修回日期: 2009-07-10
接受日期: 2009-07-13
在线出版日期: 2009-08-28
目的: 探讨炎症性肠病患者肠黏膜CD14的表达及与该病的关系.
方法: 收集活动性溃疡性结肠炎(ulcerative colitis, UC)患者25例及克罗恩病(Crohn's disease, CD)患者15例; 20例对照来自于非炎症性肠病患者手术切除的正常结肠组织(肠镜及肠黏膜病理组织学检查结果均正常). 炎症性肠病的临床诊断均依据于常规影像学、内镜学及组织学标准. 采用免疫组织化学方法检测肠黏膜组织中CD14表达量.
结果: 肠黏膜固有层单个核细胞表达CD14. UC患者CD14阳性细胞百分数高于CD患者, 但二者差异无统计学意义(P>0.05); UC患者较正常对照有显著性差异(t = 4.404, P<0.01), CD患者较正常对照亦见显著性差异(t = 3.324, P<0.01). CD14的表达与疾病活动度(disease activity index, DAI)相关, UC组轻、中、重度比较有显著性差异(F = 56.709, P<0.01); CD组轻、中、重度比较亦有显著性差异(F = 12.880, P<0.01).
结论: CD14参与了炎症性肠病的发病过程, 其表达强度反映了该病的程度.
引文著录: 吴思梦, 徐德魁, 郑长青. 炎症性肠病患者肠黏膜白细胞分化抗原-14的表达及意义. 世界华人消化杂志 2009; 17(24): 2526-2529
Revised: July 10, 2009
Accepted: July 13, 2009
Published online: August 28, 2009
AIM: To investigate the expression of CD14 in intestinal mucosa of patients with inflammatory bowel disease and its relationship with the disease.
METHODS: Specimens from 25 cases of ulcerative colitis (UC) and 15 Crohn's disease (CD) and 20 controls were studied by immunohistochemical staining. The clinical diagnosis of inflammatory bowel disease was confirmed according to the routine radiologic, endoscopic and histologic criteria. All controls had normal colonoscopy, and the mucosal biopsies were histologically normal.
RESULTS: There was expression of CD14 in lamina propria. Percent of positive cells in intestinal mucosa in ulcerative colitis was higher than that in Crohn's disease, but without significant difference (P > 0.05). There was significant difference between ulcerative colitis and controls (t = 4.404, P < 0.01). Difference was also significant between Crohn's disease and controls (t = 3.324, P < 0.01). CD14 was positively related to the disease activity index. There was significant difference among slight, moderate and severe UC (F = 56.709, P < 0.01) and as well as among the slight, moderate and severe CD (F = 12.880, P < 0.01).
CONCLUSION: CD14 may be involved in the pathogenesis of inflammatory bowel disease, and its intensity reflects the activity of the disease.
- Citation: Wu SM, Xu DK, Zheng CQ. Expression of cluster of differentiation antigen-14 in intestinal mucosa of patients with inflammatory bowel disease and its implication. Shijie Huaren Xiaohua Zazhi 2009; 17(24): 2526-2529
- URL: https://www.wjgnet.com/1009-3079/full/v17/i24/2526.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v17.i24.2526
白细胞分化抗原-14(cluster of differentiation antigen 14, CD14)是一种特异性巨噬细胞表面标志物, 是脂多糖(lipopolysaccharide, LPS)高亲和受体, 与LPS结合后激活巨噬细胞获得多种增强活性, 如抗微生物活性、细胞因子分泌、抗原递呈等, 在介导慢性肠道炎症中起着关键作用. 本实验对CD14在炎症性肠病(inflammatory bowel disease, IBD)患者体内表达及与疾病活动性的关系进行研究, 以明确CD14在炎症性肠病发病机制中的作用.
病例组选择中国医科大学盛京医院1995-10/2007-04经病理证实的溃疡性结肠炎(ulcerative colitis, UC)及克罗恩病(Crohn disease, CD)患者, IBD的临床诊断均依据常规影像学、内镜学及组织学标准, 均为活动期. UC组25例, 男13例, 女12例, 平均年龄49.73(13-79)岁, 按照Southerland[1]疾病活动指数评估, 轻度8例, 中度14例, 重度3例; CD组15例, 男10例, 女5例, 平均年龄55.92(26-66)岁, 按照Harvey和Bradshow简化标准[1]评估, 轻度6例, 中度7例, 重度2例. 正常对照20例, 选取非炎症性肠病患者手术切除的正常结肠组织, 男11例, 女9例, 平均年龄45.57(17-67)岁. 病例组及对照组均未患有血液病、过敏性疾病及其他感染性疾病.
全部蜡块均行4 μm厚连续切片, 常规脱蜡至水, 室温下30 mL/L过氧化氢去除内源性过氧化物酶, 高温高压修复抗原; 滴加一抗(CD14抗体即用型, 北京中杉公司), 4℃孵育过夜; 滴加二抗(即用型通用型, 北京中杉公司), 37℃, 30 min; DAB显色, 苏木素复染, 中性树胶封片. 组织切片中显示胞质染为淡黄色至黄棕色者为阳性细胞标志, 凡显色强度与背景无明显差别者为阴性. 肠黏膜固有层CD14表达以计数1000倍高倍镜下4个视野内平均阳性细胞百分数. 盲法计数并由两人独立判断, 取其平均值.
统计学处理 采用SPSS11.5统计软件进行t检验及One-way Anova检验, 以P<0.05为差异有意义, 以P<0.01为差异有显著意义.
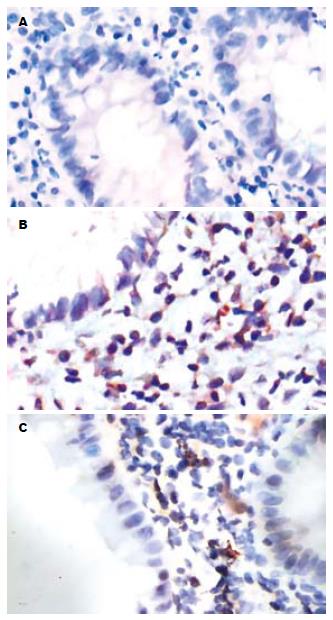
IBD患者肠黏膜固有层细胞阳性为胞质着色; 正常肠黏膜固有层未见阳性细胞(图1). UC患者肠黏膜CD14免疫组织化学染色显示阳性细胞百分数分别从0%-16.55%不等, CD患者中0%-9.38%不等, 正常肠黏膜中均为0. 比较活动期UC、CD患者肠黏膜阳性细胞百分数, 发现UC患者CD14阳性细胞百分数高于CD患者, 但二者差异无统计学意义(t = 1.393, P = 0.175>0.05); UC患者较正常对照有显著性差异(t = 4.404, P = 0.001<0.01), CD患者较正常对照亦见显著性差异(t = 3.324, P = 0.006<0.01).
IBD是一组病因不明的慢性肠道炎症性疾病, 包括UC和CD. IBD发病与环境、感染、遗传、免疫等因素有关. 其中免疫紊乱在IBD发病机制中起着重要的作用[2]. IBD被认为是由于正常肠道菌群的提呈导致黏膜免疫系统不适当/过度激活造成的. 在这个过程中, 免疫活性单核/巨噬细胞通过调节细胞及效应细胞导致并维持慢性炎症.
CD14主要存在于单核/巨噬细胞表面, 部分来源于粒细胞. CD14的表达具有相对的组织和细胞特异性, 而其表达的组织器官特异性与其细胞的组织分布有关, 多种因素可以影响CD14的表达, 离体单核细胞向巨噬细胞成熟这一过程、多种细胞因子及LPS都会影响CD14的表达. 其中以腹腔巨噬细胞高表达, 而枯否细胞、肺泡巨噬细胞和小神经胶质细胞均呈低表达. 现有文献[3]报道, 败血症、外源性过敏性肺泡炎、急性单核细胞白血病、急性髓单核细胞白血病、冠心病患者血清中CD14水平升高.
血清中存在LPS结合蛋白(lipopolysac-charide binding protein, LBP), LPS与LBP结合形成LPS/LBP复合物, 然后与细胞膜上的受体CD14相互作用, CD14进一步参与存在于细胞膜上的跨膜受体Toll样受体/核因子-κB(toll-like receptors/nuclear factor-κB, TLRs/NF-κB)通路, 导致巨噬细胞激活[4]. 有研究发现[5], 活动性UC和CD的肠黏膜巨噬细胞亚群的表型与正常黏膜的不同, 其中包括在CD的肉芽肿及活动性UC组织中发现的RFD9阳性巨噬细胞和3G8阳性巨噬细胞. 巨噬细胞激活后, 产生TNF2α、IL-21、IL-26等多种前炎症细胞因子, 导致炎症反应发生, 在介导初次免疫应答和炎症反应过程中具有多种作用[6]. 其中IL-21在消化系能调节除增强Th1细胞免疫反应外的其他的炎症路径, 并在CD中促进慢性进行性黏膜炎症[7-8]. Cario et al[9]发现UC和CD患者整个结肠及回肠末端上皮过度表达TLR4, 过量的TLR4会导致NF-κB过度激活, 产生持续扩大的炎症反应, 表现为炎症介质的过量表达. 目前研究表明[10], TLR4在CD患者回肠末端及UC患者直肠肠黏膜上调, UC患者回肠末端则是TLR2上调. 关于基因方面的研究, 国外发现[11], IBD与CD14 c.1-260C>T启动子变异相关, 而与TLR4 c.896A>G变异无关.
Rugtveit et al[12]发现在活动期IBD黏膜固有层巨噬细胞表达CD14增加. 也有研究表明[11], 活动期及缓解期CD患者回肠末端和UC患者直肠肠黏膜巨噬细胞均高度表达CD14. 本研究也证实活动期UC、CD肠黏膜固有层CD14阳性细胞百分数显著高于正常肠黏膜, 并且CD14的表达在IBD活动度间有明显差异, 病情越重, CD14表达越强. Grimm et al[5]研究显示, 活动期IBD肠黏膜表达CD14的巨噬细胞占固有层所有单个核细胞的25.1%, 而正常肠黏膜表达CD14的巨噬细胞仅占固有层所有单个核细胞的3.7%. 本实验对照组正常肠黏膜未见CD14阳性细胞, 可能与样本量较少有关.
炎症性肠病传统药物治疗存在很大的局限性, 5-氨基水杨酸和柳氮磺胺吡啶被认为是UC的治疗支柱, 但对CD患者却不能有效地维持缓解[13]. 目前, 在IBD的治疗中, 新的理念正逐渐形成, 其中分子靶标阻断炎症、免疫途径理念取得了较大进展[14].
Le Roy et al[15]研制了一种阻断LPS/LBP复合物与CD14相互作用的抗体, 结果显示有效抑制了LPS介导的TNF的产生. 而抗CD14 mAb也明显抑制了LPS与鼠巨噬细胞的结合. 在CD14参与的IBD免疫发病过程中, 阻断CD14的治疗方案有望成为治疗IBD的新手段.
炎症性肠病(IBD)是一组病因不明的慢性肠道炎症性疾病, 其中免疫紊乱在IBD发病机制中起着重要的作用. 白细胞分化抗原-14(CD14)是一种特异性巨噬细胞表面标志物, 与LPS结合后激活巨噬细胞获得多种增强活性, 在介导慢性肠道炎症中起着关键作用.
季国忠, 教授, 南京医科大学第二附属医院消化科
IBD的病因和发病机制尚不清楚, CD14可能在IBD中发挥重要作用. CD14有望控制IBD发作、维持其缓解, 提高IBD患者的生存质量.
Rugtveit et al发现在活动期IBD肠黏膜固有层巨噬细胞表达CD14增加; Grimm et al研究显示, 活动期IBD肠黏膜表达CD14的巨噬细胞占固有层所有单个核细胞的25.1%, 而正常肠黏膜表达CD14的巨噬细胞仅占固有层所有单个核细胞的3.7%.
本文检测CD14在IBD患者肠黏膜固有层的表达, 并在其表达强度与疾病活动度间进行统计学分析, 从临床角度探讨CD14在IBD发病机制中的作用.
本文探讨了IBD患者肠黏膜白细胞分化抗原-14 (CD14)的表达, 提出CD14参与了IBD免疫发病过程, 对IBD的发病机制研究和临床治疗有一定的指导价值.
编辑: 李军亮 电编:何基才
| 2. | Yoon JS, Newton SM, Wysocka M, Troxel AB, Hess SD, Richardson SK, Lin JH, Benoit BM, Kasprzycka M, Wasik MA. IL-21 enhances antitumor responses without stimulating proliferation of malignant T cells of patients with Sézary syndrome. J Invest Dermatol. 2008;128:473-480. [PubMed] [DOI] |
| 4. | Gegner JA, Ulevitch RJ, Tobias PS. Lipopolysaccharide (LPS) signal transduction and clearance. Dual roles for LPS binding protein and membrane CD14. J Biol Chem. 1995;270:5320-5325. [PubMed] |
| 5. | Grimm MC, Pavli P, Van de Pol E, Doe WF. Evidence for a CD14+ population of monocytes in inflammatory bowel disease mucosa--implications for pathogenesis. Clin Exp Immunol. 1995;100:291-297. [PubMed] |
| 6. | Hyakushima N, Mitsuzawa H, Nishitani C, Sano H, Kuronuma K, Konishi M, Himi T, Miyake K, Kuroki Y. Interaction of soluble form of recombinant extracellular TLR4 domain with MD-2 enables lipopolysaccharide binding and attenuates TLR4-mediated signaling. J Immunol. 2004;173:6949-6954. [PubMed] |
| 7. | Fina D, Caruso R, Pallone F, Monteleone G. Interleukin-21 (IL-21) controls inflammatory pathways in the gut. Endocr Metab Immune Disord Drug Targets. 2007;7:288-291. [PubMed] [DOI] |
| 8. | Shih DQ, Targan SR. Immunopathogenesis of inflammatory bowel disease. World J Gastroenterol. 2008;14:390-400. [PubMed] [DOI] |
| 9. | Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010-7017. [PubMed] [DOI] |
| 10. | Frolova L, Drastich P, Rossmann P, Klimesova K, Tlaskalova-Hogenova H. Expression of Toll-like receptor 2 (TLR2), TLR4, and CD14 in biopsy samples of patients with inflammatory bowel diseases: upregulated expression of TLR2 in terminal ileum of patients with ulcerative colitis. J Histochem Cytochem. 2008;56:267-274. [PubMed] [DOI] |
| 11. | Baumgart DC, Buning C, Geerdts L, Schmidt HH, Genschel J, Fiedler T, Gentz E, Molnar T, Nagy F, Lonovics J. The c.1-260C>T promoter variant of CD14 but not the c.896A>G (p.D299G) variant of toll-like receptor 4 (TLR4) genes is associated with inflammatory bowel disease. Digestion. 2007;76:196-202. [PubMed] [DOI] |
| 12. | Rugtveit J, Nilsen EM, Bakka A, Carlsen H, Brandtzaeg P, Scott H. Cytokine profiles differ in newly recruited and resident subsets of mucosal macrophages from inflammatory bowel disease. Gastroenterology. 1997;112:1493-1505. [PubMed] [DOI] |
| 13. | Oldenburg B, Hommes D. Biological therapies in inflammatory bowel disease: top-down or bottom-up? Curr Opin Gastroenterol. 2007;23:395-399. [PubMed] [DOI] |
| 15. | Le Roy D, Di Padova F, Tees R, Lengacher S, Landmann R, Glauser MP, Calandra T, Heumann D. Monoclonal antibodies to murine lipopolysaccharide (LPS)-binding protein (LBP) protect mice from lethal endotoxemia by blocking either the binding of LPS to LBP or the presentation of LPS/LBP complexes to CD14. J Immunol. 1999;162:7454-7460. [PubMed] |