterials. 2009;30:5061-5067. [PubMed] |
| 2. | Yang T, Tsang KS, Poon WS, Ng HK. Neurotrophism of bone marrow stromal cells to embryonic stem cells: noncontact induction and transplantation to a mouse ischemic stroke model. Cell Transplant. 2009;18:391-404. [PubMed] |
| 3. | Ghazanfari S, Tafazzoli-Shadpour M, Shokrgozar MA. Effects of cyclic stretch on proliferation of mesenchymal stem cells and their differentiation to smooth muscle cells. Biochem Biophys Res Commun. 2009;388:601-605. [PubMed] |
| 4. | Snarski E, Torosian T, Paluszewska M, Urbanowska E, Milczarczyk A, Jedynasty K, Franek E, Jedrzejczak WW. Alleviation of exogenous insulin requirement in type 1 diabetes mellitus after immunoablation and transplantation of autologous hematopoietic stem cells. Pol Arch Med Wewn. 2009;119:422-426. [PubMed] |
| 5. | Tao XR, Li WL, Su J, Jin CX, Wang XM, Li JX, Hu JK, Xiang ZH, Lau JT, Hu YP. Clonal mesenchymal stem cells derived from human bone marrow can differentiate into hepatocyte-like cells in injured livers of SCID mice. J Cell Biochem. 2009; Aug 19. [Epub ahead of print]. [PubMed] |
| 6. | Boeloni JN, Ocarino NM, Melo AB, Silva JF, Castanheira P, Goes AM, Serakides R. Dose-dependent effects of triiodothyronine on the osteogenic differentiation of rat bone marrow mesenchymal stem cells. Horm Res. 2009;72:88-97. [PubMed] |
| 7. | Chivu M, Dima SO, Stancu CI, Dobrea C, Uscatescu V, Necula LG, Bleotu C, Tanase C, Albulescu R, Ardeleanu C. In vitro hepatic differentiation of human bone marrow mesenchymal stem cells under differential exposure to liver-specific factors. Transl Res. 2009;154:122-132. [PubMed] |
| 8. | Elvenes J, Knutsen G, Johansen O, Moe BT, Martinez I. Development of a new method to harvest chondroprogenitor cells from underneath cartilage defects in the knees. J Orthop Sci. 2009;14:410-417. [PubMed] |
| 9. | Sheng Z, Fu X, Cai S, Lei Y, Sun T, Bai X, Chen M. Regeneration of functional sweat gland-like structures by transplanted differentiated bone marrow mesenchymal stem cells. Wound Repair Regen. 2009;17:427-435. [PubMed] |
| 10. | Brunskill SJ, Hyde CJ, Doree CJ, Watt SM, Martin-Rendon E. Route of delivery and baseline left ventricular ejection fraction, key factors of bone-marrow-derived cell therapy for ischaemic heart disease. Eur J Heart Fail. 2009;11:887-896. [PubMed] |
| 14. | Hong H, Chen JZ, Zhou F, Xue L, Zhao GQ. Influence of serum from liver-damaged rats on differentiation tendency of bone marrow-derived stem cells. World J Gastroenterol. 2004;10:2250-2253. [PubMed] |
| 15. | 唐 力军, 高 毅, 张 志, 李 浩, 单 毓强. 肝细胞生长因子联合成纤维细胞生长因子-4诱导人骨髓来源的多能成体祖细胞向肝样细胞分化. 中华肝脏病杂志. 2005;13:652-655. |
| 17. | Saulnier N, Lattanzi W, Puglisi MA, Pani G, Barba M, Piscaglia AC, Giachelia M, Alfieri S, Neri G, Gasbarrini G. Mesenchymal stromal cells multipotency and plasticity: induction toward the hepatic lineage. Eur Rev Med Pharmacol Sci. 2009;13 Suppl 1:71-78. [PubMed] |
| 18. | Alfaqeh H, Norhamdan MY, Chua KH, Chen HC, Aminuddin BS, Ruszymah BH. Cell based therapy for osteoarthritis in a sheep model: gross and histological assessment. Med J Malaysia. 2008;63 Suppl A:37-38. [PubMed] |
| 19. | Grässel S, Ahmed N, Göttl C, Grifka J. Gene and protein expression profile of naive and osteo-chondrogenically differentiated rat bone marrow-derived mesenchymal progenitor cells. Int J Mol Med. 2009;23:745-755. [PubMed] |
| 20. | Nakamura S, Yamada Y, Baba S, Kato H, Kogami H, Takao M, Matsumoto N, Ueda M. Culture medium study of human mesenchymal stem cells for practical use of tissue engineering and regenerative medicine. Biomed Mater Eng. 2008;18:129-136. [PubMed] |
| 21. | Lin JR, Guo KY, Li JQ, Yan DA. In vitro culture of human bone marrow mesenchymal stem cell clonies and induced differentiation into neuron-like cells. Diyi Junyi Daxue Xuebao. 2003;23:251-253, 264. [PubMed] |
| 22. | Cheng Z, Zheng Q, Wang W, Guo X, Wu Y, Zheng J. Targeted induction of differentiation of human bone mesenchymal stem cells into neuron-like cells. J Huazhong Univ Sci Technolog Med Sci. 2009;29:296-299. [PubMed] |
| 23. | Genovese JA, Spadaccio C, Rivello HG, Toyoda Y, Patel AN. Electrostimulated bone marrow human mesenchymal stem cells produce follistatin. Cytotherapy. 2009;11:448-456. [PubMed] |
| 24. | Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009; Jul 8. [Epub ahead of print]. [PubMed] |
| 25. | Xie QP, Huang H, Xu B, Dong X, Gao SL, Zhang B, Wu YL. Human bone marrow mesenchymal stem cells differentiate into insulin-producing cells upon microenvironmental manipulation in vitro. Differentiation. 2009;77:483-491. [PubMed] |
| 26. | Figliuzzi M, Cornolti R, Perico N, Rota C, Morigi M, Remuzzi G, Remuzzi A, Benigni A. Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant Proc. 2009;41:1797-1800. [PubMed] |
| 27. | 王 婧, 刘 开彦. 人骨髓间充质干细胞体外分化为雪旺样细胞的方法. 北京大学学报(医学版). 2003;35:202-206. |
| 28. | Saldanha KJ, Piper SL, Ainslie KM, Kim HT, Majumdar S. Magnetic resonance imaging of iron oxide labelled stem cells: applications to tissue engineering based regeneration of the intervertebral disc. Eur Cell Mater. 2008;16:17-25. [PubMed] |
| 29. | Reddy AM, Kwak BK, Shim HJ, Ahn C, Cho SH, Kim BJ, Jeong SY, Hwang SJ, Yuk SH. Functional characterization of mesenchymal stem cells labeled with a novel PVP-coated superparamagnetic iron oxide. Contrast Media Mol Imaging. 2009;4:118-126. [PubMed] |
| 30. | Zhang Y, Ruel M, Beanlands RS, deKemp RA, Suuronen EJ, DaSilva JN. Tracking stem cell therapy in the myocardium: applications of positron emission tomography. Curr Pharm Des. 2008;14:3835-3853. [PubMed] |
| 31. | Leiker M, Suzuki G, Iyer VS, Canty JM Jr, Lee T. Assessment of a nuclear affinity labeling method for tracking implanted mesenchymal stem cells. Cell Transplant. 2008;17:911-922. [PubMed] |
| 32. | Schormann W, Hammersen FJ, Brulport M, Hermes M, Bauer A, Rudolph C, Schug M, Lehmann T, Nussler A, Ungefroren H. Tracking of human cells in mice. Histochem Cell Biol. 2008;130:329-338. [PubMed] |
| 33. | Gu J, Shi X, Zhang Y, Chu X, Hang H, Ding Y. Establishment of a three-dimensional co-culture system by porcine hepatocytes and bone marrow mesenchymal stem cells in vitro. Hepatol Res. 2009;39:398-407. [PubMed] |
| 34. | Harb R, Xie G, Lutzko C, Guo Y, Wang X, Hill CK, Kanel GC, DeLeve LD. Bone marrow progenitor cells repair rat hepatic sinusoidal endothelial cells after liver injury. Gastroenterology. 2009;137:704-712. [PubMed] |
| 35. | Hong ZF, Huang XJ, Yin ZY, Zhao WX, Wang XM. Immunosuppressive function of bone marrow mesenchymal stem cells on acute rejection of liver allografts in rats. Transplant Proc. 2009;41:403-409. [PubMed] |